Abstract
Advances in standards of care have extended the life expectancy of patients with kidney failure. However, options for chronic vascular access for haemodialysis — an essential part of kidney replacement therapy — have remained unchanged for decades. The high morbidity and mortality associated with current vascular access complications highlights an unmet clinical need for novel techniques in vascular access and is driving innovation in vascular access care. The development of devices, biological approaches and novel access techniques has led to new approaches to controlling fistula geometry and manipulating the underlying cellular and molecular pathways of the vascular endothelium, and influencing fistula maturation and formation through the use of external mechanical methods. Innovations in arteriovenous graft materials range from small modifications to the graft lumen to the creation of completely novel bioengineered grafts. Steps have even been taken to create new devices for the treatment of patients with central vein stenosis. However, these emerging therapies face difficult hurdles, and truly creative approaches to vascular access need resources that include well-designed clinical trials, frequent interaction with regulators, interventionalist education and sufficient funding. In addition, the heterogeneity of patients with kidney failure suggests it is unlikely that a ‘one-size-fits-all’ approach for effective vascular access will be feasible in the current environment.
Despite growing recognition of and attention to the importance of chronic kidney disease (CKD) in health care, kidney failure remains a worldwide public health concern1. Globally, prevalence rates of kidney failure continue to rise2, with 1.2 million deaths from kidney failure in 2015 (REF3). The US Renal Data System (USRDS) 2018 annual report2 reported over 700,000 prevalent cases of treated kidney failure in 2016. This number has only continued to increase4, and trend estimates suggest that the number of patients with treated kidney failure will increase by 29–68% in the USA by 2030 (REF5). At a global level, the number of patients with treated kidney failure is expected to increase from 2.6 million in 2010 to 5.4 million in 2030 (REF.4). Alarmingly, these numbers do not reflect the number of patients who need kidney replacement therapy (KRT) but do not receive it; in 2010, up to 7 million people were estimated to need KRT but were not able to receive it owing to resource limitations4. This number is unfortunately likely to increase to 9 million people by 2030 (REF6).
The economic burden of kidney failure is also substantial. In the USA in 2010, total Medicare spending on KRT was $34 billion7, with hospitalizations driving up to 35% of the cost of care for patients on haemodialysis8. Worldwide, per patient costs for haemodialysis can reach ~US$100,000 (~€88,000) per year9. Increasing the use of home dialysis could potentially offset some of these costs10; however, home dialysis remains under-utilized in many regions and limits the opportunities for trained dialysis care providers and practising nephrologists to examine patients.
More than 2.6 million patients worldwide received KRT in 2010 (REFS2,11), and most of these patients received haemodialysis4. In the USA, long-term haemodialysis accounts for >60% of KRT for kidney failure (approximately 500,000 patients)2. However, despite the fact that long-term haemodialysis has been used since the introduction of the first arterial shunt in the 1960s, vascular access — the key lifeline for haemodialysis — remains difficult, with unacceptably high morbidity and mortality. Access-related complications remain associated with high rates of hospitalization12 and infection13. In addition, the cost of vascular access complications is prohibitively high2,14; in the USA in 2013, the annual total direct cost relating to vascular access management was estimated to be $5 billion15.
Given the growing prevalence of kidney failure, the extremely high morbidity, mortality and cost of dialysis vascular access, and the expectation that haemodialysis will remain the mainstay of KRT at least in the immediate future, there remains a significant unmet clinical need to address current limitations in vascular access for haemodialysis. Efforts to address this unmet need are driving critical innovation and research into novel techniques and processes of care to improve long-term vascular access for chronic haemodialysis. In this Review, we evaluate the key existing challenges in establishing and maintaining vascular access in patients receiving haemodialysis, with regard to both the underlying biology and aspects relating to the process of care and implementation. We then describe novel and innovative technologies under development that address these issues and summarize new policy initiatives that emphasize the importance of innovation in vascular access for kidney disease therapeutics.
Challenges in vascular access
Effective treatment of kidney failure with haemodialysis is dependent on reliable vascular access to enable blood exchange several times per week. Currently, vascular access for haemodialysis is achieved through creation of an arteriovenous (AV) fistula, generally in the upper arm or forearm of the patient, placement of a graft that connects an artery and vein (AV graft), or placement of a central venous catheter (CVC) (FIG. 1). However, all of these methods are associated with limitations that drive mortality and decrease the quality of life of patients with kidney failure (FIG. 2).
Fig. 1 |. Current vascular access routes for haemodialysis.
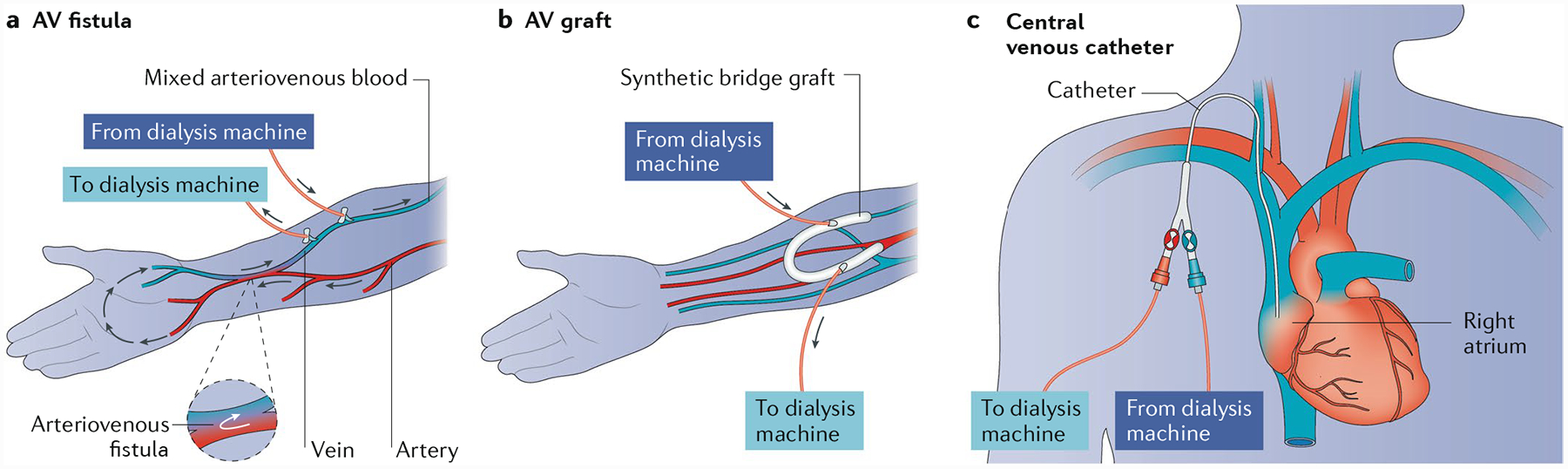
Currently, vascular access for haemodialysis is achieved through one of three methods: a | creation of an arteriovenous (AV) fistula, generally in the upper arm or forearm of the patient; b | placement of a graft that connects the artery and vein (AV graft); or c | with a central venous catheter.
Fig. 2 |. Clinical manifestations of vascular access dysfunction.
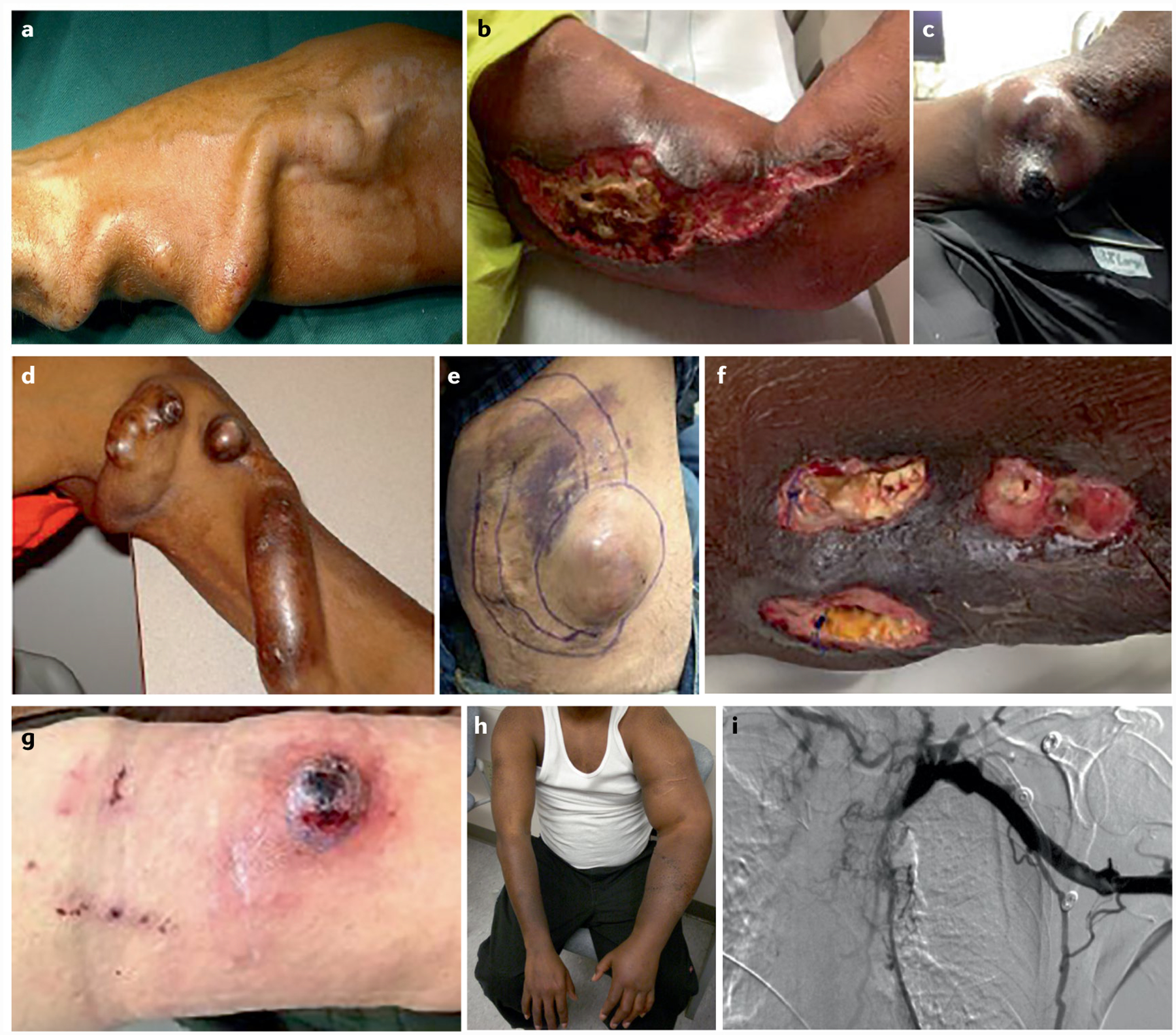
Myriad types of vascular access dysfunction can occur owing to complications of fistulas, grafts and central venous catheters. a | Tortuous aneurysmal radiocephalic fistula formed from continued dilation caused by outward remodelling159. b | Severe wound complication from a basilic vein harvest site in an attempt to create a brachiobasilic fistula via basilic vein harvest and transposition. c | Ulcerated pseudoaneurysm in dilated fistula segment. d | Dilated biological graft, demonstrating both a true aneurysm (the tube-like enlargement in the body of the graft) and pseudoaneurysms (the bubble-like out-pouches caused by needle cannulation). e | Graft aneurysm resulting from a cannulation injury. f | Open wounds over an infected expanded polytetrafluoroethylene (ePTFE) graft, which remarkably is still being used for in-centre dialysis. g | Ulcerated pseudoaneurysm with imminent rupture following cannulation of an ePTFE graft. h | The clinical sequalae of prolonged central venous catheter use, with associated central vein stenosis and chronic severe left arm swelling. i | Representative venogram demonstrating complete occlusion of the left brachiocephalic vein due to prolonged catheter dependence. Part a reprinted from REF.159, CC BY 3.0.
Arteriovenous fistula formation
The first use of the AV fistula was pioneered in 1966 by Brescia and colleagues16; however, there have been few developments since then. Indeed, the Brescia-Cimino radiocephalic AV fistula is still the procedure of choice for autogenous AV fistula creation over half a century later. Overall, the AV fistula is the preferred type of haemodialysis vascular access owing to its good long-term patency and low complication rates compared with the other options17, although its use varies across different regions (FIG. 3a). Marked differences also exist between regions with regard to the time to first AV fistula use (FIG. 3b). Of particular note, considerable disparities exist in the use of this preferred type of vascular access across genders and ethnic groups in the USA18,19 (FIG. 4).
Fig. 3 |. Regional differences in the use of different vascular access modalities.
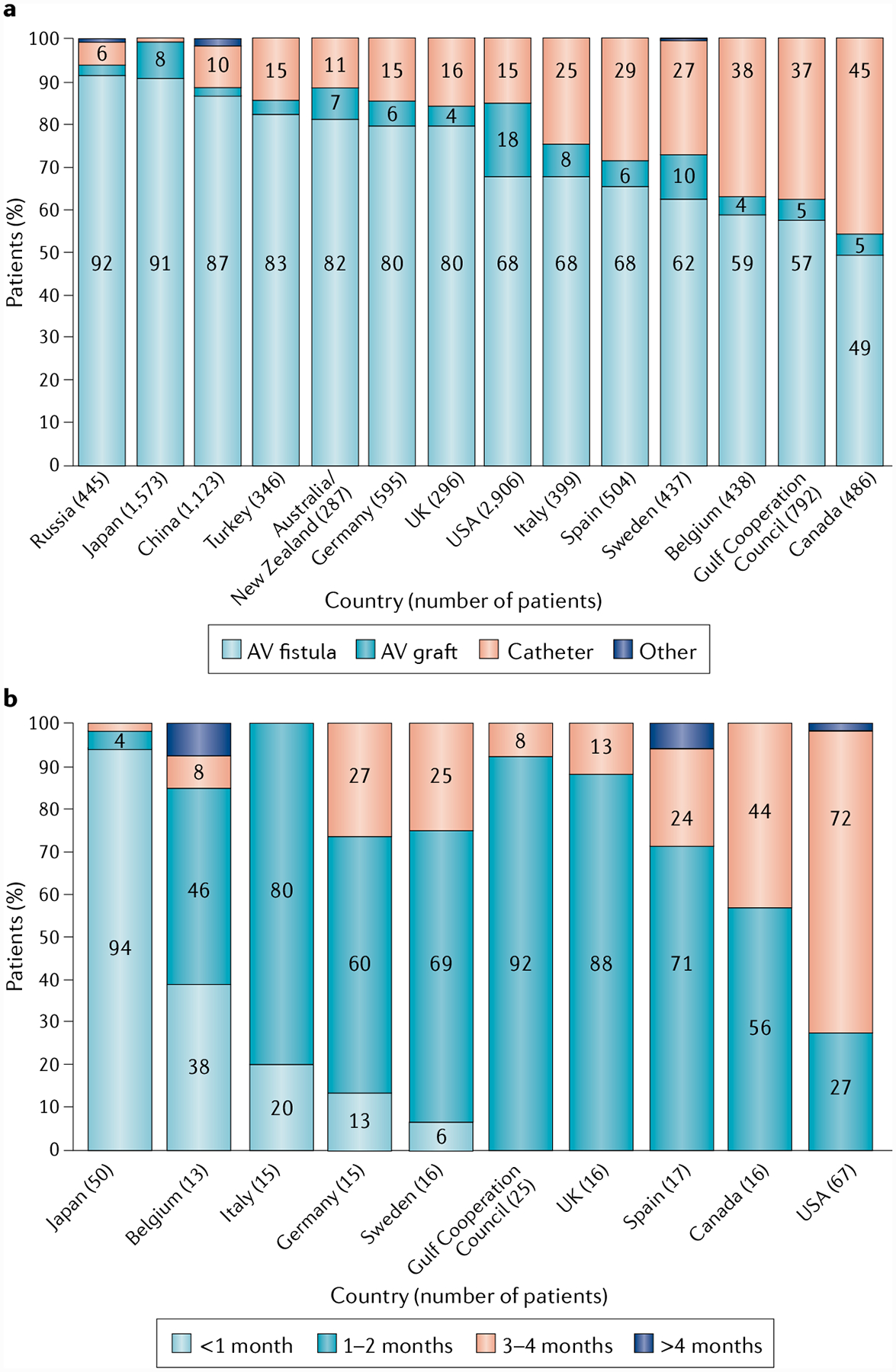
a | Regional differences in the rates of use of arteriovenous (AV) fistula, AV graft, central venous catheter and other vascular access modalities. b | Regional differences in the time to first cannulation of AV fistulae (defined as when the AV fistula was first cannulated successfully). Reprinted with permission from REF18, Elsevier.
Fig. 4 |. Health disparities in vascular access.
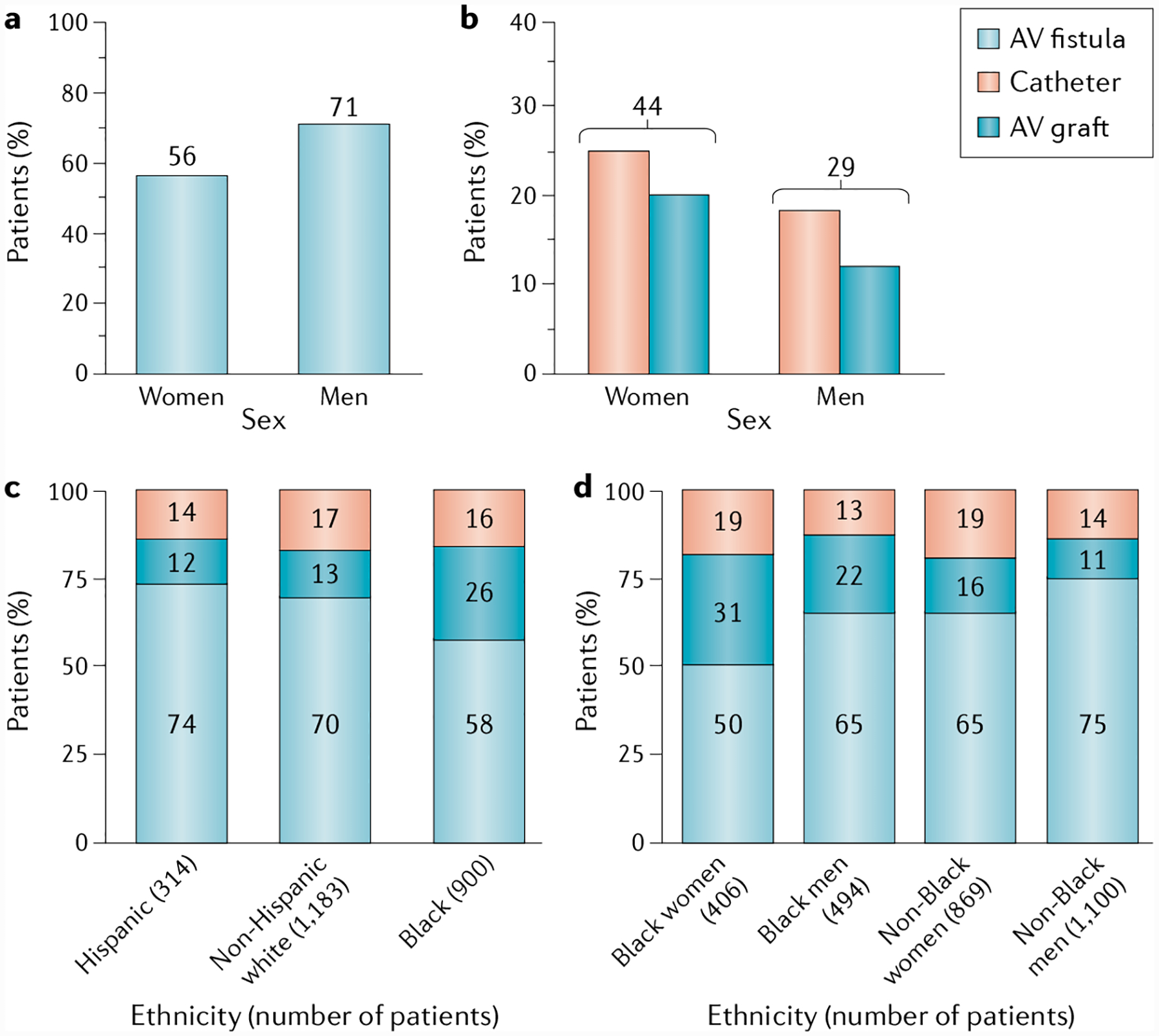
a | Rate of arteriovenous (AV) fistula use in dialysis among men and women in the USA for at least 90 days. b | Rate of catheter and expanded polytetrafluoroethylene (ePTFE) grafts used in dialysis for at least 90 days among US women and men. Data for parts a and b are from the US Renal Data System (USRDS), 2018 (REF2). c | Use of different vascular access modalities across different ethnic groups. d | Use of different vascular access modalities according to sex. Parts c and d reprinted with permission from REF18, Elsevier.
Despite the widespread use of fistulas in haemodialysis, this type of vascular access has undeniable limitations. One such limitation is the important problem of maturation of AV fistulas. In a meta-analysis of 318 studies of adult patients with AV fistulas in developed regions, the mean time to fistula maturation was 3.5 months, although only 26% of fistulas were mature at 6 months20. In that meta-analysis, maturation was defined as time before suitability for dialysis; however, it is worth noting that the studies included in the meta-analysis had varying definitions of maturation, and therefore, the article provides both the rate of fistula maturation at 6 months and the mean time to reliable usability for dialysis, and thus provides an encompassing view of fistula maturation. In a 2008 clinical trial, now recognized as a pivotal study in understanding fistula maturation, 60% of fistulas failed to mature, with maturation failure defined as failure to become suitable for dialysis (that is, use at a dialysis machine blood pump rate of ≥300 ml/min during 8 of 12 dialysis sessions occurring in a 30-day suitability ascertainment period between days 120 and 150)21. However, failure rates may vary depending on the population. For example, in older patients starting haemodialysis with a catheter, 51% of fistulas failed to mature during the 6 months following placement22.
Maturation.
Maturation in the context of fistula maturation is a dynamic remodelling process whereby the vascular wall thickens and the vein dilates in size to allow regular cannulation and blood flow for haemodialysis. Thrombosis, stenosis and poor blood flow can all contribute to a lack of clinical maturation.
At the biological level, failure of AV fistula maturation often results from a juxta-anastomotic stenosis that probably occurs secondary to vascular injury as a consequence of haemodynamic injury (such as that caused by non-laminar flow), surgical handling and/or vascular wall shear stress23. It was once thought that this stenotic lesion occurred primarily as the result of an aggressive neointimal hyperplasia predominantly involving myofibroblasts and small numbers of contractile smooth muscle cells and fibroblasts24–26. However, more recent data suggest that inward remodelling (that is, a reduction in luminal diameter/vasoconstriction of the vessel) might also have an important role in the pathogenesis of juxta-anastomotic stenosis24,27. A better understanding of the interplay and interactions between the remodelling and stenosis formation processes is critical to identify therapies that target the biological processes involved in AV fistula dysfunction.
Juxta-anastomotic stenosis.
Narrowing of the vessel at and around the anastomosis, typically due to neointimal hyperplasia. Juxta-anastomotic stenosis (that is, stenosis on the venous side within 3 cm of the anastomosis) is part of a larger collective term, ‘peri-anastomotic stenosis’, which also includes anastomotic stenosis and arterial stenosis.
An unintended consequence of low AV fistula maturation rates is an increased frequency of percutaneous and surgical interventions after fistula formation in an attempt to promote maturation. Approximately 70–86% of fistulas require two or three interventions per patient-year in the first year after fistula formation to facilitate maturation and maintain function28,29, and in a retrospective cohort study in patients >67 years old who started haemodialysis with a catheter, 42% of the patients required an intervention to make their AV fistula functional22. Other than the financial and physical burdens of these additional procedures, available evidence indicates that fistulas that mature as a result of an intervention have shorter secondary patency than those that mature without an intervention30, although the risk of 1-year access abandonment and need for further interventions does seem to be lower for fistulas that become usable than for grafts22. In addition, AV fistula maturation failure often results in a prolonged CVC contact time with its attendant complications of infection, thrombosis and central vein stenosis31.
Secondary patency.
The time from access placement until access abandonment or thrombosis, or the time from measurement of patency including intervening manipulations (surgical or endovascular interventions) designed to re-establish functionality in a thrombosed access.
Autogenous AV fistulas can also develop true aneurysmal and pseudoaneurysmal changes (FIG. 2a). A pseudoaneurysm, also sometimes called a ‘false aneurysm’, is caused by damage to the vascular wall that results in a locally contained haematoma, whereas a true aneurysm is a bulging, weakened area of the vascular wall that results in the widening of the vessel. A consensus definition of an AV fistula aneurysm is still under debate, with some advocating for the definition of an aneurysm as an increase of more than twofold in venous diameter32 and others proposing an increase of more than threefold33,34. Regardless of the definition used, rates of aneurysm formation in fistulas vary from 43% to 60%35,36. Many vascular access aneurysms are benign and remain stable, and patients are asymptomatic and potentially dialyse without problems despite the presence of a large, tortuous and cosmetically disfiguring fistula33. However, other fistulas have enlarging aneurysms that are at risk of rupture and require urgent revision or ligation. Indeed, the reported rates of AV fistula aneurysm rupture range from 0.8% to 5.6%37. Therefore, AV fistulas are prone to substantial rates of mechanical failure over time, with a small but significant risk of rupture.
AV fistula aneurysm.
A true arteriovenous (AV) fistula aneurysm is defined as an abnormal vessel dilation that may burst if not treated. The definition of a true AV access aneurysm is still under debate, but one proposed definition is an increase in vessel diameter of at least 50% with true dilation of all layers of the vessel wall.
Additional complications include ‘steal’ syndrome, which develops when there is a shunting of blood away from the distal limb, often in the presence of pre-existing peripheral vascular disease, which results in inadequate blood flow and ischaemia in tissues downstream from the AV conduit site, or wound complications from surgical procedures (FIG. 2b,c). Furthermore, high-flow AV fistulas can also result in hyperdynamic heart failure owing to the excess workload resulting from the increased cardiac output38,39.
AV graft placement
AV grafts for vascular access connect an artery and vein through the placement of a synthetic or non-autogenous tube, typically in the upper extremity, although lower extremity (for example, femoral) graft placement can also be used, especially in patients with limited availability of sites for vascular access. The most common graft materials are expanded polytetrafluoroethylene (ePTFE), polycarbonate urethane, polyurethane and multi-material grafts40.
Fistulas remain the preferred method for vascular access because for long-term use, AV grafts are associated with higher all-cause mortality and fatal infections than AV fistulas, with risk ratios of 1.18 and 1.36, respectively41; grafts also tend to be associated with a higher rate of complications. In a 2017 meta-analysis, 49% of patients with grafts experienced access-related non-infectious complications compared with 31.8% of patients with fistulas, and infectious complications were almost twice as high as with fistulas42. However, grafts remain necessary in those patients who are not candidates for AV fistulas owing to inadequate superficial venous anatomy or vascular proliferative phenotypes that complicate native fistula creation43.
Thrombosis, often due to stenosis as a result of venous neointimal hyperplasia at the venous anastomotic site or in the associated outflow vein, is a common graft complication and the predominant cause of overall graft failure44. The biological profile of this neointimal hyperplasia is similar to that of AV fistula stenosis, although greater extracellular matrix deposition occurs in the neointimal hyperplasia of grafts that are in direct contact with graft material45,46. Other potential complications of grafts (FIG. 2d–g) include steal syndrome, infection, venous hypertension and pseudoaneurysm (FIG. 2d,e), which occur more frequently in patients with a graft than in those with a fistula47.
Despite the preference for fistulas in clinical practice, grafts may offer some advantages over fistulas. First, they need much less time to mature, with newer versions of synthetic grafts ready for haemodialysis use as soon as 24–72 hours after implantation (as compared with standard ePTFE grafts, which are typically ready for use in 2–4 weeks)40,48. Grafts also require fewer interventions than AV fistulas to create a functional vascular access (although the frequency of interventions was found to be lower for AV fistulas than for grafts in the first year of successful use)22 and are considered preferable to catheter use. Importantly, grafts are essential in patients who are not candidates for fistulas and are often used in patients in whom multiple AV fistulas have failed to mature.
Central venous catheters
CVCs are used to provide vascular access for both short-term and long-term use. Non-tunnelled CVCs are typically for short-term, in-hospital use and are made of comparatively stiff material. Long-term (tunnelled) CVCs are made of softer materials (for example, polyurethane–polycarbonate copolymer or silicone) and are placed under the skin and into the jugular vein (most commonly), from where they traverse to the brachiocephalic vein and superior vena cava before terminating in the right atrium. Long-term catheter use is generally a concern owing to the numerous complications associated with this approach31 (FIG. 2h,i). The most common complication of CVCs is infection49, which can be localized to the catheter or occur in the bloodstream. In addition, CVC use is associated with higher rates of all-cause mortality, fatal infection and cardiovascular events than the use of either AV fistulas or grafts41. Catheter-related infections are associated with a decreased quality of life, as a result of increased hospitalization and the need for intravenous antibiotic therapy50. This high level of morbidity and access failure explains why emphasis has been placed on catheters being a ‘last use’ vascular access strategy for haemodialysis over the past two decades31,51.
Another complication of CVC use is catheter dysfunction as a result of fibrin sheath formation or thrombosis that requires repeated interventions to maintain the utility of the catheter. These interventions have variable success rates52,53, and the rates of primary patency failure and removal of CVCs is estimated at 91% and 52%, respectively, in the first year54,55. CVCs also often result in a central vein stenosis, which can limit the success of future life-saving AV or CVC access.
Primary patency.
The time from access placement until any intervention designed to maintain or re-establish patency, access thrombosis, or the time from measurement of patency.
Globally, the prevalence of CVC use tends to vary depending on geographical region and ranges from 2% to 49%18. Fistulas are used more frequently in Japan than in many other regions, with better time to maturation and time to first use for AV fistulas in Japan, Europe, Australia and New Zealand than in the USA19. In general, North America has a high rate of new CVC use, with the highest rate of total CVC use observed in Canada (FIG. 3a). Despite the recognized clinical inferiority of CVCs compared with fistulas and grafts, an estimated 80% of patients in the USA started dialysis with a catheter in 2016, equivalent to rates observed in 2010 (REF2), indicating a lack of substantial change over time. The reason for the high rate of CVC use in the USA is unclear, but the high rate may be due to delays in referral of patients with stage 4 CKD and low fistula maturation rates56. Furthermore, in the USA, health-care coverage for patients with kidney failure is not available to non-Medicare patients until 3 months after the start of dialysis57. Of note, CVC use is increasing slightly in regions that currently have high rates of fistula use, such as Japan58,59, suggesting that CVC use remains necessary with the current technologies and treatments, even in regions with higher fistula maturation success rates.
Limitations across access methods
In addition to specific challenges that are unique to each individual vascular access method, limitations also exist that are shared across fistulas, grafts and CVCs, albeit with somewhat different frequencies. Some of these limitations may also be unique to specific populations and relate to differences in access to care.
Occurrence of infection and sepsis.
As mentioned earlier, an important challenge for vascular access is the prevalence and burden of infection on patients receiving haemodialysis, as infection rates affect quality of life, morbidity and mortality, and also result in economic hardship for patients. Infection and sepsis risk across different vascular access modalities is not equal. The rate of vascular access infections in patients with a fistula for dialysis is only 0.5–1.5% per patient-year60, which is markedly lower than that observed in patients with an ePTFE graft (septicaemia rates of 10–15% per patient-year61,62) and those with a catheter (septicaemia rates up to 200% per patient-year63). Patients with a tunnelled catheter have a risk of death from septicaemia about sevenfold higher than patients with a graft or fistula64.
However, despite differences in infection risk across access methods, the collective risk of infection remains problematic. In the USA alone, the total number of hospitalizations for access-related infections is approximately 58,000 per year2. These hospitalized patients require treatment for systemic complications from sepsis, and undergo prolonged administration of intravenous antibiotics and repeat operations to remove and/or replace infected accesses. Of the $35 billion spent on treating patients with kidney failure in 2016, Medicare spent more than $3 billion solely on hospitalizations for infections2.
Among patients with kidney failure, sepsis accounts for 9.3% of hospital admissions, and complications associated with the access constitute a further 9.2% of hospital admissions. In the USA, sepsis is the most expensive reason for hospitalization. Patients admitted with sepsis typically remain hospitalized for a week or more, at a cost of $13,000–44,000, depending on the severity of the infection65.
Infection is also associated with an increased risk of death. Among patients on dialysis, mortality associated with sepsis ranges from 5% to 31%65, and septicaemia accounts for roughly 8% of the ~78,000 deaths of patients on haemodialysis in the USA each year2, corresponding to a staggering 6,000 patients per year. In many cases, the cause of sepsis is an infected access, often an ePTFE graft or an access catheter.
Gender and race disparities in vascular access.
Although fistulas are the current gold standard for dialysis vascular access, certain subgroups of patients are burdened by higher rates of ePTFE graft and catheter use. According to the USRDS database, women with kidney failure have lower rates of fistula use and higher rates of ePTFE graft and catheter use than men with kidney failure66. This difference may be in part because women are often poorer candidates for fistula use than men because of their smaller venous anatomy, which makes fistula surgery and maturation technically more challenging67,68. USRDS data also show that at 1 year after dialysis initiation, the rate of fistula use is only 56% in women compared with 71% in men (FIG. 4a). Consequently, ePTFE graft use is higher among women (20%) than among men (12%), as is catheter use (24% and 17%, respectively)2. In total, 44% of women dialyse through an ePTFE graft or a catheter compared with only 29% of men (FIG. 4b).
This higher use of grafts and catheters among women also corresponds to a higher risk of infection. Women have a 36% increased relative risk of hospitalization for infection of a vascular access than men, with rates of hospitalization for vascular access infection of 15% and 11% per patient-year for women and men, respectively, according to the USRDS data2. Overall, higher graft and catheter use drive an increased risk of bloodstream infections, sepsis and hospitalization in women, which demonstrates an important gender-based disparity in the health care of women with kidney failure and burdening women with inferior haemodialysis access.
Differences in vascular access and outcomes also exist between ethnic groups. In the USA, Black Americans have the highest rate of failed fistula maturation and the highest proportion of AV graft use at haemodialysis initiation and at 1 and 2 years after initiation2 (FIG. 4c); this disparity is even greater among Black women (FIG. 4d). Studies in the USA indicate that African American and Hispanic patients start haemodialysis with an AV fistula less frequently than white (non-Hispanic) patients, regardless of insurance status69,70. This disparity persists as patients age, despite Medicare coverage71. A 2020 analysis of patients in the USA who started dialysis using a tunnelled CVC indicates that Black patients had earlier and more frequent access failures (both graft and fistula) than white patients, independent of access type, age or underlying comorbidities72. Although more research is needed to understand the reasons for the differences in vascular access type and outcomes, data generally suggest that these differences are due to a confluence of socioeconomic factors, health-care disparities and process-of-care factors as opposed to underlying biological differences66.
Advances in vascular access
Although AV fistulas are the preferred mode of vascular access for dialysis, the above discussion highlights clear limitations and complications associated with each of the three types of permanent vascular access. Given the essential role of haemodialysis in health care, advances in vascular access technology are needed to reduce morbidity and mortality and increase quality of life for the growing number of patients with kidney failure. This unmet need is driving the development of novel approaches to address key issues relating to fistula maturation and innovations in graft materials (FIGS 5 and 6). The overall goal is to generate an approach that can be used in all patients to maintain chronic adequate vascular access with a low risk of complications. In the context of this Review, it is important to remember that a technology can completely alter existing clinical paradigms. For example, a tunnelled dialysis CVC without the complications of infection, thrombosis or central vein stenosis could completely change the clinical care process from a ‘Fistula First’ approach to a ‘Catheter First’ paradigm.
Fig. 5 |. Innovations in approaches to manipulating fistula geometry and haemodynamics.
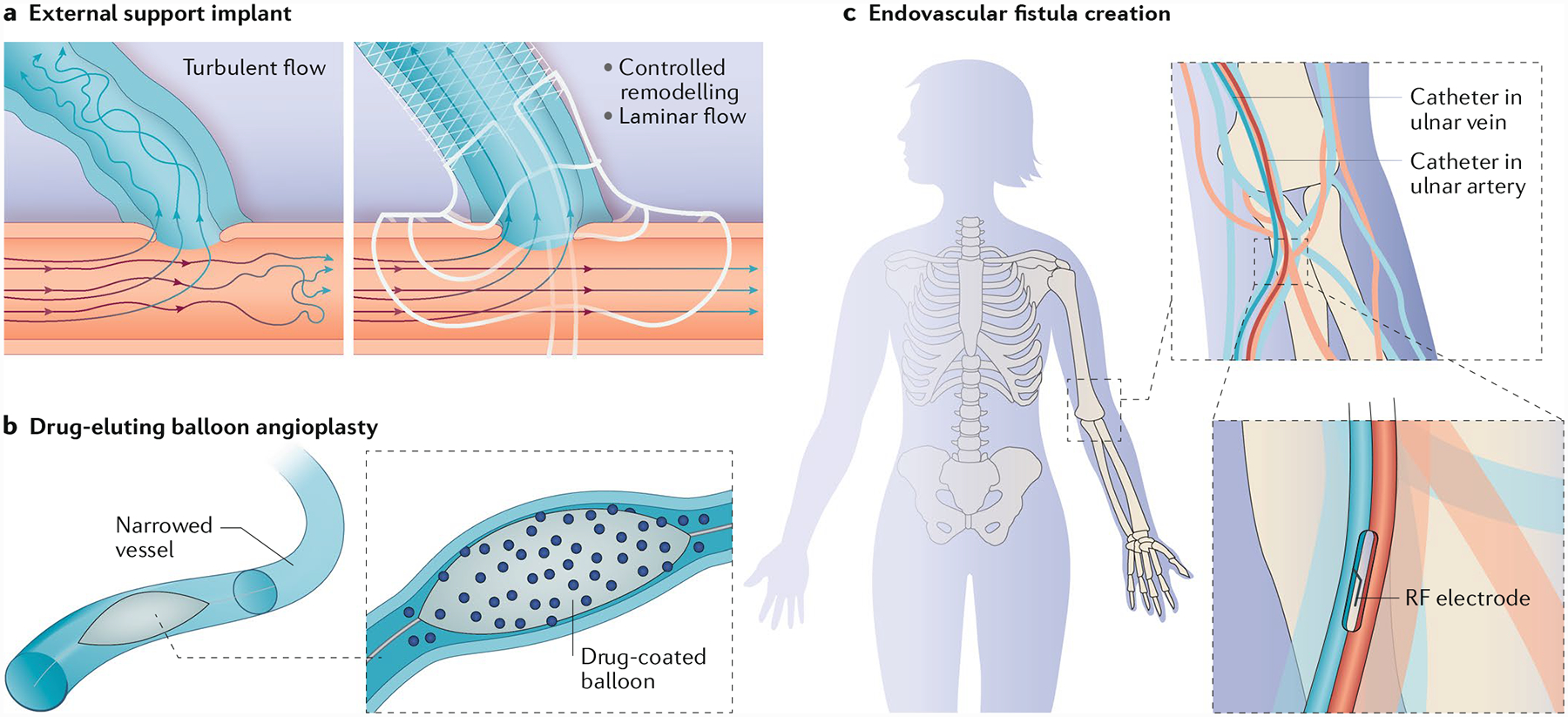
One area of innovation involves the development of approaches to manipulating fistula geometry. a | External support devices can address fistula geometry by reducing wall tension and modulating haemodynamic flow patterns in the blood vessels. b | Balloon-assisted maturation, including drug-coated or drug-eluting balloons, can reduce stenosis and support fistula maturation. c | Endovascular approaches, including the creation of anastomoses by thermal or radiofrequency (RF) methods, can reduce trauma to the blood vessels and subsequently reduce intimal hyperplasia. Part a adapted with permission from http://www.laminatemedical.com/vasq/, VasQ, Laminate Medical.
Fig. 6 |. Innovations in graft materials.
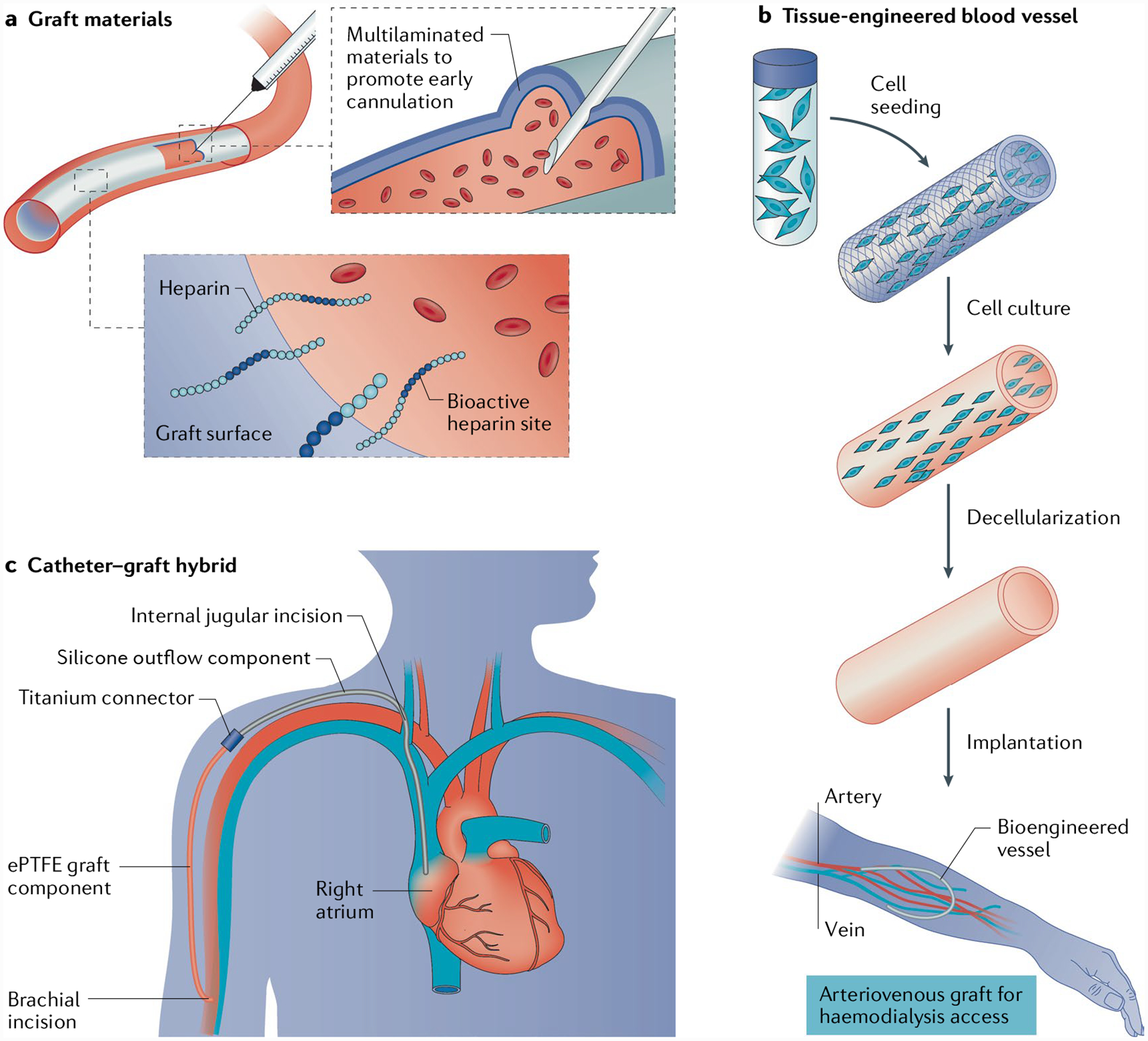
Improvements in graft and graft–catheter materials include simple improvements to grafts as well as the creation of new vessel materials. a | The addition of heparin to the blood-contacting surface of grafts may lead to a reduction in thrombosis and intimal hyperplasia, whereas the development of multilayered, rapidly sealing grafts may allow cannulation as early as 24–72 hours after implantation. b | Innovations in tissue-engineered blood vessels have enabled the creation of decellularized vessels that may mimic the host vasculature. c | A graft–catheter hybrid device, made from ePTFE graft connected via a titanium coupler to a silicone venous outflow component, can be placed through severely diseased or stenotic segments of central vein anatomy. Part b adapted with permission from REF139, AAAS.
Supporting AV fistula maturation
A key issue with AV fistula use is the high rate of maturation failure, especially in the USA where maturation failure drives the initiation of haemodialysis with a CVC in many patients. The likelihood of fistula maturation failure is influenced by a number of underlying factors, including patient age, blood vessel size, blood flow, extent of vessel remodelling and the presence of comorbidities, such as diabetes mellitus and/or cardiovascular disease43. At a pathogenic level, maturation failure might result from vascular injury, which leads to both neointimal hyperplasia and inward remodelling. Despite the role of these factors in fistula maturation, the ability to predict maturation failure remains elusive. In the USA, only 55% of AV fistulae are used within 4 months of placement43, and data from the USA-based Human Fistula Maturation Consortium indicate unassisted and assisted maturation rates of 40% and 66%, respectively. Regions outside the USA tend to have better maturation rates and fistula use, with successful fistula use rates of 67% in Europe, Australia and New Zealand combined, and approximately 90% in Japan19,58 (FIG. 3a). Patients who dialyse with a catheter while their fistula is maturing are at increased risk of adverse events (such as infection) for the duration of catheter use than they would otherwise be if they started dialysis with a fistula or graft. The median annual cost is also higher for patients who start haemodialysis with CVC than for those who start with an AV fistula, driven predominantly by hospitalization as a result of catheter-related bacteraemia and the underlying cost of an indwelling catheter inducing central vein pathology, although this cost is unknown73. Advances in improving the time to fistula maturation and the overall rate of maturation have generally focused on improving fistula geometry and haemodynamics, biological approaches and mechanical approaches.
Manipulating fistula geometry and haemodynamic flow.
Despite the complex processes underlying fistula maturation, in-clinic evaluation of fistula geometry suggests that the key variables associated with maturation failure — stenosis due to neointimal hyperplasia24,74,75 and failed vascular remodelling24 — can be manipulated to improve blood flow and, subsequently, fistula maturation (BOXES 1; 2). For example, a novel vascular anastomosis technique that relieves torsional stress on the vessel wall in an effort to reduce the stenotic response — the piggyback straight-line onlay technique (SLOT) — led to lower rates of juxta-anastomotic stenosis and overall fistula failure compared with traditional end-to-side techniques in the creation of a radiocephalic fistula76, indicating the importance of the outflow vein configuration in fistula maturation.
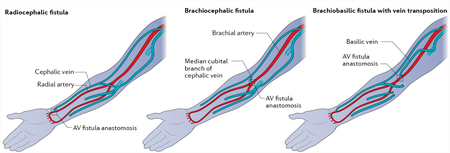
Box 1 |. Types of fistula.
Autologous arteriovenous fistulae (AVF) are generally created in the wrist or in the forearm (see the figure). the Brescia–Cimino radiocephalic AvF, first used in 1966, is still the procedure of choice today for most patients; a radiocephalic fistula is formed in the wrist by fusing the cephalic vein and radial artery, with the location where they meet termed the anastomosis. In the forearm, the types of fistula formed are generally brachiocephalic (fistula formed from the brachial artery and cephalic vein) and brachiobasilic transposition (fistula formed from the basilic vein and the brachial artery). Figure adapted with permission from REF160, Elsevier.
Box 2 |. Creation of a fistula.
The most common surgical technique for fistula formation is an end-to-side anastomosis formation, where the end of the vein is connected to the side of the donor artery (see the figure). In a side-to-side technique, the sides of the vein and artery are connected, and in the end-to-end technique, the ends of the vein and artery are connected. The formation of the anastomosis results in torsional stress, which is compounded by blood flow in the vessels and may affect fistula maturation. Novel surgical techniques and devices that manipulate the angles of the fistula may affect blood flow and stress on the vessels, subsequently affecting stenosis formation and fistula maturation. Figure adapted with permission from REF76, Elsevier.
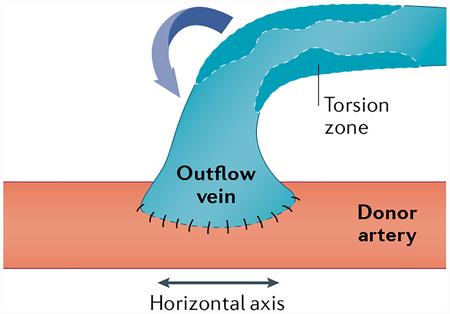
Piggyback straight-line onlay technique.
A fistula creation technique where the vein ‘piggybacks’ on the artery at the site of anastomosis, and the arterial blood flows into a straight cylindrical lumen. The cephalic vein in the subcutaneous plane is dissected and divided, and the cut end is over-sewn with prolene; it is moved medially over the artery, which lies in a deeper plane. A fistula is created between the posterior aspect of the vein and the anterior aspect of the artery. The outflow vein is dissected further in the subcutaneous tissue to obtain a straight line.
End-to-side techniques.
Techniques in which the fistula is formed by connecting the end of the vein to the side of the artery. This is the most common technique for creation of a radiocephalic fistula by connecting the end of the cephalic vein to the side of the radial artery.
Radiocephalic fistulae with anastomotic angles of <30° have demonstrated reduced primary patency and secondary patency, increased rates of juxta-anastomotic stenosis and the need for interventions compared with fistulae with angles of >30°77. This finding is further supported by a modelling study in 60 patients with autologous AV fistula placement, in whom a greater degree of arterial non-planarity and a larger bifurcation angle (that is, the angle formed between the proximal artery and the tangent to the vein at the bifurcation point) were associated with a higher likelihood of fistula maturation78. Collectively, these studies indicate that fistula geometry can have an important impact on clinical outcomes, with non-planar and anastomotic angles of >30° potentially improving the rate of fistula maturation in the clinic.
Manipulation of anastomotic angles and geometry might be amenable to control through a medical device. The VasQ (Laminate Medical Technologies) is an external support nitinol implant designed to reduce vein wall tension and control flow patterns (FIG. 5a), and the Optiflow (Bioconnect Systems) is an implantable siliconized polyurethane anastomotic connector designed to standardize fistula surgical placement. Both devices have demonstrated success in early clinical trials. The VasQ was associated with a high maturation rate in a small number of patients at a single site (20 patients; >70% unassisted maturation at 6 months after AV fistula creation)79. Enrolment of 144 patients has now been completed for a multicentre US-based trial of VasQ80. The Optiflow also had promising results in an initial study in 41 patients81,82, but development was stopped because of funding constraints, and at the time of writing, the business seemed to have ceased operations.
Other methods for influencing haemodynamic properties have also been evaluated in attempts to improve fistula maturation but have not generally been successful. For example, the development of a small blood pump system that maintained wall shear stress — a factor demonstrated to affect AV fistula remodelling and neointimal hyperplasia83–85 — stalled at the preclinical stage86, and no method that targets haemodynamic flow, other than VasQ, was at the time of writing in clinical development or successfully adopted in the market.
Improving fistula maturation through biological approaches.
Molecular and cellular processes of the vascular endothelium regulate vascular physiology. Given that disruptions in the vascular endothelium may impair the ability of the endothelium to outwardly or inwardly remodel and/or generate neointimal hyperplasia following fistula formation procedures, these biological processes could represent a target to improve fistula maturation. A detailed description of all ongoing research in this field is outside the scope of this Review; however, some methods have advanced sufficiently to enter clinical studies.
One approach involves the culture and application of endothelial cells at or near the location of fistula formation to influence vessel development and response to injury87. Vascugel, for example, is a system involving allogenic human aortic endothelial cells cultured on a gelatin matrix. In an early phase I/II study in eight patients, Vascugel placed adjacent to the venous anastomosis and outflow vein was safe and was associated with a slight improvement in assisted primary patency rate, although this benefit has not been confirmed in larger studies88. Further analysis demonstrated particular improvement in vascular remodelling in patients with diabetes, which was thought to potentially be related to abnormal glucose homeostasis or poor pre-existing endothelial function, although the underlying mechanism was not explored89. Development of this product has since been halted due to an alloimmune response in approximately 20% of treated patients88, highlighting the need to consider immune reactions when developing cellular therapies.
A recombinant human type I pancreatic elastase, PRT-201, has also been evaluated in human trials, with the underlying molecular hypothesis that direct application of this solution (by drops applied directly to the exposed graft–vein anastomosis and outflow vein) would result in enhanced degradation of elastin that would then result in persistent vasodilation and allow increased blood flow. In addition, it was proposed that the degraded elastin fragments might inhibit the cellular migration of cells from the adventitia into the intima, thus potentially reducing neointimal hyperplasia. Although small initial studies demonstrated safety and also some efficacy of specific doses of PRT-201, two large multicentre randomized studies were not able to demonstrate differences in their primary end points90–93. Another clinical study that investigated the effects of vasculature relaxation using papaverine — an opioid derivative that relaxes the smooth muscles of the vessel wall — also failed to demonstrate improvement in maturation rates over placebo94. This finding is in line with evidence suggesting that vessel elasticity rather than vessel dilation is related to AV fistula maturation74. Although more research is needed, these studies suggest futility in vasculature dilation as a mechanism for improving fistula maturation.
Currently, a sirolimus-eluting collagen implant (SeCI) is in phase III clinical trials to assess efficacy in facilitating AV fistula maturation95. This device is wrapped around the AV fistula during its formation and releases the anti-proliferative agent, sirolimus (also known as rapamycin), with the overall goal of reducing narrowing of the vein. A small first-in-human pilot clinical study of this device showed significant improvement in fistula maturation as compared with historical controls96.
Overall, approaches to manipulating molecular and cellular pathways involved in fistula maturation and remodelling have so far remained limited with minimal success, potentially owing to the complexity of these biological pathways and the challenges associated with the delivery of appropriate modulators to the local vascular environment.
Mechanical methods to assist fistula maturation.
Balloon-assisted maturation (BAM) is a mechanical method that aims to enhance AV fistula maturation through repeated long-segment angioplasty procedures to sequentially dilate the peri-anastomotic venous segment. Interestingly, BAM techniques seem to have been adopted prior to the emergence of supportive published studies, and evidence that BAM improves maturation success97,98 or affects maturation times99 remains limited. BAM techniques can be associated with complications, such as fibrosis and stenosis97; common injuries include haematoma, rupture, spasm and thrombosis, with significantly more complications occurring with BAM procedures performed in forearm fistulas and with the use of large (7–12 mm) balloons. Except for haematomas, which occur following ~40% of procedures, complications tend to be associated with <10% of procedures100.
The use of drug-coated and drug-eluting balloons has also been explored for the reduction of post-angioplasty stenosis in AV access (FIG. 5b). To date, paclitaxel and sirolimus — chemotherapeutic drugs with anti-proliferative properties — have been the most investigated, and results with these drug-device combinations have been fairly promising. Angioplasty with paclitaxel-coated and eluting balloons demonstrated higher rates of successful treatment of stenosis and juxta-anastomotic stenosis than plain old balloon angioplasty (POBA)101 and might decrease the number of re-intervention procedures102,103. A large randomized study of the Lutonix paclitaxel-coated balloon showed a trend towards efficacy in the treatment of post-angioplasty stenosis in AV fistulae. Although that study did not reach its primary efficacy end point of post-intervention patency (that is, target lesion primary patency at 6 months defined as no need for clinically driven re-intervention on the target lesion or access thrombosis), use of the balloon was associated with a reduction in the number of interventions needed to maintain patency104. By contrast, a very similar study using the Medtronic IN.PACT paclitaxel-eluting balloon demonstrated an improvement in the primary end point of post-intervention primary patency as compared with POBA105.
Plain old balloon angioplasty.
(POBA). also known as classic balloon angioplasty, this technique mechanically widens narrowed vessels by using a balloon to dilate the identified stenosis.
Several endovascular approaches have also been developed to facilitate fistula creation by reducing vessel trauma and thereby reducing intimal hyperplasia (FIG. 5c). The past few years have seen a number of exciting advances in this field, including FDA approval of the Ellipsys and the everlinQ devices in 2018. The Ellipsys uses a single catheter inserted under ultrasound guidance to create a secure thermal fused anastomosis between the proximal radial or ulnar artery and a deep communicating vein. By contrast, the everlinQ endoAVF system uses fluoroscopic guidance; magnetic catheters are used to align the artery and vein, and a radiofrequency electrode is used to create the anastomosis.
Clinical studies of the Ellipsys have demonstrated high rates of functional patency, with 88% of107 patients achieving two-needle dialysis over a mean of 114.3 days106. A retrospective study of 34 patients with percutaneous fistula formed using the Ellipsys also found high patency, with 82% of patients achieving two-needle dialysis by 6 weeks107.
The everlinQ endoAVF has been evaluated in two studies. In the single-centre, single-arm, prospective EASE study, 78% of 32 patients achieved successful two-needle cannulation by 90 days108. In the prospective, single-arm, multicentre NEAT study, 87% of 60 patients had an AV fistula that was physiologically suitable for dialysis within 3 months (defined as brachial artery flow ≥500 ml/min and vein diameter ≥4 mm), although functional usability (usability for prescribed dialysis via two-needle cannulation) was only 64% in patients who received dialysis109. Additional evaluation also suggested that the everlinQ reduced the number of interventions compared with those needed following surgical fistula formation, and the reduction in event rate per patient-year corresponded to reduced overall costs of $13,400–16,500 (REF110). The everlinQ has since been modified and renamed as the WavelinQ, which received FDA 510(k) premarket clearance in 2019. The updated model has demonstrated improvements in mean primary patency compared with radiocephalic AV fistulas (362 days versus 235 days, respectively) and slightly improved primary patency and secondary patency rates compared with radiocephalic AV fistula at 6 and 12 months after fistula formation111.
Although endovascular techniques seem to offer an improvement over currently available surgical techniques, it is worth noting that patients receiving endovascular AV fistula procedures still require a catheter for haemodialysis until the fistula matures. Also, since the data used for FDA clearance comprised single-arm studies only, analysis of real-world data is needed as these devices begin to be used in routine clinical practice.
Adoption of novel approaches for fistula maturation in the clinic.
Fistula maturation failure results in prolonged catheter use. However, despite the development of approaches to improving fistula maturation, including targeting fistula geometry and biology, manipulating haemodynamic flow, and altering mechanical methods, translation of novel techniques to the clinic has remained limited. The most commonly adopted innovation seems to be BAM procedures, despite a paucity of supporting evidence from large-scale clinical trials, and endovascular AV fistula procedures, which have demonstrated improvements in overall rates of fistula maturation, but still require catheter use during the maturation period. Thus, while advances have been made, the field of fistula maturation remains open for novel interventions.
Novel graft material and procedures
Grafts are considered preferable to catheters for vascular access. They are often more easily cannulated than fistulas and are essential for patients who are not candidates for fistulas. The key challenge with grafts is long-term patency, which can be lost as a consequence of vein neointimal hyperplasia, stenosis, thrombosis, infection and deterioration of the graft material due to repeated use (for example, damage done by needles during cannulation or from interventions to restore patency). Loss of primary unassisted patency is estimated to be as high as 75% by 1 year112. Therefore, new, more durable graft materials are needed to overcome the current limitations.
Modifications to the graft lumen and luminal chemistry.
Modification of the ‘blood-contacting’ surface of AV grafts has been assessed as a simple attempt to improve patency and reduce infection rates. Based on the hypothesis that heparin would prevent clotting in the graft and subsequently improve patency, in 2006, vascular grafts with heparin covalently bonded to the luminal surface (such as Propaten) were introduced (FIG. 6a). Such heparin-coated grafts demonstrated positive outcomes in reducing thrombosis and intimal hyperplasia in a canine model113; however, they were not found to be superior to standard synthetic ePTFE grafts in clinical studies114,115 and may offer limited benefit at a higher cost.
Additional investigations with other potential pharmacological agents are ongoing and at the preclinical stage. In porcine models, coating of the terminal ends of an ePTFE graft or throughout the length of the ePTFE graft with paclitaxel reduced neointimal hyperplasia at sites of anastomosis116–118. Similarly, sirolimus-coated and eluting grafts also suppressed neointimal hyperplasia in the venous anastomosis of a porcine model without occurrence of infection119,120. At the clinical level, however, a large randomized study of a paclitaxel wrap placed around the graft–vein anastomosis (Angiotech Pharmaceuticals) was stopped at the 25% Data Safety Monitoring Board review because of an increase in the rate of infections in the paclitaxel wrap arm121,122.
Advances in graft material.
The approval of ‘early’ cannulation grafts — for example, Flixene (Getinge), AVflo (Nicast), Rapidax (Vascutek Terumo) and ACUSEAL (Gore) — also represents a step forward in innovation for AV grafts40 (FIG. 6a). Early cannulation grafts generally rely on multilaminated materials that allow haemodialysis cannulation as early as 24–72 hours after implantation; importantly, use of these grafts could potentially avoid the need for a catheter. However, although these grafts can be used earlier than standard ePTFE grafts, they do not seem to offer improvements in patency or a reduction in infection rates40.
The ‘hybrid vascular graft’ was another innovative graft product that was developed by Gore. This product included a constrained nitinol reinforced terminal section that allowed the formation of an endoluminal anastomosis where the venous anastomosis is created by deployment of a covered stent graft directly into the target vein. The graft received FDA approval in 2010, and clinical outcomes were favourable123,124, particularly in complex revascularizations involving a stenotic vein that would have made creation of a surgical anastomosis extremely difficult125. However, clinical adoption was low, and the product has been discontinued.
The above-mentioned grafts in general offer only incremental advances over standard ePTFE grafts, indicating a need for new, innovative graft materials. Novel materials could address any of the limiting factors of current grafts, including poor durability, the delay between implantation and cannulation, and potential for injury (for example, from needles during cannulation or surgical implantation).
The InnAVasc AV graft (InnAVasc Medical) is a new device that has been designed to address two of these factors: the risk of needle-related vascular access injuries and the delay between implantation and cannulation. The device comprises two cannulation chambers with self-sealing properties and uses materials that prevent side and back wall needle punctures. In experimental studies in sheep, cannulation could be started immediately, with patency that was similar to that in the ePTFE study control126. The device is currently being tested in an early-stage clinical trial in approximately 26 patients127 to determine its ability to reduce the risk of cannulation-associated access injuries. Although demonstration of improved patency compared with standard ePTFE grafts is yet to be determined, the current clinical trial will also provide insights into this outcome based on literature reports of ePTFE patency.
Other products in development include the STARgraft (Healionics), an artificial blood vessel made from a precision-pore structured silicone128,129 that is currently in first-in-human trials in patients with kidney failure receiving haemodialysis; a sirolimus (drug)-eluting cuff graft system by Cylerus that is being tested in an animal model; and a number of nitric oxide-releasing grafts that are currently being explored in non-clinical studies130,131.
Innovations in creating novel vessels.
Bioengineering approaches are also being used to create new graft materials in the form of allogenic and xenogenic vessels. Data regarding the use of bovine carotid artery (BCA) grafts are conflicting; some, but not all, studies found slightly improved secondary patency outcomes compared with that of ePTFE grafts132,133. However, to date, there is no evidence that BCA grafts improve primary or primary unassisted graft outcomes132, and overall, the slight potential benefit in terms of secondary patency outcomes is unlikely to offset the cost difference. Autologous and allogenic vascular conduits have also not demonstrated superiority over current approaches, with evidence of structural degradation and aneurysm formation134,135. Collectively, these limitations probably explain why these grafts have not been widely adopted.
Bovine carotid artery (BCA) grafts.
A BCA graft (such as the Artegraft (Artegraft, Inc.)) is a xenograft used for vascular access in haemodialysis. These biological conduits are chemically fixed bovine carotid arteries, and no longer have the ability to remodel, repopulate with host cells or heal.
Another biological approach that involved seeding of genetically modified endothelial cells onto ePTFE grafts demonstrated evidence of improved primary patency in a porcine model136. However, a concern with this approach, as with all approaches that use cellular technologies, is the potential for an immunological response that could lead to rejection of the graft and/or potentially rule out a future kidney transplant for the patient owing to sensitization against a broad range of alloantigens. Ideally, a biological AV graft would mimic the host vasculature, allowing remodelling of the vessel into the host tissue without immunological limitations.
Substantial effort has gone into the development of approaches to create bioengineered vessels that are mechanically strong and immunologically inert. However, very few technologies have advanced beyond preclinical development. Tissue-engineered vascular grafts comprising autologous bone marrow cells grown on a biodegradable scaffold have demonstrated the viability of this approach, with successful use in a limited number of patients with cardiovascular disease137,138. The human acellular vessel (HAV) is another bioengineering approach that has shown promise for haemodialysis vascular access. This vessel is generated by seeding human vascular cells onto a polymer scaffold composed of polyglycolic acid, on which the cells are cultured to form tissue. During incubation, the cells produce extracellular matrix and the polymer scaffold degrades, creating a biological vessel that mimics the human vasculature. The HAV is decellularized to remove the cells and associated antigens. The final result is a robust tube of extracellular matrix139 devoid of cellular components (FIG. 6b).
The HAV has been evaluated in phase II trials in patients with kidney failure receiving haemodialysis, and has demonstrated high rates of secondary patency at 6 and 12 months after implantation (97% and 89%, respectively)140. In addition, the HAV seems to have potential to reduce rates of infection in grafts, with studies in rats showing significantly lower rates of abscess formation and bacterial burden compared with ePTFE grafts141. Interestingly, histological examination of explanted HAVs from patients in the rare cases in which the HAV was removed, show recellularization and remodelling of the graft142, with infiltration, maturation and circumferential alignment of α-smooth muscle actin-expressing cells, together with infiltration of host myogenic, endothelial and nestin+ and CD90+ progenitor cells. These findings suggest that the acellular HAV may convert into a multilayered living tissue that facilitates self-healing after cannulation injury. HAVs are currently being evaluated in phase III clinical trials in patients with kidney failure receiving haemodialysis, comparing clinical outcomes with outcomes following the use of ePTFE grafts143 and AV fistulas144.
Infiltration.
Migration of ceils from the outer layer of the blood vessel to the inner layers that mainly impacts inward remodelling and/or the development of neointimal hyperplasia.
HeRO: the graft–catheter hybrid.
Despite the acknowledgement that catheters are a last resort for vascular access, they remain necessary in some circumstances, and this necessity has driven innovation in the development of late-stage vascular access options for patients who face long-term catheter use. The Hemodialysis Reliable Outflow (HeRO) device is a subcutaneous, implantable graft–catheter hybrid that consists of an ePTFE graft connected via a titanium coupler to a silicone venous outflow component (FIG. 6c). This device is able to be placed through severely diseased or stenotic segments of central vein anatomy, but often requires advanced endovascular skills to safely deploy the catheter component of the device, which ideally terminates in the right atrium. Also the graft component requires 2–4 weeks of tissue incorporation (similar to standard ePTFE AV grafts) and is accessed in the same manner as conventional grafts.
The HeRO has been commercially available in the USA since 2008 (achieving a CE Mark in 2013) and is approved for use in catheter-dependent patients with central vein stenosis and/or occlusion. This approach is predominantly reserved for patients whose venous outflow stenosis has progressed too far centrally to implant a standard upper extremity access, those who have central vein stenosis and those with central vein occlusion.
CE Mark.
A certification used in the european Union (Eu) to indicate that devices conform with health, safety and environmental standards set by the european Commission for products sold in the european economic Area (EEA). Many devices require Ce marking before they are able to be sold in the EU.
A retrospective review of 164 patients who received a HeRO implant suggested that it is comparable to standard AV grafts and is superior to tunnelled catheters in terms of patency, interventions and infection rate145. However, the device has some limitations. A 2013 review of 19 patients at a single institution who underwent HeRO implantation identified potential complications from steal syndrome, immediate device failure and thromboses; however, these results may in part have been complicated by the technical learning curve associated with hybrid vascular devices, the low number of patients and the high-risk nature of the patients owing to the presence of advanced stenosis and venous occlusion146. A contrasting 2019 review of quality-of-life outcomes in patients with kidney failure using the HeRO graft for haemodialysis access found a lower incidence of infection and complications compared with those following the use of tunnelled catheters, which equated to an overall reduction in long-term costs despite higher initial costs associated with the HeRO device and placement147. That study also found that the HeRO device required a higher number of interventions to maintain patency than lower extremity grafts and AV grafts, although these interventions were found to be beneficial in the context of quality of life for the patient. Overall, the results support HeRO as a beneficial technology for ‘last-resort’ implantation, where all other upper extremity vascular accesses have failed.
Innovation in process of care
In the setting of dialysis vascular access, it is important to emphasize that innovation in process of care is as important as, if not more important than, innovation in devices, materials and techniques. For example, in the context of AV fistula creation and maturation, a multidisciplinary process of care for patients with advanced CKD is likely to be more effective than any single novel intervention for AV fistula maturation if it includes the following elements: early referral to a nephrologist; institutional vein preservation programmes that prohibit placement of intravenous access and peripheral intravenous central catheters in patients with a reduced glomerular filtration rate; appropriate referral to a dedicated and committed access surgeon; a post-surgery evaluation at 4–6 weeks after creation of the AV fistula to assess maturation with referral for intervention as needed; and the use of experienced cannulators for the initial needling and/or the possible enhanced use of ultrasound-guided cannulation (FIG. 7a). In particular, the identification and subsequent dismantling of barriers to processes of care could result in more holistic and integrated practices that may facilitate AV fistula maturation. Of note, individual vascular access programmes are likely to have at least some unique barriers, such as the lack of a vascular access coordinator or an interventionalist whose clinic is geographically distant or over-booked, which could represent opportunities for innovation in local processes of care in vascular access.
Fig. 7 |. Innovation in vascular access and process of care.
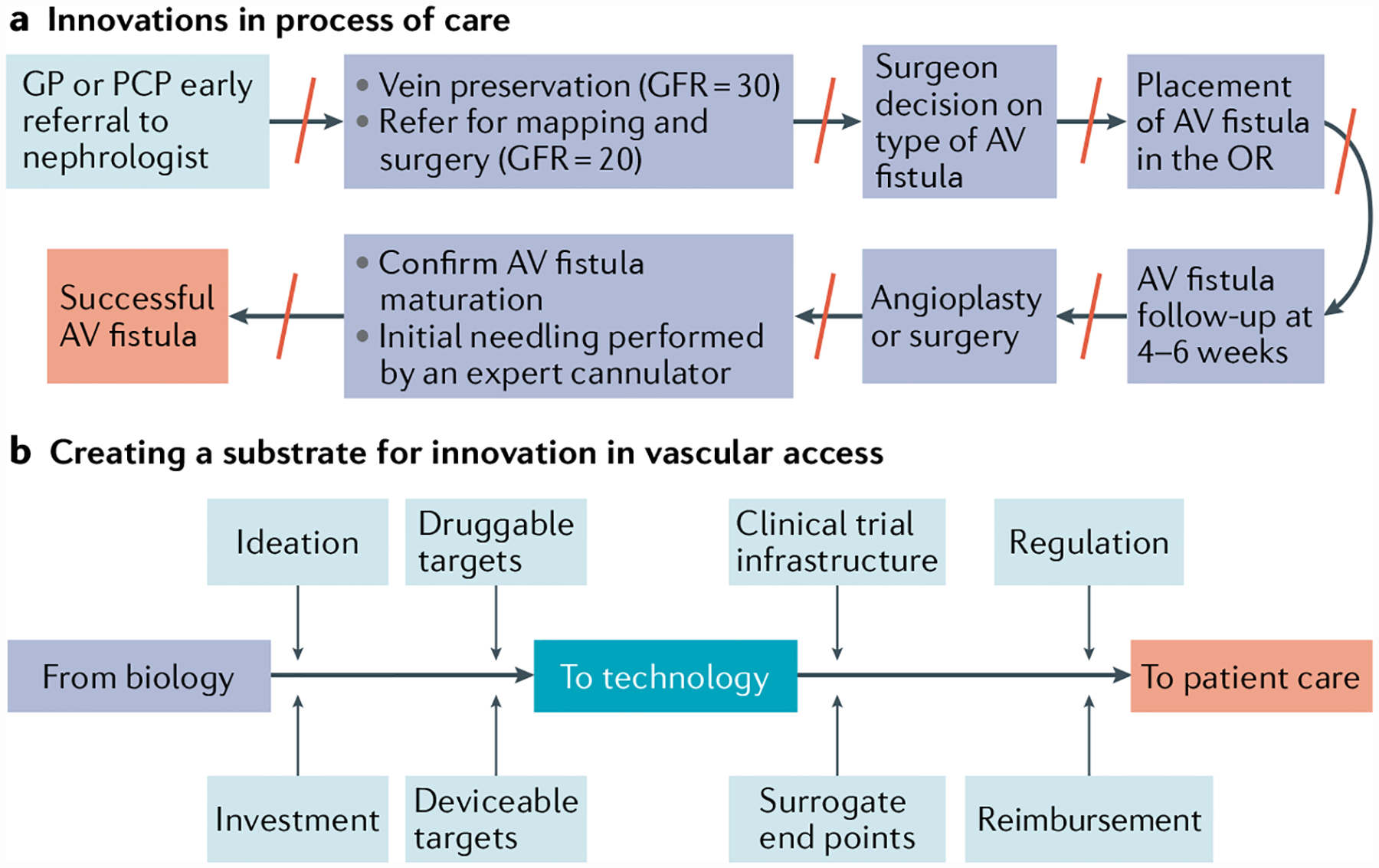
a | Innovation in process of care to increase incident arteriovenous (AV) fistula use. Note the multiple barriers (red lines) in the process of care multistep pathway, all of which could also potentially be opportunities for local process of care innovation. b | The process of innovation in the vascular access field requires a multipronged and multidisciplinary approach right from the point at which the concept is first ideated based on an understanding of biological and physiological processes, through investment, the identification of druggable and deviceable targets, to clinical trial design and infrastructure development, and completion of well-defined regulatory and reimbursement pathways. As a community the field needs coordination of these aspects to truly create an innovation substrate for the development and adoption of novel and effective patient-centred therapies and process of care pathways. GFR, glomerular filtration rate (mi/min/1.73 m2); GP, general practitioner; OR, operating room; PCP, primary care physician.
In addition to adopting the best process of care pathways for individual programmes, it is critically important that different regions adopt the process of care pathways that are best suited to the available expertise and resources. In many parts of the world outside North America, Europe, Australia and Japan, it is far more cost-effective to use a predominantly surgical pathway for AV fistula creation and maintenance as opposed to an endovascular pathway. Also, in many emerging economies (for example, Brazil, China, India, Egypt and South Africa), process of care pathways could be quite different for different segments of the population based on geography (for example, urban versus rural) and socioeconomic status.
Despite the substantial morbidity, mortality and economic costs associated with dialysis vascular access, the current environment is a sweet spot for discovery and innovation in processes of care in vascular access owing to a number of policy initiatives, particularly in the USA.
An important step forward in the USA has been the Executive Order on Advancing American Kidney Health148, which was signed in July 2019 and encourages the implementation of global payment systems whereby overall health-care costs for patients with advanced kidney disease would be disbursed as a single overall payment with asymmetrical risk. This global payment system could incentivize innovation in vascular access over the previous fee-for-service model, in which more interventions means more profit; for example, a new $3,000 device that enhances AV fistula maturation, consequently saving $20,000 in downstream infection, angioplasty and surgery costs, would be more in demand in a global payment system where the reduction in admissions due to lower infection and interventional procedures would reduce costs and increase potential profits, which could then be returned to the health-care system.
In addition, the USA kidney community has over the past 7 years created a patient-centred substrate that is designed to address the key steps in the development of new innovative approaches for the treatment of kidney disease, including innovative approaches to vascular access (FIG. 7b). This innovative substrate is largely the result of organizations such as the Kidney Health Initiative (KHI) and the KidneyX Innovation Accelerator.
The KHI is a public–private partnership between the American Society of Nephrology (ASN) and the FDA and various other companies that aim to facilitate the passage of novel drugs, devices and biologic agents into the kidney disease space149,150. In the context of vascular access, the KHI has published a series of white papers on clinical trial end points for vascular access150–153, as well as a roadmap that describes the components needed for an ideal vascular access in the context of future portable, wearable, implantable and bioengineered kidneys154. These publications are likely to serve as catalysts for increased interest, investment and innovation in vascular access.
The KidneyX Innovation Accelerator on the other hand is a public–private partnership between the ASN and the US Department of Health and Human Services (HHS), which aims to fund innovative products through a series of competitions, improve coordination across federal agencies, de-risk commercialization and create a sense of urgency driven by people living with kidney diseases. KidneyX has already instituted an initial series of prize competitions focused around the Redesign Dialysis competition155. Interestingly, vascular access innovation comprised almost one-third of the 15 awards in the first phase of prizes.
These initiatives aim to promote innovation in the process of care and in dialysis vascular access for kidney disease. However, it is critically important that the entire dialysis vascular access community — including nurses, technicians, facility managers, innovators, engineers, epidemiologists, surgeons, radiologists, nephrologists and patients — take advantage of this opportunity to truly change the way we care for this ‘lifeline’ for patients on haemodialysis.
Hurdles for emerging technologies
The current dialysis vascular landscape is such that only small, incremental changes are being successfully approved and transitioned to the clinic, resulting in what is arguably stagnation in the field of vascular access care. This stagnation is due, at least in part, to hurdles that exist in product development, testing, approval, and clinical implementation and adoption. Innovative ideas have the greatest potential to help patients; however, the underlying idea must be scientifically sound as well as creative. Non-clinical testing should support transition of the innovation to the clinic, as well as model potential benefits in patients. Adoption of an appropriate non-clinical model is therefore essential156, and the option to use canine, porcine or other animal models (including the need for models of uraemia in large animals) should be critically evaluated.
An additional, often overlooked challenge is the need to co-develop processes for clinical manufacturing, which needs to occur in conjunction with non-clinical testing of the technology. The ability to scale manufacturing is essential for the development of a viable commercial product, and the overall production costs will have far-reaching implications on the use of the product in the clinic. Haemodialysis is an expensive part of care for patients with kidney failure, and as mentioned earlier, vascular access procedure costs represent a substantial proportion of these costs2,14. Excessive costs associated with new products may prevent adoption or support of the technology once it reaches the market.
Regulatory approval is also a large hurdle. Well-designed, large-scale clinical studies are often needed to clearly demonstrate efficacy, and trial end points should be carefully considered152,153,157. The selection of trial end points is especially critical for truly innovative treatments where a comparator may not be available. Interactions with the relevant regulatory authority should be initiated early and occur frequently to guide development. After approval, concerted efforts in interventionalist education may be required, especially for novel treatments, and it might be difficult to navigate existing options for reimbursement. Across the whole spectrum of development, funding and financial support are essential. This support might require partnering with academic institutions, government and/or private companies to support preclinical testing, appropriately powered clinical studies, commercial manufacturing and scaling, as well as necessary education for market adoption.
Finally, another important and often overlooked aspect of innovation in the vascular access field is that it needs to be patient-centred and address issues that are important to patients as opposed to issues that are important to other stakeholders such as physicians, industry partners, regulators and payers. As an example, data suggest that patients with dialysis vascular access dysfunction use CVCs because of concern about the physical appearance of their vascular access and for other non-medical reasons, as well as concern about previous access failure158. Similarly, primary or secondary (cumulative) patency, which are currently the clinical gold standard for evaluation of dialysis vascular access, might be less important to patients than cannulation issues such as pain and infiltration, and the need for repeated procedures to maintain a functional access. At a practical level, these differences suggest that developers need to create vascular access therapies that address the issues that are important to patients, which may be different from those that are important to health professionals.
Conclusions
Options for chronic vascular access in patients on haemodialysis have remained unchanged over many decades, and this has contributed to high patient morbidity and mortality, and high health-care costs. The unmet clinical need for novel techniques in vascular access is driving a wave of innovation in vascular access. New devices, biological therapies and techniques have resulted in new approaches to controlling fistula geometry, manipulating the underlying cellular and molecular pathways involved in vascular endothelium remodelling, and influencing fistula maturation and formation. Changes in AV graft materials range from small modifications to the graft lumen to the creation of completely novel bioengineered grafts. Steps have also been taken to create new devices for treatment of patients with central vein stenosis.
Despite these advances, clinical and market adoption of novel technologies has been low. Emerging therapies for clinical use face difficult hurdles, and it is clear that truly creative approaches to vascular access will need critical resources, including well-designed clinical trials, frequent regulatory interaction, interventionalist education and greater financial investment, to ensure the success of these innovations. In addition, given the heterogeneity of patients with kidney failure, it is unlikely that a single ‘one-size-fits-all’ approach will be able to provide effective vascular access for all patients. Therefore, future research should pursue all avenues of vascular care, including approaches to improving fistula maturation, the provision of durable graft options for patients in whom fistulas are not a viable option, and the generation of creative options for patients with late-stage disease. Last but not least, it is critically important for all innovations to be integrated into well-defined patient-centred process of care pathways that are likely to vary across programmes and regions.
Key points.
Globally, effective treatment of kidney failure depends on reliable vascular access so that patients can receive long-term kidney replacement therapy.
current vascular access options are arteriovenous fistula, graft or central venous catheter, each of which is associated with high mortality, morbidity and economic burden.
For fistulas, new devices, biological approaches and techniques are in development that control fistula geometry, manipulate underlying cell and molecular pathways and influence maturation.
New graft and catheter materials are also in development, encompassing both incremental changes in current technologies and novel bioengineered vascular materials.
Process of care innovations are also important in order to generate patient-centred approaches that will be applicable to all individuals with kidney failure.
Acknowledgements
The authors acknowledge the support of K.L. Hamilton (Humacyte, Inc., Durham, NC, USA) in collating information and references for this article.
Footnotes
Competing interests
J.H.L. is the CEO of Humacyte, Inc. and is a co-founder of InnAVasc. L.E.N. is the founder of Humacyte, Inc. P.R.-C. is a consultant and adviser for WL Gore, BD, Medtronic, Cormedix, Humacyte, Akebia, Vifor-Relypsa and Bayer; the founder and Chief Scientific Officer of Inovasc LLC; the founding co-chair of the Kidney Health Initiative; and currently a member of the steering committee of the KidneyX Innovation Accelerator. P.R.-C. is also a co-PI on phase II of NIH Small Business award programs with Cylerus.
Informed consent
The authors affirm that human research participants provided written informed consent for publication of the images in Fig. 2.
References
- 1.Hill NR et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases. United States Renal Data System https://www.usrds.org/2018/view/Default.aspx (2018).
- 3.GBD 2015 Mortality and Causes of Death Collaborators. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liyanage T et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1972–1982 (2015). [DOI] [PubMed] [Google Scholar]
- 5.McCullough KP, Morgenstern H, Saran R, Herman WH & Robinson BM Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol 30, 127–135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Society of Nephrology. ISN Global Kidney Health Atlas. 2nd ed. https://www.theisn.org/global-atlas (2019). [Google Scholar]
- 7.Swaminathan S, Mor V, Mehrotra R & Trivedi A Medicare’s payment strategy for end-stage renal disease now embraces bundled payment and pay-for-performance to cut costs. Health Aff. 31, 2051–2058 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golestaneh L Decreasing hospitalizations in patients on hemodialysis: time for a paradigm shift. Semin. Dial 31, 278–288 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JK, Neovius M, Jacobson SH, Elinder C-G & Hylander B Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open 6, e012062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu FX, Treharne C, Culleton B, Crowe L & Arici M The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrol. 15, 161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan AA Peritoneal dialysis or hemodialysis: present and future trends in the United States. Contrib. Nephrol 189, 61–64 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Feldman HI et al. Hemodialysis vascular access morbidity in the United States. Kidney Int. 43, 1091–1096 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Dalrymple LS et al. Risk factors for infection-related hospitalization in in-center hemodialysis. Clin. J. Am. Soc. Nephrol 10, 2170–2180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman HI, Kobrin S & Wasserstein A Hemodialysis vascular access morbidity. J. Am. Soc. Nephrol 7, 523–535 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Thamer M et al. Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am. J. Kidney Dis 72, 10–18 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Brescia MJ, Cimino JE, Appel K & Hurwich BJ Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N. Engl. J. Med 275, 1089–1092 (1966). [DOI] [PubMed] [Google Scholar]
- 17.Enzler MA, Rajmon T, Lachat M & Largiader F Long-term function of vascular access for hemodialysis. Clin. Transplant 10, 511–515 (1996). [PubMed] [Google Scholar]
- 18.Pisoni RL, Zepel L, Port FK & Robinson BM Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am. J. Kidney Dis 65, 905–915 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Pisoni RL et al. International differences in the location and use of arteriovenous accesses created for hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis 71, 469–478 2018). [DOI] [PubMed] [Google Scholar]
- 20.Bylsma LC, Gage SM, Reichert H, Dahl SLM & Lawson JH Arteriovenous fistulae for haemodialysis: a systematic review and meta-analysis of efficacy and safety outcomes. Eur. J. Vasc. Endovasc. Surg 54, 513–522 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Dember LM et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 299, 2164–2171 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T, Qian J, Thamer M & Allon M Tradeoffs in vascular access selection in elderly patients initiating hemodialysis with a catheter. Am. J. Kidney Dis 72, 509–518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy-Chaudhury P et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am. J. Kidney Dis 50, 782–790 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Roy-Chaudhury P, Sukhatme VP & Cheung AK Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J. Am. Soc. Nephrol 17, 1112–1127 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Dixon BS Why don’t fistulas mature? Kidney Int. 70, 1413–1422 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Lee T & Roy-Chaudhury P Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv. Chronic Kidney Dis 16, 329–338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajabi-Jagahrgh E et al. Influence of temporal variation in wall shear stress on intima-media thickening in arteriovenous fistulae. Semin. Dial 26, 511–519 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Falk A Maintenance and salvage of arteriovenous fistulas. J. Vasc. Interv. Radiol 17, 807–813 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Lok C, Arnold R, Rajan D & Glickman M Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J. Vasc. Access 18, 8–14 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J & Allon M Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J. Vasc. Surg 64, 155–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacson E, Lazarus JM, Himmelfarb J, Ikizler TA & Hakim RM Balancing Fistula First with Catheters Last. Am. J. Kidney Dis 50, 379–395 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Rajput A et al. Venous aneurysms in autogenous hemodialysis fistulas: is there an association with venous outflow stenosis. J. Vasc. Access 14, 126–130 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Mudoni A et al. Aneurysms and pseudoaneurysms in dialysis access. Clin. Kidney J 8, 363–367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasklinsky G et al. Management of true aneurysms of hemodialysis access fistulas. J. Vasc. Surg 53, 1291–1297 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Valenti D, Mistry H & Stephenson M A novel classification system for autogenous arteriovenous fistula aneurysms in renal access patients. Vasc. Endovasc. Surg 48, 491–496 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Jankovic A et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron. Clin. Pract 124, 94–98 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Shiraya S et al. Successful surgical remodeling of a giant venous aneurysm formed in an autogenous arteriovenous fistula: a case report. Yonago Acta Med. 61, 142–144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saleh MA, El Kilany WM, Keddis VW & El Said TW Effect of high flow arteriovenous fistula on cardiac function in hemodialysis patients. Egypt. Heart J 70, 337–341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequeira A & Tan T-W Complications of a high-flow access and its management. Semin. Dial 28, 533–543 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Al Shakarchi J, Houston G & Inston N Early cannulation grafts for haemodialysis: a systematic review. J. Vasc. Access 16, 493–497 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Ravani P et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J. Am. Soc. Nephrol 24, 465–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravani P et al. Examining the association between hemodialysis access type and mortality: the role of access complications. Clin. J. Am. Soc. Nephrol 12, 955–964 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodside KJ et al. Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: a national study. Am. J. Kidney Dis 71, 793–801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beathard GA The treatment of vascular access graft dysfunction: a nephrologist’s view and experience. Adv. Ren. Replace. Ther 1, 131–147 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Collins MJ et al. Therapeutic strategies to combat neointimal hyperplasia in vascular grafts. Expert. Rev. Cardiovasc. Ther 10, 635–647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muto A, Model L, Ziegler K, Eghbalieh SDD & Dardik A Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ. J 74, 1501–1512 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ & King LR Comparison of autogenous fistula versus expanded polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am. J. Surg 152, 238–243 (1986). [DOI] [PubMed] [Google Scholar]
- 48.Maytham GGD, Sran HK & Chemla ES The use of the early cannulation prosthetic graft (Acuseal) for angioaccess for haemodialysis. J. Vasc. Access 16, 467–471 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Allon M Dialysis catheter-related bacteremia: treatment and prophylaxis. Am. J. Kidney Dis 44, 779–791 (2004). [PubMed] [Google Scholar]
- 50.Klevens RM et al. Dialysis surveillance report: National Healthcare Safety Network (NHSN)-data summary for 2006. Semin. Dial 21, 24–28 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Lynch JR, Wasse H, Armistead NC & McClellan WM Achieving the goal of the fistula first breakthrough initiative for prevalent maintenance hemodialysis patients. Am. J. Kidney Dis 57, 78–89 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clase CM, Crowther MA, Ingram AJ & Cina CS Thrombolysis for restoration of patency to haemodialysis central venous catheters: a systematic review. J. Thromb. Thrombolysis 11, 127–136 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Zacharias JM, Weatherston CP, Spewak CR & Vercaigne LM Alteplase versus urokinase for occluded hemodialysis catheters. Ann. Pharmacother 37, 27–33 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Jassal SV, Pierratos A & Roscoe JM Venous stenosis and thrombosis associated with the use of internal jugular vein catheters for hemodialysis. ASAIO J. 45, 356–359 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Hodges TC et al. Longitudinal comparison of dialysis access methods: risk factors for failure. J. Vasc. Surg 26, 1009–1019 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Allon M & Lok CE Dialysis fistula or graft: the role for randomized clinical trials. Clin. J. Am. Soc. Nephrol 5, 2348–2354 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Lin E, Mell MW, Winkelmayer WC & Erickson KF Health insurance in the first 3 months of hemodialysis and early vascular access. Clin. J. Am. Soc. Nephrol 13, 1866–1875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rayner HC et al. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines. Am. J. Kidney Dis 44, 22–26 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Rayner HC & Pisoni RL The increasing use of hemodialysis catheters: evidence from the DOPPS on its significance and ways to reverse it. Semin. Dial 23, 6–10 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Stevenson KB et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am. J. Kidney Dis 39, 549–555 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Akoh JA & Patel N Infection of hemodialysis arteriovenous grafts. J. Vasc 11, 155–158 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Katzman HE, Glickman MH, Schild AF, Fujitani RM & Lawson JH Multicenter evaluation of the bovine mesenteric vein bioprostheses for hemodialysis access in patients with an earlier failed prosthetic graft. J. Am. Coll. Surg 201, 223–230 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Miller LM et al. Hemodialysis tunneled catheter-related infections. Can. J. Kidney Health Dis 3, 2054358116669129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bray BD et al. Vascular access type and risk of mortality in a national prospective cohort of haemodialysis patients. QJM 105, 1097–1103 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Paoli CJ, Reynolds MA, Sinha M, Gitlin M & Crouser E Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit. Care Med 46, 1889–1897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah S, Leonard AC, Meganathan K, Christianson AL & Thakar CV Gender and racial disparities in initial hemodialysis access and outcomes in incident end-stage renal disease patients. Am. J. Nephrol 48, 4–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson WJ, Barker J & Allon M Disparities in fistula maturation persist despite preoperative vascular mapping. Clin. J. Am. Soc. Nephrol 3, 437–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee H-W & Allon M When should a patient receive an arteriovenous graft rather than a fistula? Semin. Dial 26, 6–10 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Zarkowsky DS et al. Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg. 150, 529–536 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Nee R et al. Impact of poverty and health care insurance on arteriovenous fistula use among incident hemodialysis patients. Am. J. Nephrol 42, 328–336 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Patibandla BK et al. Disparities in arteriovenous fistula placement in older hemodialysis patients. Hemodial. Int 18, 118–126 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Copeland TP, Hye RJ, Lawrence PF & Woo K Association of race and ethnicity with vascular access type selection and outcomes. Ann. Vasc. Surg 62, 142–147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Balas A et al. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J. Am. Soc. Nephrol 28, 3679–3687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kheda MF et al. Influence of arterial elasticity and vessel dilatation on arteriovenous fistula maturation: a prospective cohort study. Nephrol. Dial. Transpl 25, 525–531 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Beathard GA, Arnold P, Jackson J & Litchfield T Aggressive treatment of early fistula failure. Kidney Int. 64, 1487–1494 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Bharat A, Jaenicke M & Shenoy S A novel technique of vascular anastomosis to prevent juxta-anastomotic stenosis following arteriovenous fistula creation. J. Vasc. Surg 55, 274–280 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Sadaghianloo N et al. Increased reintervention in radial-cephalic arteriovenous fistulas with anastomotic angles of less than 30 degrees. J. Vasc. Surg 62, 1583–1589 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Corbett RW et al. Heterogeneity in the nonplanarity and arterial curvature of arteriovenous fistulas in vivo. J. Vasc. Surg 68, 152S–163S (2018). [DOI] [PubMed] [Google Scholar]
- 79.Chemla E, Velazquez CC, D’Abate F, Ramachandran V & Maytham G Arteriovenous fistula construction with the VasQ external support device: a pilot study. J. Vasc. Access 17, 243–248 (2016). [DOI] [PubMed] [Google Scholar]
- 80.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03242343 (2020). [DOI] [PubMed]
- 81.Chemla E et al. Arteriovenous fistula creation using the Optiflow vascular anastomotic connector: the OPEN (Optiflow PatEncy and MaturatioN) study. J. Vasc. Access 15, 38–44 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Nikam M et al. Prospective controlled pilot study of arteriovenous fistula placement using the novel Optiflow device. J. Vasc. Surg 61, 1020–1025 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Jia L et al. Effects of wall shear stress in venous neointimal hyperplasia of arteriovenous fistulae. Nephrology 20, 335–342 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Fitts MK, Pike DB, Anderson K & Shiu Y-T Hemodynamic shear stress and endothelial dysfunction in hemodialysis access. Open Urol. Nephrol. J 7, 33–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robbin ML et al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation Study. J. Am. Soc. Nephrol 29, 2735–2744 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loree HM II et al. In vitro study of a medical device to enhance arteriovenous fistula eligibility and maturation. ASAIO J. 61, 480–486 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Majesky MW Vascular development. Arterioscler. Thromb. Vasc. Biol 38, e17–e24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conte MS et al. Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: the V-HEALTH study. J. Vasc. Surg 50, 1359–1368.e1 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Conte MS, Nugent HM, Gaccione P, Roy-Chaudhury P & Lawson JH Influence of diabetes and perivascular allogeneic endothelial cell implants on arteriovenous fistula remodeling. J. Vasc. Surg 54, 1383–1389 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Peden EK et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J. Vasc. Access 14, 143–151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwivedi AJ et al. Application of human type I pancreatic elastase (PRt-201) to the venous anastomosis of arteriovenous grafts in patients with chronic kidney disease. J. Vasc. Access 15, 376–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bleyer AJ et al. A randomized trial of vonapanitase (PATENCY-1) to promote radiocephalic fistula patency and use for hemodialysis. J. Vasc. Surg 69, 507–515 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Proteon Therapeutics. Proteon Therapeutics announces top-line results from phase 3 PATENCY-2 clinical trial of vonapanitase in radiocephalic arteriovenous fistulas: investigational vonapanitase did not meet co-primary endpoints. https://www.globenewswire.com/news-release/2019/03/28/1781199/0/en/Proteon-Therapeutics-Announces-Top-Line-Results-From-Phase-3-PATENCY-2-Clinical-Trial-of-Vonapanitase-in-Radiocephalic-Arteriovenous-Fistulas.html (2019).
- 94.Kazemzadeh G, Saberi A, Manani R, Sadeghipour F & Rahmani A Effect of local papaverine on arteriovenous fistula maturation in patients with end-stage renal disease. J. Bras. Nefrol 41, 185–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02513303 (2020). [DOI] [PubMed]
- 96.Paulson WD et al. Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: first human experience with a sirolimus-eluting collagen membrane (Coll-R). Nephrol. Dial. Transpl 27, 1219–1224 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Derderian T et al. To BAM or not to BAM?: a closer look at balloon-assisted maturation. Ann. Vasc. Surg 27, 104–109 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Park SC, Ko SY, Kim J II, Moon IS & Kim SD Balloon-assisted maturation for arteriovenous fistula maturation failure: an early period experience. Ann. Surg. Treat. Res 90, 272–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rizvi SA et al. The clinical efficacy of balloon-assisted maturation of autogenous arteriovenous fistulae. Ann. Vasc. Surg 41, 41–45 (2017). [DOI] [PubMed] [Google Scholar]
- 100.DerDerian T, Hingorani A, Boniviscage P, Carollo A & Ascher E Acute complications after balloon-assisted maturation. Ann. Vasc. Surg 28, 1275–1279 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Patane D et al. Treatment of juxta-anastomotic stenoses for failing distal radiocephalic arteriovenous fistulas: drug-coated balloons versus angioplasty. J. Vasc. Access 20, 209–216 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Swinnen JJ, Zahid A & Burgess DCA Paclitaxel drug-eluting balloons to recurrent in-stent stenoses in autogenous dialysis fistulas: a retrospective study. J. Vasc. Access 16, 388–393 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Boitet A et al. Drug-coated balloon angioplasty for dialysis access fistula stenosis. Semin. Vasc. Surg 29, 178–185 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Trerotola SO, Lawson J, Roy-Chaudhury P & Saad TF Drug coated balloon angioplasty in failing AV fistulas. Clin. J. Am. Soc. Nephrol 13, 1215–1224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03041467 (2020). [DOI] [PubMed]
- 106.Hull JE et al. The pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J. Vasc. Interv. Radiol 29, 149–158.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Hebibi H, Achiche J, Franco G & Rottembourg J Clinical hemodialysis experience with percutaneous arteriovenous fistulas created using the Ellipsys® vascular access system. Hemodial. Int 23, 167–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berland TL, Clement J, Griffin J, Westin GG & Ebner A Endovascular creation of arteriovenous fistulae for hemodialysis access with a 4 Fr device: clinical experience from the EASE study. Ann. Vasc. Surg 60, 182–192 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Lok CE et al. Endovascular proximal forearm arteriovenous fistula for hemodialysis access: results of the prospective, multicenter novel endovascular access trial (NEAT). Am. J. Kidney Dis 70, 486–497 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Arnold RJG et al. Comparison between surgical and endovascular hemodialysis arteriovenous fistula interventions and associated costs. J. Vasc. Interv. Radiol 29, 1558–1566.e2 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Inston N, Khawaja A, Tullett K & Jones R WavelinQ created arteriovenous fistulas versus surgical radiocephalic arteriovenous fistulas? A single-centre observational study. J. Vasc. Access 10.1177/1129729819897168 (2020). [DOI] [PubMed] [Google Scholar]
- 112.Dixon BS et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N. Engl. J. Med 360, 2191–2201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freeman J et al. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded polytetrafluoroethylene Propaten graft in a chronic canine femoral artery bypass model. Ann. Vasc. Surg 49, 295–303 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Lazarides MK, Argyriou C, Antoniou GA, Georgakarakos E & Georgiadis GS Lack of evidence for use of heparin-bonded grafts in access surgery: a meta-analysis. Semin. Vasc. Surg 29, 192–197 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Shemesh D et al. A prospective randomized study of heparin-bonded graft (Propaten) versus standard graft in prosthetic arteriovenous access. J. Vasc. Surg 62, 115–122 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Baek I et al. Paclitaxel coating of the luminal surface of hemodialysis grafts with effective suppression of neointimal hyperplasia. J. Vasc. Surg 55, 806–814. e1 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Lee BH et al. Paclitaxel-coated expanded polytetrafluoroethylene haemodialysis grafts inhibit neointimal hyperplasia in porcine model of graft stenosis. Nephrol. Dial. Transpl 21, 2432–2438 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Baek I et al. Paclitaxel coating on the terminal portion of hemodialysis grafts effectively suppresses neointimal hyperplasia in a porcine model. J. Vasc. Surg 61, 1575–1582.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Baek I et al. Suppression of neointimal hyperplasia by sirolimus-eluting expanded polytetrafluoroethylene (ePTFE) haemodialysis grafts in comparison with paclitaxel-coated grafts. Nephrol. Dial. Transpl 27, 1997–2004 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Cagiannos C et al. Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model. J. Vasc. Surg 42, 980–988 (2005). [DOI] [PubMed] [Google Scholar]
- 121.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00448708 (2011). [DOI] [PubMed]
- 122.Medgadget. Angiotech Suspends Vascular Wrap Trial Enrollment. https://www.medgadget.com/2008/04/angiotech_suspends_vascular_wrap_trial_enrollment.html (2008).
- 123.Nguyen-Lee JJ et al. Clinical experience with gore hybrid vascular graft in complex revascularizations demonstrates safety and efficacy. Ann. Vasc. Surg 66, 646–653 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Gomez LF & Peden EK Description and early outcomes of the hybrid graft for dialysis. J. Vasc. Access 18, 64–67 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Gage SM & Lawson JH Challenging hybrid cases: how we do them. Endovasc. Today 2014, 13–16 (2014). [Google Scholar]
- 126.Gage SM et al. An immediate access dialysis graft designed to prevent needle-related complications: results from the initial pre-clinical studies. J. Vasc. Access 21, 328–335 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03645681 (2020). [DOI] [PubMed]
- 128.Kammer JA & Mundy KM Suprachoroidal devices in glaucoma surgery. Middle East Afr. J. Ophthalmol 22, 45–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bryers JD, Giachelli CM & Ratner BD Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol. Bioeng 109, 1898–1911 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kabirian F, Brouki Milan P, Zamanian A, Heying R & Mozafari M Nitric oxide-releasing vascular grafts: a therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta Biomater. 92, 82–91 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Fleser PS et al. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J. Vasc. Surg 40, 803–811 (2004). [DOI] [PubMed] [Google Scholar]
- 132.Arhuidese I et al. Bovine carotid artery biologic graft outperforms expanded polytetrafluoroethylene for hemodialysis access. J. Vasc. Surg 65, 775–782 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Pineda DM et al. Bovine carotid artery xenografts for hemodialysis access. J. Vasc. Surg 65, 1729–1734 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Wystrychowski W et al. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J. Vasc. Surg 60, 1353–1357 (2014). [DOI] [PubMed] [Google Scholar]
- 135.McAllister TN et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440–1446 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Tzchori I et al. Improved patency of ePTFE grafts as a hemodialysis access site by seeding autologous endothelial cells expressing fibulin-5 and VEGF. Mol. Ther 26, 1660–1668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matsumura G, Hibino N, Ikada Y, Kurosawa H & Shin’oka T Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials 24, 2303–2308 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Geelhoed WJ et al. A novel method for engineering autologous non-thrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials 229, 119577 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Dahl SLM et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med 3, 68ra9 (2011). [DOI] [PubMed] [Google Scholar]
- 140.Lawson JH et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387, 2026–2034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kirkton RD et al. Susceptibility of ePTFE vascular grafts and bioengineered human acellular vessels to infection. J. Surg. Res 221, 143–151 (2018). [DOI] [PubMed] [Google Scholar]
- 142.Kirkton RD et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci. Transl. Med 11, eaau6934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02644941 (2020). [DOI] [PubMed]
- 144.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03183245 (2020). [DOI] [PubMed]
- 145.Gage SM et al. Multi-center experience of 164 consecutive Hemodialysis Reliable Outflow [HeRO] graft implants for hemodialysis treatment. Eur. J. Vasc. Endovasc. Surg 44, 93–99 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Wallace JR, Chaer RA & Dillavou ED Report on the hemodialysis reliable outflow (HeRO) experience in dialysis patients with central venous occlusions. J. Vasc. Surg 58, 742–747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maqsood MH & Rubab K Quality of life of patients using the hemodialysis reliable outflow (HeRO) graft in hemodialysis. Cureus 11, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trump DJ Executive order on advancing American kidney health https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/ (The White House, 2019). [Google Scholar]
- 149.Linde PG et al. Overcoming barriers in kidney health-forging a platform for innovation. J. Am. Soc. Nephrol 27, 1902–1910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ & Roy-Chaudhury P Fostering innovation, advancing patient safety: the kidney health initiative. Clin. J. Am. Soc. Nephrol 8, 1609–1617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hurst FP, Lee RE, Thompson AM, Pullin BD & Silverstein DM FDA regulatory perspectives for studies on hemodialysis vascular access. Clin. J. Am. Soc. Nephrol 13, 513–518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beathard GA et al. Definitions and end points for interventional studies for arteriovenous dialysis access. Clin. J. Am. Soc. Nephrol 13, 501–512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shenoy S et al. Clinical trial end points for hemodialysis vascular access: background, rationale, and definitions. Clin. J. Am. Soc. Nephrol 13, 490–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nexight Group. Kidney Health Initiative: Technology Roadmap for innovative approaches to renal replacement therapy https://www.asn-online.org/membership/BlastEmails/files/KHI_RRT_Roadmap1.0_FINAL_102318_web.pdf (2018).
- 155.Commins J HHS names KidneyX redesign phase 1 winners. HealthLeaders https://www.healthleadersmedia.com/innovation/hhs-names-kidneyx-redesign-phase-1-winners (2019). [Google Scholar]
- 156.Abbott WM et al. Evaluation and performance standards for arterial prostheses. J. Vasc. Surg 17, 746–756 (1993). [DOI] [PubMed] [Google Scholar]
- 157.Allon M et al. Recommended clinical trial end points for dialysis catheters. Clin. J. Am. Soc. Nephrol 13, 495–500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaudhry M et al. Seeing eye to eye: the key to reducing catheter use. J. Vasc. Access 12, 120–126 (2011). [DOI] [PubMed] [Google Scholar]
- 159.Dorobantu LF, Stiru O, Bulescu C, Bubenek S & Iliescu VA in Hemodialysis Ch. 30 (ed. Suzuki H) (IntechOpen, 2013). [Google Scholar]
- 160.Allon M & Robbin ML Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 62, 1109–1124 (2002). [DOI] [PubMed] [Google Scholar]


