Abstract
Background:
Significant disparities in hepatitis C (HCV) treatment existed in the interferon treatment era, such that patients with mental health and substance use disorders were less likely to be treated. We aimed to evaluate whether these perceptions continue to influence HCV treatment decisions.
Methods:
We emailed HCV providers a survey to assess their perceptions of barriers to HCV treatment adherence and initiation. We assessed the frequency of perceived barriers and willingness to initiate HCV treatment in patients with these barriers. We identified a group of providers more willing to treat patients with perceived barriers to adherence and determined the associated provider characteristics using Spearman rho and Wilcoxon rank-sum tests.
Results:
A total of 103 providers (29%) responded to the survey. The most commonly endorsed perceived barriers to adherence were homelessness (65%), ongoing drug (58%), and ongoing alcohol use (33%). However, 90%, 68%, and 90% of providers were still willing to treat patients with these comorbidities, respectively. Ongoing drug use was the most common reason providers were never or rarely willing to initiate HCV treatment. Providers who were less willing to initiate treatment more frequently endorsed patient-related determinants of adherence, while providers who were more willing to initiate treatment more frequently endorsed provider-based barriers to adherence (e.g., communication).
Conclusions:
Most responding providers were willing to initiate HCV treatment in all patients, despite the presence of perceived barriers to adherence or previous contraindications to interferon-based treatments. Ongoing substance use remains the most prominent influencer in the decision not to treat.
Keywords: attitude of health personnel, compliance, direct-acting antiviral agents, addiction
Introduction
Hepatitis C virus (HCV) affects nearly 2.4 million people in the United States (US) and continues to pose a national public health risk.1 Chronic HCV is associated with high rates of cirrhosis, hepatocellular carcinoma, and liver-related mortality.1 HCV disproportionately affects Veterans2 such that the Veterans Health Administration (VA) is currently the largest national provider of HCV care. In the past, interferon was the backbone of HCV treatment; however, interferon-based treatment was associated with numerous systemic side effects and had many contraindications to therapy. Many Veterans were deemed illegible for treatment; thus few patients with chronic HCV were offered interferon-based regimens and even fewer were cured of HCV in the interferon-era.3 Mental health and substance use disorders were the most common reasons to exclude patients from interferon treatment, despite evidence that patients with these disorders had adequate rates of treatment adherence.4
When direct-acting antiviral (DAA) medications entered the HCV treatment sphere, they became the standard of care for all genotypes of HCV.5–9 Since this shift, VA has provided DAAs to more than 110,000 Veterans with HCV infection, with overall sustained virologic response rates exceeding 95%.10 DAAs have an improved side effect profile and shorter treatment courses that range from 8–24 weeks, allowing providers more ease in prescribing and managing the increasing number of patients able to tolerate these medications.
The World Health Organization has set an HCV treatment target of 80% worldwide by 2030, with the goal of global HCV eradication.11 However, HCV treatment eligibility can be subjective and depends on provider perceptions of patient adherence, such that persistent concerns about early discontinuation and non-adherence among providers could limit access to treatment. Early discontinuation and medication non-adherence have been associated with treatment failure, which in turn can be associated with drug resistance, disease progression, and viral spread.12–15 Gaps in the literature still exist concerning physician perspectives of patient DAA adherence and their impacts on medical decision making with regard to treatment eligibility.
In an effort to address this gap, our group performed a qualitative study in which we found that HCV care providers considered specific patient populations to be less likely to adhere to treatment, including patients with substance use disorders, mental disorders, and housing instability.16 However, this study was limited to 24 providers from one VA Medical Center. HCV providers’ perceptions of barriers to and facilitators of HCV treatment eligibility and adherence have neither been fully evaluated quantitatively or with a larger cohort. Thus, the primary goal of this study was to assess the provider-perceived barriers to and facilitators of DAA medication adherence and the factors associated with treatment decisions in a national cohort.
Methods
Recruitment and Consent
Institutional Review Board approval was obtained from VA Pittsburgh Healthcare System (VAPHS). Providers for whom we had a working email address who were known to be involved in HCV care were emailed for study recruitment. This list included a range of provider types, including pharmacists, since pharmacists provide HCV treatment and education to many Veterans. An introductory email to the providers explained the study, including the risks, and benefits, and ensured respondents of their confidentiality. Informed consent was obtained from all individual participants included in the study.
Survey
A web-based questionnaire was developed using Research Electronic Data Capture (REDCap) to assess the perceived importance of various factors regarding adherence and treatment eligibility. The survey was adapted from the AIDS Clinical Trial Group Adherence Questionnaire17 using the additional potential barriers to treatment identified through our prior qualitative work.16 We chose to adapt the AIDS Clinical Trial questionnaire as it is a well-established questionnaire for assessing treatment adherence that has been used in VA and it addresses similarities between the populations with HIV and HCV. The survey was initially designed for patients, so we removed the patient-specific questions (e.g., “what are the most likely ways that you became infected with HIV?”) and focused on the question that assess barriers to adherence. We added several other questions based on our prior qualitative study. These included questions about treatment barriers (e.g., homelessness, transportation, substance use/addiction, and medical comorbidities)16 and facilitators of adherence based on our previous study (e.g., provider encouragement, education, substance use counseling, and positive feedback).
Providers were asked about demographics including age, gender, race, specialty, years of clinical practice, and number of HCV patients seen. We then asked providers the following: 1) how often they thought Veterans would have difficulty adhering to HCV treatment based on 12 different psychosocial factors and medical comorbidities; 2) how willing they were to start Veterans on treatment based on 12 different issues provided; 3) how important nine factors were in motivating patients to take the treatment; 4) to what extent 18 different factors were barriers to patient adherence; and 5) how helpful each of 14 provider practices were in facilitating treatment adherence. Responses were based on four- and five-point Likert scales.
The survey was emailed to providers via REDCap18 between November 2016 and January 2017. Three weekly email reminders were sent to encourage survey completion by non-responders.
Analysis
The frequency of survey responses was calculated for each survey item. Survey responses were reported as both continuous variables using means and standard deviations and categorical variables using percentages. For responses with large inter-cohort variation, we performed a Kruskal-Wallis test with Dunn post-hoc (Bonferroni corrected) to evaluate associations between high standard deviation variables (defined a priori as a standard deviation > 0.9) and demographic characteristics. Spearman rho was used to examine relationships between question responses.
We sought to understand characteristics that would make a provider more or less willing to treat patients perceived to be at risk for non-adherence. To capture “willingness to treat,” we separated providers who reported that they were at least sometimes willing to treat any group of patients. We used Wilcoxon rank sum tests to compare the characteristics of these providers with those of providers who answered they “rarely” or “never” treated patients with perceived risks of treatment non-adherence. All analyses were performed using R statistical software.
Results
Provider characteristics
Of the 357 emailed providers, 103 (29%) responded to the survey (Table 1). Provider respondents included gastroenterologist/hepatologists (34%), infectious disease specialists (23%), pharmacists (26%), and primary care providers (10%). Most respondents were female (65%) and Caucasian (74%). Years of clinical experience was bimodal with 39% of respondents having one to five years of experience and 40% of respondents with over 10 years of experience. Most providers cared for >40 patients with HCV (82%).
Table 1.
Provider Characteristics (N = 103)
| Characteristic | N (%) |
|---|---|
| Age | |
| 20–30 | 5 (5) |
| 31–40 | 21 (20) |
| 41–50 | 28 (27) |
| 51–60 | 26 (25) |
| 61+ | 22 (21) |
| Sex | |
| Female | 67 (65) |
| Race | |
| White/Caucasian | 76 (74) |
| Asian | 9 (9) |
| Black/African-American | 6 (6) |
| Other | 9 (9) |
| Clinical Role | |
| Primary Care | 10 (10) |
| Gastroenterology | 7 (7) |
| Hepatology | 28 (27) |
| Infectious Disease | 24 (23) |
| Pharmacy | 27 (26) |
| Other | 5 (5) |
| Years of Clinical Experience | |
| 0-1 | 0 |
| 1–5 | 40 (39) |
| 5–10 | 20 (19) |
| >10 | 41 (40) |
| HCV Treatment Candidates Seen this Year | |
| 1–10 | 7 (7) |
| 11–20 | 3 (3) |
| 20–40 | 7 (7) |
| >40 | 84 (82) |
Missing data excluded when calculating column percentages.
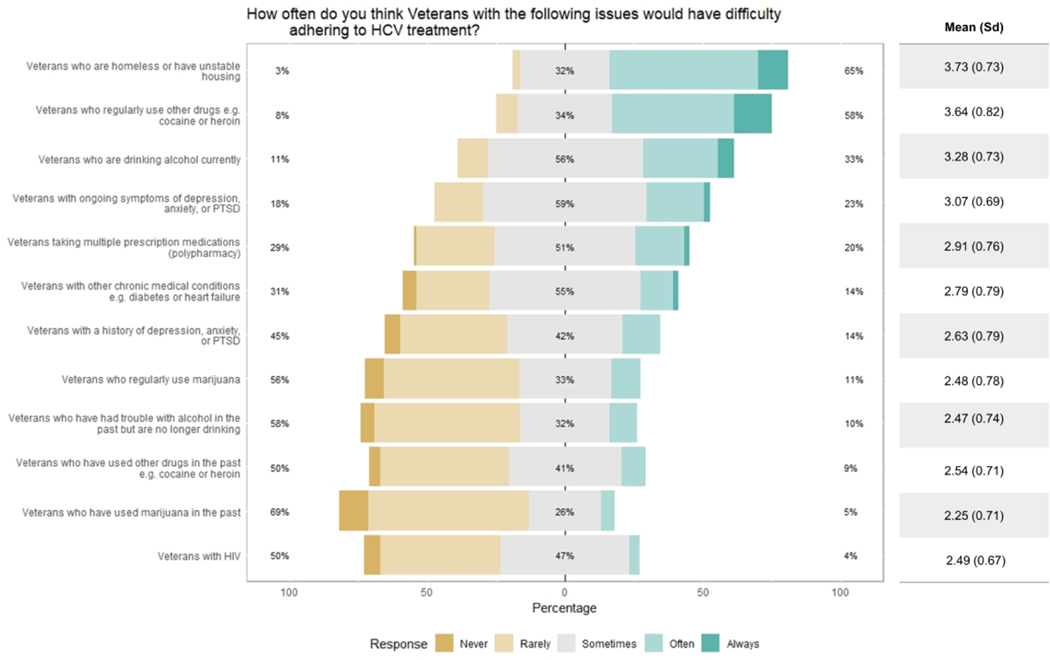
Providers were asked how often they believed Veterans with the prespecified psychosocial factors and medical comorbidities had difficultly adhering to HCV treatment. Most providers noted that homelessness/unstable housing (65%), ongoing drug use (58%), and ongoing alcohol use (33%) “often” or “always” cause Veterans difficulty in adhering to treatment (Figure 1). Here, ongoing drug use refers to drugs other than marijuana, such as heroin and cocaine. Conversely, most providers perceived Veterans to “rarely” or “never” have adherence difficulty from past marijuana (70%), alcohol (58%), or drug use (51%).
Figure 1.
Provider perceptions of patient subpopulations most likely to experience adherence difficulty (n= 103).
Barriers to adherence
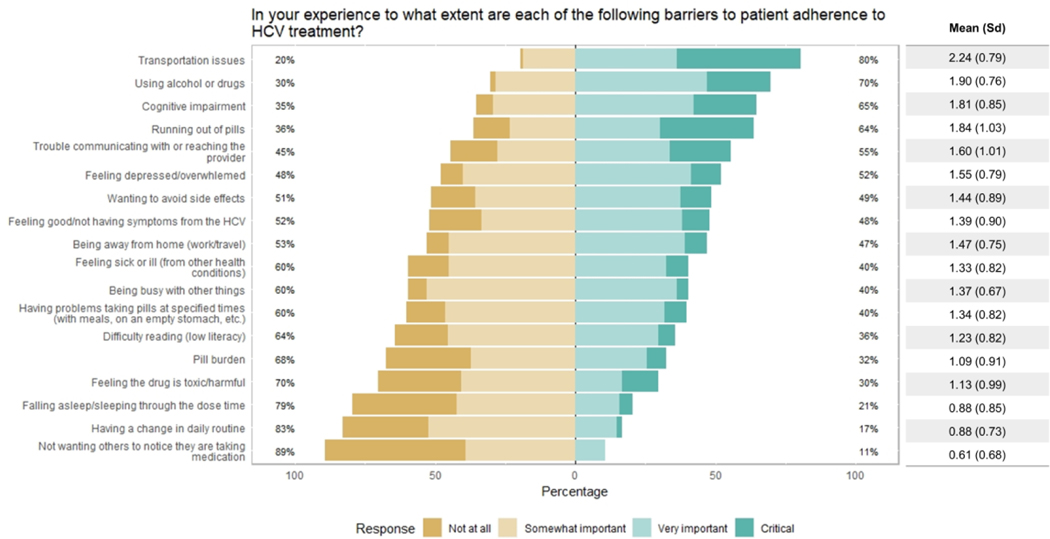
When asked about the importance of 18 potential barriers to treatment adherence, most providers believed that transportation issues (80%), using alcohol or drugs (70%), cognitive impairment (65%), and running out of pills (64%) were “very important” or “critical” barriers (Figure 2). The least important barriers identified were not wanting others to notice they are taking medication (11%), having a change in daily routine (17%), falling asleep/sleeping through the dose time (21%), and feeling the drug is toxic or harmful (30%). Using an a priori threshold of a standard deviation (sd) > 0.9 as a marker of provider disagreement, we found that the following were points of non-consensus as to whether they were associated with non-adherence: running out of pills (sd 1.03), trouble communicating with or reaching the provider (sd 1.01), feeling the drug is toxic/harmful (sd 0.99), pill burden (sd 0.91), and not having symptoms from HCV (sd 0.9).
Figure 2.
Provider perceptions of barriers to treatment adherence (n= 102).
Facilitators of adherence
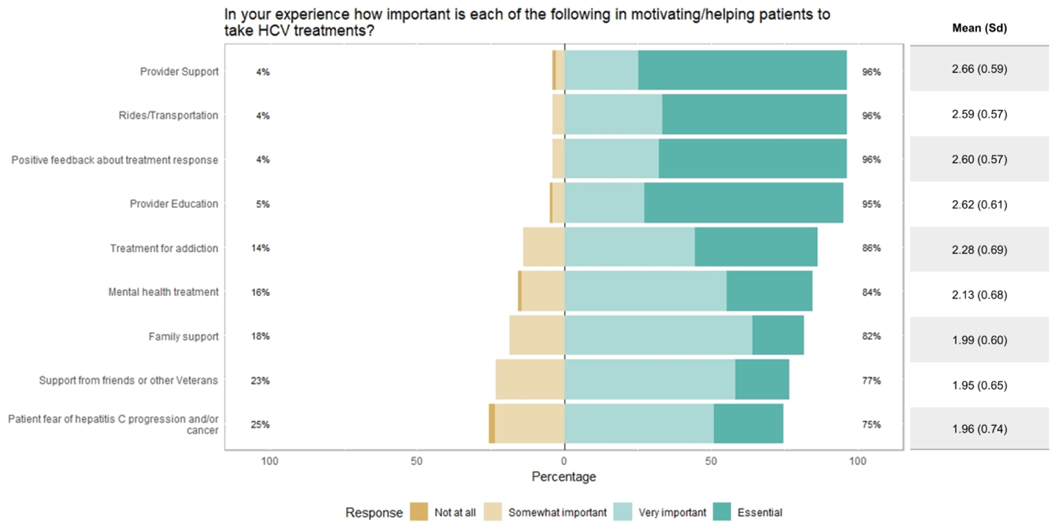
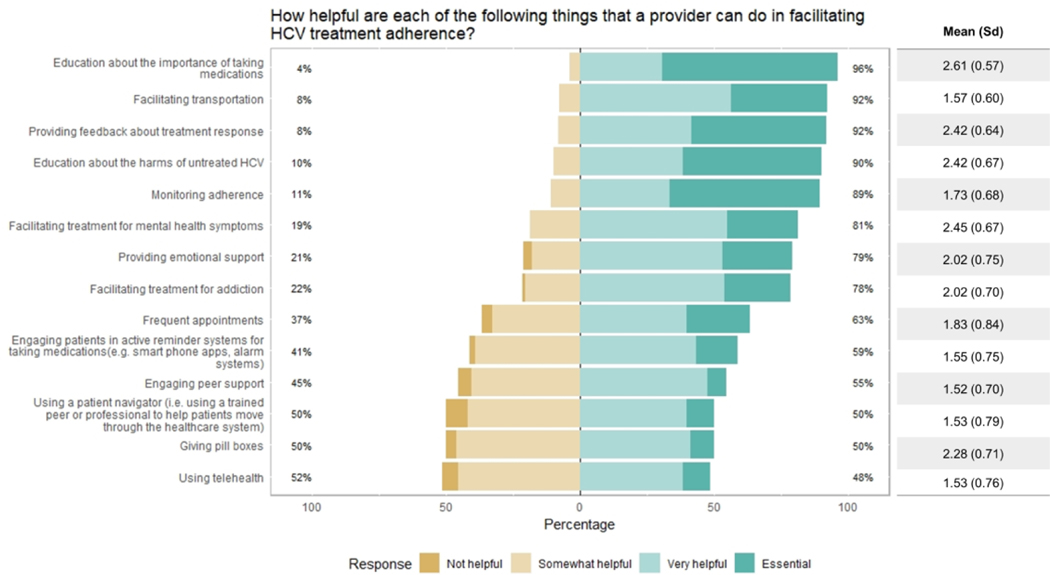
Regarding potential motivators of treatment adherence, at least 95% of providers found the following to be “very important” or “essential”: provider support, rides/transportation, positive feedback about treatment response, and provider education (Figure 3). Patients’ fear of HCV progression and/or cancer was judged to be the least important factor, with 25% of providers considering it “not at all” or only “somewhat important” for motivating adherence. When asked about ways that providers could facilitate treatment adherence (Figure 4), respondents considered education about the importance of taking medications to be the most helpful or essential (96%), followed by facilitating transport (92%), feedback about treatment response (92%), education about the harms of untreated HCV (90%), and monitoring adherence (89%). Opinion was split on using a patient navigator (50%), using telehealth (48%), and giving pill boxes (50%).
Figure 3.
Provider perceptions of factors motivating patients’ treatment adherence (n= 102).
Figure 4.
Perceived helpfulness of provider-initiated strategies to facilitate treatment adherence (n= 100).
Factors associated with willingness to treat
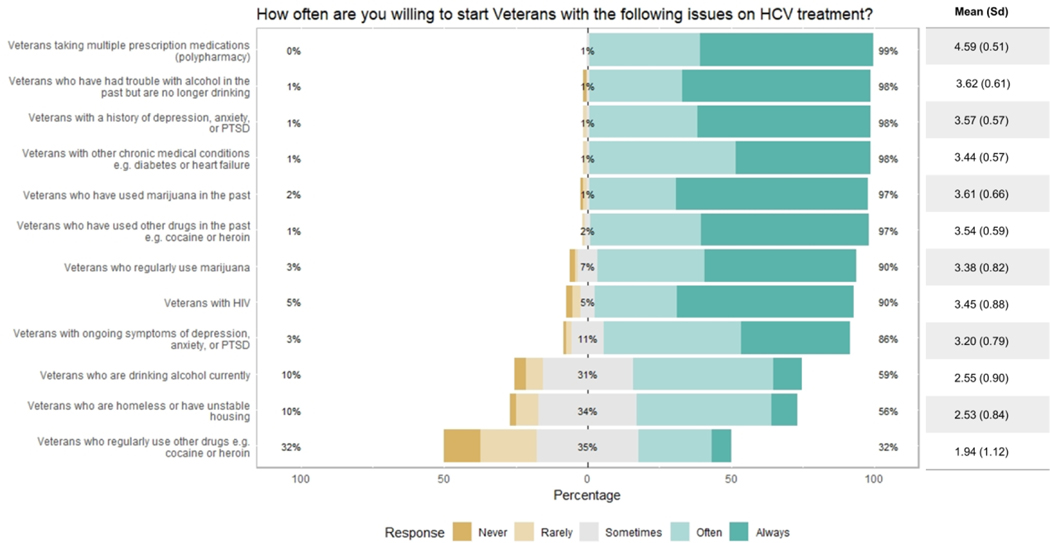
Providers were generally willing to initiate HCV treatment for Veterans with any of the 12 potential barriers (Figure 5), including taking multiple prescription medications (99%), past alcohol use (98%), a history of mental illness (98%), other chronic medical conditions (98%), past marijuana use (97%), or past drug use (97%). Providers were least likely to treat Veterans currently drinking alcohol (59%), who were homeless (56%), or currently using other drugs such as heroin or cocaine (32%). Because responses for current use of other drugs had a high standard deviation (1.12), we evaluated possible associations with provider demographics to explain this variation. We found no significant associations with age, specialty, gender, or years of experience; however, willingness to start DAA treatment of Veterans currently using drugs positively correlated with willingness to start Veterans who are currently drinking (Figure S1, Spearman rho r = 0.66, p <0.0001).
Figure 5.
Provider willingness to initiate treatment (n= 100).
We noted that a subset of providers (38%) answered they were “rarely” or “never” willing to treat patients for one or more of the potential barriers. This subset was designated as the “unwilling to treat” group. The 62% of providers who at no time answered “rarely” or “never” for this question were designated as the “willing to treat” group. The unwilling and willing to treat groups did not significantly differ by age, race, gender, specialty, years of experience, or number of patients seen with HCV. There was a statistical difference in the number of “always” and “often” responses to Question 1 (“How often do you think Veterans with the following issues would have difficulty adhering to HCV treatment?”) (p = 0.015); however, this statistical difference disappeared (p = 0.199) when the responses to “ongoing drug use” were excluded (Figure S2). We found no significant correlation between general willingness to treat and perceived adherence issues.
The willing group was more likely to start Veterans who were homeless, previously or currently using alcohol, previously or currently using marijuana, previously or currently using other drugs, or experiencing ongoing mental health symptoms (Table 2, p < 0.05). They were also more likely to consider trouble communicating with or reaching the provider as a barrier to adherence (p < 0.05), and to view giving pillboxes and using reminder systems as helpful for facilitating adherence (p < 0.05). On the other hand, 83% of providers in the unwilling group “rarely” or “never” were willing to initiate treatment for Veterans with ongoing drug use. The unwilling group was also more likely to believe Veterans who regularly use drugs such as cocaine or heroin would experience adherence difficulty (p < 0.05), and to rate treatment for addiction as important in motivating adherence (p < 0.05).
Table 2.
Comparison of survey responses between provider groups
| Survey Question | Willing to treat | Unwilling to treat | p-value |
|---|---|---|---|
| How often do you think Veterans with the following issues would have difficulty adhering to HCV treatment? | |||
| • Veterans who regularly use other drugs | 2.44 | 2.94 | 0.002 |
| In your experience to what extent are each of the following barriers to patient adherence to HCV treatment? | |||
| • Trouble communication with or reaching the provider | 1.78 | 1.32 | 0.024 |
| In your experience how important is each of the following in motivating/helping patients to take HCV treatments? | |||
| • Facilitating treatment for addiction | 3.17 | 2.45 | 0.044 |
| How helpful are each of the following things that a provider can do in facilitating HCV treatment adherence? | |||
| • Engaging in reminder systems | 1.87 | 1.48 | 0.019 |
| • Giving pill boxes | 1.67 | 1.36 | 0.038 |
| How often are you willing to start Veterans with the following issues on HCV treatment? | |||
| • Veterans who are homeless | 2.81 | 2.10 | <0.001 |
| • Veterans who have had trouble with alcohol in the past but are no longer drinking | 3.76 | 3.40 | 0.006 |
| • Veterans who are currently drinking alcohol | 2.92 | 1.95 | <0.001 |
| • Veterans who have used marijuana in the past | 3.74 | 3.40 | 0.029 |
| • Veterans who regularly use marijuana | 3.56 | 3.10 | 0.015 |
| • Veterans who have used other drugs in the past | 3.69 | 3.32 | 0.005 |
| • Veterans who regularly use other drugs | 2.60 | 0.93 | <0.001 |
| • Veterans with ongoing symptoms of depression, anxiety, or PTSD | 3.39 | 2.90 | 0.006 |
Discussion
Most VA providers were willing to initiate HCV treatment in patients with prior contraindications to interferon-based treatments. There did exist a minority group of providers, however, who were less willing to treat patients with perceived barriers to care. The providers in this group were mainly influenced by perceptions of ongoing drug use as a barrier to adherence in their decision not to treat Veterans with HCV.
The issue of whether patients with active drug use should receive HCV treatment was the subject of ongoing disagreement among providers in this cohort. Current VA guidelines do not exclude such patients from HCV treatment.19 In fact, studies have shown that drug use before and during treatment did not affect sustained virologic response (SVR) rates in clinical trials and that people who inject drugs (PWID) can also be successfully treated in a real-world setting.20–22 Some have argued that PWID should be a high-priority population to treat given the risk of ongoing disease transmission and the mounting US opioid epidemic.23 As noted above, provider willingness to start patients with ongoing drug use also positively correlated with willingness to start patients who were currently drinking alcohol. While this is consistent with prior literature demonstrating an association between alcohol use and treatment ineligibility, alcohol use has not been associated with reduced SVR with DAA medications.24,25 These data collectively indicate that provider perceptions vary in this arena and that a targeted provider-level intervention may increase treatment for patients with alcohol and substance use.
We found that providers who were more willing to treat patients with broad psychosocial backgrounds tended to focus more on the role providers played in adherence and were more likely to focus on provider-based treatment/adherence barriers (e.g., provider communication and accessibility), rather than patient-based barriers (e.g., substance abuse). Our group previously described an association between HCV treatment ineligibility and poor relationships between patient and provider in the interferon era.26 In that study, the described barriers included poor provider communication and feelings of stigmatization. The importance of communication and patient-provider relationships spans many facets of clinical care. Though we previously found that hepatology providers focused almost exclusively on provider-level facilitators (e.g., more frequent contact with the provider) while primary care providers focused more on the role of patient-based facilitators,16 we did not find similar associations in this study. This was likely due to small numbers of primary care providers who participated in the study, limiting our ability to compare these groups.
Interestingly, 25% of providers believed that fear of progression of the disease was not a strong adherence motivator, but 90% of providers thought that education about the harms of untreated HCV was an important provider-level facilitator for adherence. We believe this reflects that while some providers did not find fear of disease progression to be a helpful motivator, it was generally still a part of routine care.
HCV disproportionately affects homeless Veterans. HCV was identified in 12.1% of homeless Veterans compared to 2.7% of non-homeless Veterans in 2015.27 Homelessness has long been a barrier to interferon-based HCV treatment, with some literature suggesting that this barrier still exists despite DAA availability.2,28–30 In contrast, we found that while providers perceived homelessness/unstable housing as a barrier to adherences, they were generally willing to treat homeless Veterans. This aligns with the literature describing that the SVR rates achieved among homeless Veterans are generally comparable to those of non-homeless Veterans.31 The success of VA in treating this population may be in part due to the extensive services available to support such Veterans. It is unclear whether these results generalize to other populations.
Transportation was another commonly reported barrier to adherence among providers in this study. Similar findings were reported by Rich et al. in a review of qualitative literature on facilitators to HCV treatment adherence among PWID,32 in which they identified several qualitative studies (of patients as well as providers) that cited transportation as a critical mediator of adherence. In contrast, another study found that ensuring transportation and offering rideshare services to patients did not improve primary care clinic attendance among low-income patients with Medicaid.33 While this cohort did not include Veterans, these data indicate that transportation may be necessary but not sufficient to overcome barriers to clinic attendance.
There were several key limitations to this study. First, aligning with survey-based methodology, we used convenience sampling, which may not be generalizable to the entire population of HCV providers in or outside of VA. Despite sampling limitations, the response rate of 29% was still within range of the typical survey response rates for clinicians.34 We recognize that self-reported responses on a survey may differ from how providers practice, given the strong social desirability bias in completing surveys. The anonymity of the survey somewhat mitigates this concern. However, the anonymity of the survey precluded our ability to compare the demographic characteristics of respondents vs. non-respondents and required that the HCV treatment rates be self-reported. Second, we had small numbers of certain subgroups of providers, which limited our ability to compare across these groups. Despite these small numbers, we were able to capture responses from a diverse group of providers. Another limitation was that we did not address all possible barriers with our survey. For example, we asked about mental health but not specifically about schizophrenia. However, we did capture some of these concerns through a free-text option at four points during the survey (see Supplemental Tables S1-S4). The perception of other psychiatric disorders as barriers to adherence should be evaluated in future studies. Lastly, we did not address DAA cost as a potential barrier. While cost-related issues such as high copayments can contribute to early discontinuation and nonadherence to medications in general, VA is a unique environment, given that HCV treatment is universally available with minimal copayments. The fact that Veterans have access to HCV treatment and homelessness services may limit generalizability to providers outside VA whose patients may be more likely to experience cost and homelessness-related barriers to adherence. Future non-VA studies should assess the role of cost. Despite these limitations, this study was able to provide important insights about provider perceptions of DAA-based HCV treatment eligibility and adherence.
In conclusion, we found that while substance use, alcohol use, and unstable housing remain as important perceived barriers to HCV medication adherence, only active substance use was reported to influence providers’ decision to start treatment. Providers who were more likely to perceive substance use as a barrier to treatment adherence were also less likely to start DAA treatment for Veterans with more complex issues. Most providers believed they played an active role in promoting treatment adherence among their patients both through education and support but also through actively referring patients for help with transportation and substance use disorder treatment. Future studies should determine if provider perceptions align with those of patients in order to identify and address remaining gaps in HCV treatment and eradication.
Supplementary Material
Acknowledgments:
The contents of this article are the views of the authors alone and do not represent the views of the Department of Veterans Affairs or the United States Government.
Compliance with Ethical Standards
Funding: This study was funded by an investigator-initiated research grant from Gilead Sciences to the institution.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA Network Open. 2018;1(8):e186371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88–96. [DOI] [PubMed] [Google Scholar]
- 3.Lam BP, Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therapeutic Advances in Gastroenterology. 2015;8(5):298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chainuvati S, Khalid SK, Kancir S, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat. 2006;13(4):235–241. [DOI] [PubMed] [Google Scholar]
- 5.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373(27):2599–2607. [DOI] [PubMed] [Google Scholar]
- 6.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973–1982. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163(1):1–13. [DOI] [PubMed] [Google Scholar]
- 9.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. [DOI] [PubMed] [Google Scholar]
- 10.Sustained Virologic Response in Veterans in VHA Care Starting DAA Therapy in 2014 or Later for the Nation. Accessed January 6th, 2019. [Google Scholar]
- 11.World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. 2016; http://www.who.int/iris/handle/10665/206453. Accessed March 14, 2019.
- 12.Rossi C, Young J, Martel-Laferriere V, et al. Direct-Acting Antiviral Treatment Failure Among Hepatitis C and HIV-Coinfected Patients in Clinical Care. Open Forum Infect Dis. 2019;6(3):ofz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano V, Vispo E, Poveda E, Labarga P, Barreiro P. Treatment failure with new hepatitis C drugs. Expert Opin Pharmacother. 2012;13(3):313–323. [DOI] [PubMed] [Google Scholar]
- 14.Benitez-Gutierrez L, Barreiro P, Labarga P, et al. Prevention and management of treatment failure to new oral hepatitis C drugs. Expert Opin Pharmacother. 2016;17(9):1215–1223. [DOI] [PubMed] [Google Scholar]
- 15.Buti M, Esteban R. Management of direct antiviral agent failures. Clin Mol Hepatol. 2016;22(4):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogal SS, McCarthy R, Reid A, et al. Primary Care and Hepatology Provider-Perceived Barriers to and Facilitators of Hepatitis C Treatment Candidacy and Adherence. Dig Dis Sci. 2017;62(8):1933–1943. [DOI] [PubMed] [Google Scholar]
- 17.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–266. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer RE, Carrell DS, Cronkite D, et al. The prevalence of problem opioid use in patients receiving chronic opioid therapy: computer-assisted review of electronic health record clinical notes. Pain. 2015;156(7):1208–1214. [DOI] [PubMed] [Google Scholar]
- 20.Grebely J, Alavi M, Micallef M, et al. Treatment for hepatitis C virus infection among people who inject drugs attending opioid substitution treatment and community health clinics: the ETHOS Study. Addiction. 2016;111(2):311–319. [DOI] [PubMed] [Google Scholar]
- 21.Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153–161. [DOI] [PubMed] [Google Scholar]
- 22.Read P, Lothian R, Chronister K, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy. 2017;47:209–215. [DOI] [PubMed] [Google Scholar]
- 23.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of Treatment Eligibility in Veterans With Hepatitis C Viral Infection. Clin Ther. 2017;39(1):130–137. [DOI] [PubMed] [Google Scholar]
- 25.Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogal SS, Arnold RM, Chapko M, et al. The Patient-Provider Relationship Is Associated with Hepatitis C Treatment Eligibility: A Prospective Mixed-Methods Cohort Study. PLoS One. 2016;11(2):e0148596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of Human Immunodeficiency Virus, Hepatitis C Virus, and Hepatitis B Virus Among Homeless and Nonhomeless United States Veterans. Clin Infect Dis. 2017;65(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37:131–143. [DOI] [PubMed] [Google Scholar]
- 29.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100(8):1772–1779. [DOI] [PubMed] [Google Scholar]
- 30.Mishra G, Sninsky C, Roswell R, Fitzwilliam S, Hyams KC. Risk factors for hepatitis C virus infection among patients receiving health care in a Department of Veterans Affairs hospital. Dig Dis Sci. 2003;48(4):815–820. [DOI] [PubMed] [Google Scholar]
- 31.Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Engagement in the Hepatitis C Care Cascade Among Homeless Veterans, 2015. Public Health Rep. 2017;132(2):136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich ZC, Chu C, Mao J, et al. Facilitators of HCV treatment adherence among people who inject drugs: a systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health. 2016;16:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaiyachati KH, Hubbard RA, Yeager A, et al. Association of Rideshare-Based Transportation Services and Missed Primary Care Appointments: A Clinical Trial. JAMA Intern Med. 2018;178(3):383–389. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.