Abstract
Objective
To determine differences in clinical presentation and disease progression between patients with dementia due to AD with visually normal and abnormal EEG recordings. We hypothesized that patients with normal electroencephalographs (EEGs) are a representation of the heterogeneity of AD. We expected this group to have a phenotype with relatively predominant hippocampal atrophy, memory deficits, and a slower disease progression.
Methods
Patients were included based on diagnosis of dementia due to AD, positive amyloid and tau cerebrospinal fluid (CSF) biomarkers, and the availability of EEG recordings. Patients were categorized in groups of normal (N = 208) and abnormal (N = 336) EEG recordings based on visual assessment by experienced neurophysiologists. At baseline demographics, cognitive, MRI, and CSF measures were compared between groups. Cognitive data from follow‐up visits were assessed by linear mixed‐effects models (LMMs), and corrected for baseline value, sex, age, and educational level, to compare cognitive deterioration over time between groups.
Results
About 1 in 4.5 patients with AD dementia had a visually normal EEG and this group showed better overall cognitive performance compared to the abnormal group, where memory was the most prominent affected domain. The normal group showed less global and parietal but similar medial temporal atrophy. Follow‐up data showed a slower deterioration on all tested cognitive domains in the normal EEG group.
Interpretation
Patients with dementia due to AD and visually normal EEG recordings showed a milder clinical presentation and had a milder disease progression compared to patients with an abnormal EEG. These results provide evidence of clinical and biological heterogeneity within AD dementia.
Introduction
Visual evaluation of electroencephalography (EEG) recordings in diagnostic care of neurodegenerative diseases has value for determining a differential diagnosis. 1 , 2 , 3 However, although literature has shown that the EEGs of patients with dementia due to Alzheimer's disease (AD) generally show diffuse slowing of the posterior dominant rhythm, 4 normal EEG recordings on visual inspection are not uncommonly encountered in patients with AD dementia 5 . Even though this may hamper the discriminative value of EEG, the occurrence of visually normal EEGs might provide us other valuable information about the current disease stage and the prediction of disease progression.
In this study, we aimed to better understand the clinical value of visually normal EEG recordings in patients with AD dementia. In particular, we investigated the clinical profile and predictive value of normal EEGs in patients with AD dementia. We compared baseline demographics, magnetic resonance imaging (MRI) markers, cerebrospinal fluid (CSF) profile, cognition, and the clinical progression between patients with normal and abnormal EEGs.
Based on evidence for heterogeneity in AD, 5 , 6 , 7 , 8 , 9 , 10 , 11 we hypothesized that the visually normal and abnormal EEGs represent two different clinical groups in dementia due to AD. The normal EEG group would resemble a phenotype with a late onset of the disease, memory loss as the core symptom, predominant hippocampal atrophy and low disease activity represented by low CSF tau levels, and slow disease progression. This is in contrast with the abnormal EEG group that would have a young age of disease onset, more deterioration in other cognitive domains than memory, more global and parietal atrophy, and higher disease activity represented by high CSF tau and faster disease progression.
Methods
Population
The local Medical Ethics Committee approved a general protocol for using the clinical data for research purposes. Written informed consent was obtained from all participants. A total of 949 patients were included from the Amsterdam dementia cohort (ADC) 12 based on the clinical diagnosis of dementia due to AD 13 and positive amyloid and tau CSF biomarkers in concordance with the research criteria for AD. 14 An additional inclusion criterion was the availability of EEG recordings at baseline. Information was requested from baseline and all available follow‐up visits. The baseline visit was part of the diagnostic process and consisted of clinical evaluation by a clinician, a neuropsychological test battery, a MRI‐scan (or a computed tomography scan when MRI was not possible), an EEG recording, and a lumbar puncture. Diagnosis was determined during a consensus meeting with experienced neurologists, neurophysiologists, neuropsychologists, and radiologists. Follow‐up visits consisted of clinical evaluation by a clinician and a standardized neuropsychological test battery.
EEG recordings
Twenty minutes of eyes‐closed resting‐state EEG with O.S.G. digital equipment (Brainlab or BrainRT; O.S.G. B.V. Belgium) was recorded at baseline. To observe the reactivity of the rhythmic background activity to opening of the eyes, patients were asked to open and close their eyes for two to three times during the recording. Electrodes were placed at the positions of the 10–20 system (Fp2, Fp1, F8, F7, F4, F3, A2, A1, T4, T3, C4, C3, T6, T5, P4, P3, O2, O1, Fz, Cz, Pz). Sample frequency was 500 Hz. Electrode impedance was kept below 5 kΩ and high‐ and low‐pass filters were set at 0.5–70 Hz. Patients sat in a slightly reclined chair in a sound‐attenuated room and were monitored by an experienced technician. When necessary, sound stimuli were used by the technician to keep the patient awake.
Visual assessment of EEG recordings
The EEGs were visually assessed and scored by an experienced clinical neurophysiologist as part of the clinical routine. Parts of the EEG that were influenced by the opening of the eyes, monitored by the technician, or drowsiness, represented by slow horizontal eye movement or slowing of the posterior alpha rhythm, were excluded for the assessment. Scoring was done using a standardized severity scale (range 1–4, corresponding with no to severe abnormalities). 1 , 5 , 15 Additionally, the abnormalities were categorized (focal abnormalities, diffuse abnormalities, epileptiform activity, or any combination). Focal abnormalities were defined as (transients of) slow or sharp wave activity in one or more EEG leads, but excluding benign temporal theta of the elderly. Diffuse abnormalities were defined as either diminished reactivity of the rhythmic background activity to opening of the eyes, global slow wave activity, or a posterior dominant frequency below 8 Hz. Spikes and spike‐and‐slow‐wave complexes were defined as epileptiform activity. 16 Recent work showing increased incidence of epileptiform activity in AD moved us to not exclude patients with focal epileptiform activity from our population. 17 , 18 , 19 , 20 This was because our aim was to include a clinically representative sample. The interobserver agreement of this EEG evaluation method has previously been investigated and resulted in kappa scores of 0.60–0.87. 1
Neuropsychological assessment
All patients underwent an extensive neuropsychological test battery at baseline and a standardized set of tests was repeated during the follow‐up visits. We selected a set of neuropsychological tests for analysis with the aim to cover important cognitive domains. Overall cognition was measured by the Mini‐Metal State Examination (MMSE) and Clinical Dementia Rating (CDR), whereas specific domains were covered by the following tests: Memory: the immediate and delayed response of the Rey Auditory Verbal Learning Test (RAVLT); Executive functioning: the Frontal Assessment Battery (FAB) and Controlled Oral Word Association Test (COWAT); Language: the Animal Fluency test and naming of 20 images of the Arizona Battery for Communication Disorders of Dementia (ABCD); Attention: the Trail‐Making Test A (TMT‐A, inverted scores); Visual performance: Dot Counting and Fragmented Letters tests.
Magnetic resonance imaging
MRI scans were performed at baseline and scored by an experienced radiologist as part of the diagnostic process. Three commonly used neurodegenerative markers were used for analyses: medial temporal atrophy (MTA, range 0–4), global cortical atrophy (GCA, range 0–3), and parietal atrophy (range 0–3). All scores were rated visually and low and high scores represented, respectively, no and severe atrophy. MTA scored was rated on T1‐weighted coronal images. GCA was rated using axial FLAIR images. Parietal atrophy was scored using axial, coronal, and sagittal T1‐ and FLAIR‐weighted images. MTA and parietal atrophy scores were averaged over left and right.
Cerebrospinal fluid and APOE genotyping
All patients underwent a lumbar puncture and blood sampling at baseline. Amyloid beta 1–42 (Aβ1–42), total tau (t‐tau), and phosphorylated tau (p‐tau) were measured from the CSF sample (Innotest, Fujirebio, Ghent, Belgium). Correction for center‐specific amyloid beta 1–42 drift was applied and the following cut‐offs were used: Aβ1–42 < 813 ng/L, t‐tau > 375 ng/L, and p‐tau > 52 ng/L. 21 Due to a change in memory clinic protocol, more recent CSF Aβ1–42, t‐tau, and p‐tau values were measured by Elecsys immunoassays (Roche Diagnostics GmbH, Penzberg, Germany) (Aβ1–42 N = 97, 10%; t‐tau N = 65, 7%; p‐tau N = 65, 7%). Elecsys values were transformed to Innotest values by conversion rates from literature 22 . Apolipoprotein E (APOE) genotyping was performed on DNA isolated from the blood samples. Patients were dichotomized into carriers (hetero‐ and homozygous) and non‐carriers of the ε4 allele. This was repeated for the ε2 allele. Additionally, patients were dichotomized into carriers and non‐carriers of the ε2 allele.
Statistical analyses
Statistical analyses were performed using RStudio (version 1.1.463) 23 , the lme4 package for R 24 , and SPSS statistics (IBM SPSS Statistics for Windows, version 26.0.0.1) software. To contrast clearly visually normal EEGs with clearly abnormal EEGs, patients with no abnormalities (severity score 1) were compared to moderate abnormalities (severity score 3) at baseline and follow‐up. Patients with missing data or a severity score of 5 were excluded (N = 5). The distribution of the baseline data was checked. Differences in baseline demographics, CSF profile, MRI, and cognition were compared between groups by Chi‐square test, independent t‐test, and Mann–Whitney‐U test where appropriate. P‐values were FDR‐corrected and considered significant at pcorr < 0.05 25 . P‐values were considered significant at p < 0.05. To compare the relative decrease in different cognitive domains between different EEG groups, Z‐scores were additionally calculated for each cognitive test over the entire population and plotted for the different EEG groups. The progression of each cognitive test over time was predicted by linear mixed‐effects models (LMMs) using baseline and follow‐up data. The LMM was chosen due to its robustness in the presence of missing values 26 . Another important benefit of LMMs is the possibility to introduce an assumption of dependence between measures (e.g., repeated measures of cognitive tests within patients are typically not independent). For each cognitive test, a LMM was build using follow‐up time and/or follow‐up time squared (depending on which factor fitted best) as a fixed factor and using random slopes (when allowed by the number of observations) and random intercepts for every subject. The covariates age, sex, educational level, and baseline score were added as fixed effects to the model. Then, different models were built by adding EEG status (normal vs abnormal) as a fixed effect, fixed interaction with time, or both. The final model was selected based on the goodness of fit as indicated by the Akaike Information Criterion (AIC) 27 . The AICs were compared by analysis of variance (ANOVA) and the significantly (p < 0.05) smallest AIC was chosen for the final model. Whenever two or more not significantly different models remained, the importance of the interaction effect was determined (either p < 0.05 significance or 10% effect on the regression coefficient) or the simplest model was chosen. Additionally, the effect of baseline use of acetylcholinesterase inhibitors or memantine (as a fixed factor) on the model was evaluated. Finally, the contrast of the EEG score was extended by adding patients with EEGs scored as having mild abnormalities to the analysis (i.e., three EEG categories representing severity score 1 to 3).
Results
Population
The prevalence of visually normal EEG recordings in patients with dementia due to AD was calculated over the total sample (N = 949). Approximately 22% (1 in 4.5 patients) had a normal EEG over a time period of 16.4 years (2002–2019). In total, 208 patients were included in the normal EEG group and 336 patients were included in the abnormal EEG group. Four hundred patients were allocated to the mild abnormalities group. Of the abnormal EEG group, most patients had either a combination of focal and diffuse abnormalities (N = 200, 60%) or only diffuse abnormalities (N = 79, 24%). The occurrence of each type of abnormality within the abnormal EEG group can be found in Table S1. Follow‐up data were available for 133 patients (64%) of the normal EEG group and 193 patients (57%) of the abnormal EEG group. Median follow‐up duration was 2.0 years (interquartile range (IQR) 1.0–2.8) with a median number of follow‐up visits of 3 (IQR 2–4). Respectively, for the normal and abnormal EEG groups, the median follow‐up durations were 2.0 (IQR 1.1–3.1) and 1.8 years (IQR 1.0–2.3) with a median number of follow‐up visits of 2 (IQR 3–5) and 2 (IQR 2–4). The amount of follow‐up data that were available for each individual cognitive task can be found in Table S2.
Baseline
At baseline demographics, CSF profile, MRI markers, and cognitive performance were compared between the normal EEG and abnormal EEG patients. The results of these comparisons can be found in Table 1. The normal EEG group was on average 2 years older (pcorr = 7.0*10−4) but did not differ on sex, educational level, or duration of complaints from the abnormal EEG group. Prevalence of the APOE ε2 or ε4 allele did not differ between the groups, neither did the amount of homozygous APOE ε4 carriers (homozygous ε4 carriers/other APOE genotypes, normal N = 47/158, abnormal N = 57/268, p = 0.16). Based on the CSF results, both groups showed typical amyloid and tau burden but the levels of Aβ1–42, t‐tau, and p‐tau did not differ between the groups.
Table 1.
Baseline values of demographic variables, CSF profile, MRI markers, and cognitive tests.
| Characteristics | Normal EEG | N (%) | Abnormal EEG | N (%) |
|---|---|---|---|---|
| Sex (m/f) | 98/110 | 208 (100) | 156/180 | 336 (100) |
| Age | 66 ± 7 | 208 (100) | 64 ± 8*** | 336 (100) |
| Education | 5 (4–6) | 193 (93) | 5 (4–6) | 316 (94) |
| Duration of complaints | 3 (2–4) | 208 (100) | 3 (2–4) | 332 (99) |
| AChEI | 13/195 | 208 (100) | 18/318 | 336 (100) |
| CDR | 1 (0.5–1) | 184 (88) | 1 (1–1)*** | 291 (87) |
| APOE ε4 pos/neg | 146/59 | 205 (99) | 209/116 | 325 (97) |
| APOE ε2 pos/neg | 10/195 | 205 (99) | 20/305 | 325 (97) |
| CSF markers | ||||
| Amyloid‐beta 42 | 604 ± 107 | 208 (100) | 587 ± 105 | 336 (100) |
| Total tau | 667 (490–932) | 208 (100) | 608 (441–938) | 336 (100) |
| P‐tau | 90 ± 36 | 208 (100) | 89 ± 39 | 336 (100) |
| MRI markers | ||||
| GCA | 1 (1–1) | 160 (77) | 1 (1–2)* | 247 (74) |
| MTA | 1.5 (1–2) | 161 (77) | 1.5 (1–2) | 247 (74) |
| Parietal atrophy | 1 (1–2) | 159 (76) | 1.5 (1–2)** | 245 (73) |
| Cognitive tests | ||||
| MMSE | 22 ± 4 | 205 (99) | 18 ± 5*** | 325 (97) |
| RAVLT immediate | 22 ± 8 | 178 (86) | 20 ± 8** | 258 (77) |
| RAVLT delayed | 1 (0–3) | 177 (85) | 1 (0–3) | 253 (75) |
| FAB | 14 (11–16) | 175 (84) | 10 (8–13)*** | 248 (74) |
| COWAT | 29 ± 12 | 167 (80) | 22 ± 12*** | 231 (69) |
| Animal fluency | 13 ± 5 | 180 (87) | 11 ± 5*** | 278 (83) |
| Naming | 17 (14–19) | 153 (74) | 16 (13–18)** | 219 (65) |
| TMT‐A | −59 (−42 to −81) | 186 (89) | −94 (−61 to −173)*** | 257 (76) |
| Dot count | 10 (9–10) | 145 (70) | 9 (7–10)*** | 200 (60) |
| Fragmented letters | 18 (16–19) | 149 (72) | 16 (9–18)*** | 199 (59) |
Values for the normal and abnormal EEG groups are shown (count, mean ± standard deviation, or median with interquartile range). Group differences were tested by Chi‐square test, independent t‐test, or Mann–Whitney‐U test where appropriate. Significant differences are indicated in bold and by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). The count of AChEI includes the use of memantine. Abbreviations: Acetylcholinesterase inhibitors (AChEI), Clinical dementia rating (CDR), Apolipoprotein E (APOE), global cortical atrophy (GCA), medial temporal atrophy (MTA), Mini‐Metal State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), Frontal Assessment Battery (FAB), Controlled Oral Word Association Test (COWAT), Trail‐Making Test A (TMT‐A, inverted scores).
The MRI markers showed less parietal and global atrophy in the normal EEG group. Compared to the abnormal EEG patient group, the normal EEG patient group had a lower GCA score (normal EEG median = 1 (IQR 1–1); abnormal EEG median = 1 (IQR 1–2); pcorr = 0.04), a lower parietal atrophy score (normal EEG median = 1 (IQR 1–2); abnormal EEG median = 1.5 (IQR 1–2); pcorr = 0.02) but a similar MTA score (normal EEG median = 1.5 (IQR 1–2); abnormal EEG median = 1.5 (IQR 1–2); pcorr = 0.81).
Patients in the normal EEG group showed better performance on global cognition and on all tested individual cognitive domains compared to patients in the abnormal EEG group. There was a mean difference (Δμ) of 4 MMSE points (pcorr < 1*10−10) in favor of the normal EEG group. The distribution of CDR score was lower in the normal EEG group (normal EEG median = 1 (IQR 0.5–1), abnormal EEG median = 1 (IQR 1–1), pcorr = 7.1*10−8). The normal EEG group performed better in the memory (RAVLT immediate recall Δμ = 2, pcorr = 0.003), executive functioning (FAB median difference (Δm) = 4, pcorr < 1*10−10, COWAT Δμ = 7, pcorr = 4.4*10−8), language (animal fluency Δμ = 2, pcorr = 4.1*10−7, naming Δm = 1, pcorr = 0.005), attention (TMT‐A Δm = −35 s, pcorr < 1*10−10), and visual domains (dot counting Δm = 1, pcorr = 1.1*10−6, fragmented letters Δm = 2, pcorr = 1.0*10−6) compared to the abnormal EEG group. Z‐scores of the cognitive tests for the different EEG groups can be found in Figure S1. The normal EEG group showed prominent low Z‐scores in the memory (RAVLT) domain where the abnormal EEG group showed low scores in all domains with the lowest scores in the executive domain (FAB and COWAT).
Follow‐up
In comparison with the abnormal EEG group, the normal EEG group declined less rapidly in global cognitive performance as indicated with the MMSE score. As shown in Table 2, the LMM predicted that for all patients, MMSE declined over time with −0.82 points per year with an acceleration of −0.37*(per year 2 ) (p = 0.0002 and p < 1*10−10, respectively). This indicates that the rate of decline in MMSE points increases over time for all patients. Additionally, an interaction effect between time and EEG group was found. Over time, the normal EEG group decreased −1.15 MMSE point per year less than the abnormal EEG group (p = 0.0004). This effect was independent of the baseline MMSE score. The trajectories of both EEG groups were plotted to visualize this effect (Fig. 1).
Table 2.
Prediction of cognitive decline over time.
| Time | Time2 | Time * EEG | |
|---|---|---|---|
| Beta (std. error)/std. Beta (CI) | Beta (std. error)/std. Beta (CI) | Beta (std. error)/std. Beta (CI) | |
| MMSE | −0.82 (0.26)/−0.38 (−0.50; −0.27) | −0.37 (0.03)/−0.12 (−0.14; −0.10) | −1.15 (0.34)/−0.28 (−0.43; −0.12) |
| RAVLT immediate | −1.42 (0.17)/−0.22 (−0.27; −0.17) | – | −0.78 (0.25)/−0.12 (−0.20; −0.04) |
| FAB | −0.64 (0.09)/−0.18 (−0.23; −0.14) | – | −0.28 (0.03)/−0.08 (−0.15; −0.01) |
| COWAT | −1.68 (0.23)/−0.17 (−0.21; −0.12) | – | −1.11 (0.34)/−0.11 (−0.18; −0.04) |
| Animal fluency | −0.64 (0.20)/−0.19 (−0.25; −0.12) | −0.08 (0.04)/−0.02 (−0.05; 0.00) | −1.13 (0.16)/−0.27 (−0.34; −0.20) |
| Naming | −1.14 (0.19)/−0.26 (−0.33; −0.18) | 0.16 (0.04)/0.05 (0.02; 0.07) | −0.47 (0.15)/−0.13 (−0.21; −0.05) |
| TMT‐A | −8.65 (1.60)/−0.14 (−0.19; −0.09) | – | −21.41 (2.46)/−0.35 (−0.43; −0.27) |
| Dot count | −0.16 (0.05)/−0.25 (−0.34; −0.17) | – | −0.47 (0.08)/−0.25 (−0.34; −0.17) |
| Fragmented letters | −0.68 (0.12)/−0.14 (−0.18; −0.09) | – | −0.34 (0.18)/−0.07 (−0.14; 0.00) |
Estimations of the coefficients of the linear mixed‐effects models are shown for each cognitive test. Beta, standard error and standardized beta with 95% confidence interval (CI) of the coefficients are shown. Higher scores represent better performance (TMT‐A values were inverted). For the EEG interaction effect (Time * EEG), negative values represent better performance for the normal EEG group. Abbreviations: Mini‐Metal State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), Frontal Assessment Battery (FAB), Controlled Oral Word Association Test (COWAT), Trail‐Making Test A (TMT‐A, inverted scores).
Figure 1.
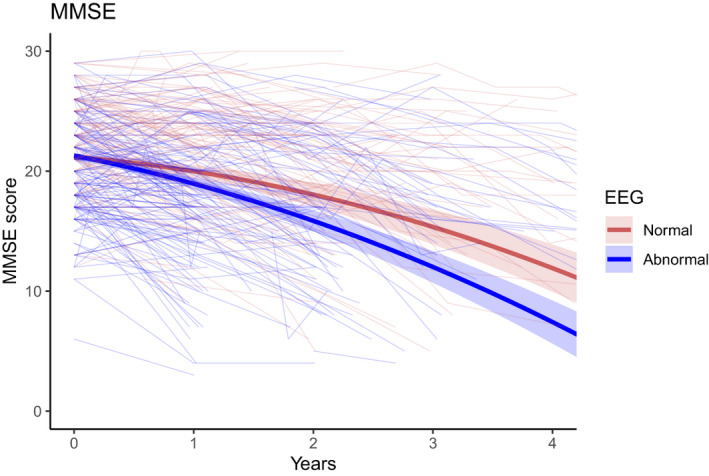
Evolution of MMSE score between EEG groups over time. Linear mixed‐effects model of MMSE score. The two heavy‐weighted lines represent the trajectories of decline of MMSE score for both the normal (red) and abnormal (blue) EEG groups. 95% confidence intervals are indicated by the shaded area. The model was corrected for the covariates age, sex, educational level, and baseline value. The multiple light‐weighted lines represent the individual trajectories of all patients in the normal (red) and abnormal (blue) EEG groups.
The results of the LMMs for the different cognitive tests are shown in Table 2 and Figure 2. Independent of baseline scores, all models showed an interaction effect between EEG group and time, indicating that patients in the normal EEG group declined less rapidly in cognitive performance compared to the abnormal EEG group. The addition of the use of acetylcholinesterase inhibitors or memantine at baseline as a covariate to the model did not change these results (Table S3).
Figure 2.
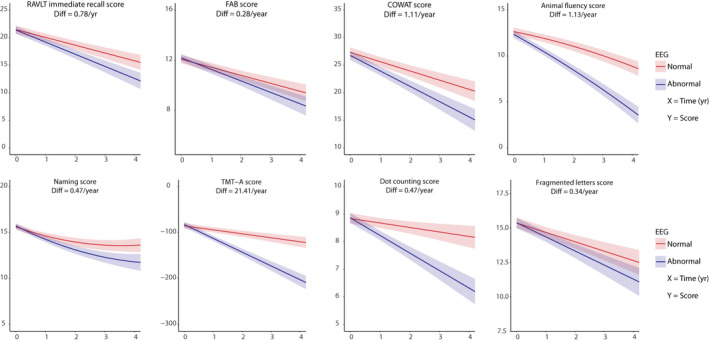
Cognitive performance over time between EEG groups. Linear mixed‐effects models of cognitive performance. Trajectories of decline in performance are shown for both the normal (red) and abnormal (blue) EEG groups. 95% confidence intervals are indicated by the shaded area. Each different cognitive test is depicted by a separate figure and the difference between the groups is indicated above each figure. All models were corrected for the covariates age, sex, educational level, and baseline value.
The LMMs were repeated for each cognitive test while adding a third EEG group with mild EEG abnormalities (severity score of 2). The baseline characteristics of this group can be found in Table S4. Apart from the memory domain, the mild EEG abnormalities group performed worse on global cognition and all individually tested cognitive domains compared to the normal group. The results of the repeated LMMs at three levels can be found in Figure 3 and Table S5. Overall, the EEG group with mild abnormalities performed either similar to the abnormal EEG group or at intermediate levels between the normal and abnormal EEG groups. These results support the previous LMM models, indicating that a normal EEG has a favorable outcome in terms of baseline cognition and rate of cognitive decline.
Figure 3.
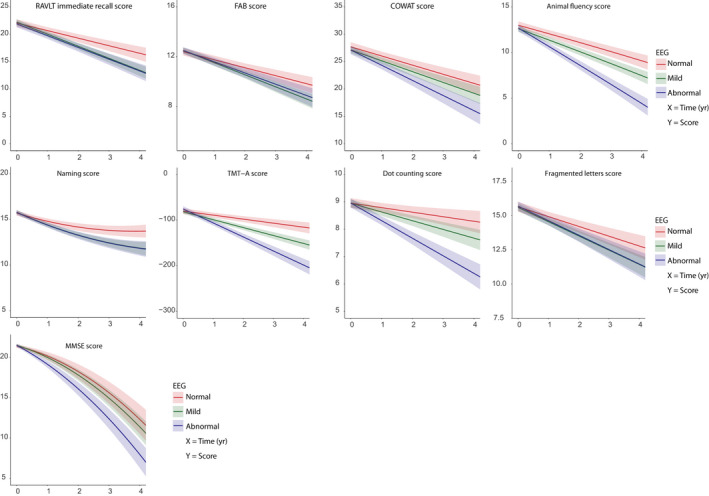
Cognitive performance over time with three severity scores. Trajectories of decline and difference in decline of performance are shown for both the normal (red), mild (green), and abnormal (blue) EEG groups. 95% confidence intervals are indicated by the shaded area. Each different cognitive test is depicted by a separate figure. All models were corrected for the covariates age, sex, educational level, and baseline value.
Discussion
The results of the current study confirmed our hypothesis that the patients with visually normal resting‐state EEG recordings in dementia due to AD are clinically different from patients with abnormal recordings. Patients with a normal EEG showed better cognitive performance, relatively prominent impaired memory, and less global and parietal atrophy on MRI compared to patients with an abnormal EEG. Importantly, patients with a normal EEG showed a slower cognitive decline over time compared to patients with an abnormal EEG—an effect which was independent of baseline cognitive performance. These results show that the clinical presentation of dementia due to AD is heterogeneous. With a prevalence in a considerable proportion of patients with dementia due to AD, a normal EEG is a clinical favorable finding in terms of clinical progression.
In an attempt to find evidence for a different clinical phenotype of the visually normal EEG group, some differences in affected cognitive domains were found. Based on evidence from multiple studies, it has been established that different subtypes of AD exist. 11 Recurrent proposed phenotypes are the “typical” AD, the dysexecutive subtype, posterior cortical atrophy, and the aphasic variant of AD. It was previously reported that patients with visually normal EEGs showed overall the best cognitive performance compared to patients with abnormalities on the EEG. However, the same normal EEG group showed similar impaired memory performance compared to the other groups, which indicated that this profile was in line with the “typical” AD phenotype. 6 Additionally, previous research has shown that early onset and APOE ε4 negative AD patients show more severe abnormalities on their EEGs. 5 More profound slowing of the posterior EEG channels in posterior cortical atrophy has been shown as well. 28 Hence, we expected our groups to display a phenotype with predominant memory deficits and hippocampal atrophy in the patients with normal EEGs compared to more deterioration of other cognitive domains and more global and parietal atrophy in patients with abnormal EEGs. We did indeed observe less parietal and global atrophy and a better cognitive performance in the normal EEG group. The hippocampal atrophy was similar in both groups and memory was the relatively most impaired domain in the normal EEG group. Although the abnormal EEG group showed the lowest overall scores, the results showed that patients in the normal EEG group did have global cortical and parietal atrophy together with deficits in most cognitive domains and similar decreases in performance over time in most domains. On the other hand, the abnormal EEG group showed decreased cognitive performance across all tests and showed the strongest decreases over time in the attention, language, and visual domains. The difference in cognitive profiles between groups could be caused due to the presence of more patients with a more “typical” AD phenotype in combination with patients who are in an earlier stage of the disease in the normal EEG group. The abnormal EEG group potentially contains more patients with a more “atypical” AD phenotype and patients who are in a more advanced disease stage.
Interestingly, although the normal EEG group showed overall better cognitive performance, the self‐reported duration of complaints indicated a similar disease duration in the two EEG groups. This suggested that the rate of cognitive decline was different between the groups and, therefore, a less aggressive disease course in patients with a normal EEG. Our LMMs confirmed this by showing a slower rate of decline in the normal EEG group for each of the investigated cognitive tests. When comparing the results of the prognostic value of normal EEGs in dementia due to AD in the present study with previous literature, only a few are directly comparable. Recent literature mostly describes prognostic EEG markers in terms of progression from normal cognition to mild cognitive impairment (MCI) or MCI to dementia. For example, several studies have found a decrease in oscillatory power of the alpha frequency band to be predictive of progression of MCI to AD dementia. 29 , 30 , 31 The value of comparing these studies with our results is, however, limited because amyloid and tau biomarkers are absent in these studies. Studies lacking these markers could be selecting predictors that are prognostic for the diagnosis of AD and not rate of cognitive decline within AD. Previous research has described the conversion rates of amyloid‐positive patients with subjective cognitive decline (SCD) to MCI or dementia with a follow‐up of about 1–3 years. SCD patients had a higher hazard ratio of converting to MCI or dementia when having higher relative delta or theta power and lower alpha or beta power. 32 This would be in line with our results that patients with normal EEGs decline less rapidly. However, the patients of the study mentioned above are in a much earlier disease stage and it has been shown that amyloid accumulation can precede years before neurodegeneration. 33 In AD demented patients, the neurodegeneration is in a more advanced stage and it would be expected that the EEG recordings would be affected. Unfortunately, the common issue with studies in the dementia stage of AD is either a lack of amyloid or tau biomarkers, the absence of longitudinal data, and small investigated populations. 34 Longitudinal studies without biomarker confirmation have shown that, in line with our results, more severe EEG abnormalities correlate with a faster cognitive decline. In a 1 year follow‐up study of 88 patients with probable AD and 35 controls, a decrease in MMSE was correlated with an increase in temporal relative delta power. 35 Additionally, a smaller study was done with 15 probable AD patients and 15 controls with a follow‐up of a year. Within the AD group, a reduction in synchronization, assessed by the S‐estimator, in the left frontotemporal cortex over time was associated with a faster cognitive decline. 36 The current study builds upon previous knowledge by using AD biomarkers, a large study sample, and by using another point of view. Our results show that the feature of having a visually normal EEG in AD dementia has a favorable prognosis for patients.
Some studies have indicated that AD patients with higher CSF tau levels show a faster cognitive decline. 37 , 38 , 39 Our abnormal EEG group had a faster cognitive decline but not a higher CSF tau. Although this is not in line with each other, it could be due to the absence of reverse causality or dependence on the levels of p‐tau. For example, previous studies found that very high levels of t‐ and p‐tau in particular were predictive of a faster cognitive decline. 7 , 39 T‐ or p‐tau levels of this height were rare in our sample. The relation between CSF tau and EEG is difficult as these markers seem to develop differently over time. As literature is sparse, future studies should investigate the cross‐sectional and longitudinal correlations between EEG and CSF AD markers. 34
Another marker that has been reported to be predictive of disease progression or severity is APOE ε4. In our study, APOE ε4 status was not different between the normal and abnormal EEG groups. Previous studies have reported conflicting results on APOE ε4. Some have shown more EEG abnormalities, that is, more severe slowing of the oscillatory activity, in APOE ε4 carriers, 40 , 41 where others have shown a higher occurrence of abnormal EEGs in APOE ε4 non‐carriers. 5 Similar discrepancies have been found in functional connectivity analyses. 42 , 43 Different factors could explain why no difference in APOE ε4 allele carriage was found between groups. Firstly, APOE ε4 and visually normal EEGs may be independent predictors of cognitive decline. We have not analyzed whether APOE ε4 carriage is associated with cognitive decline in our cohort and whether EEG changes are associated with APOE ε4‐related cognitive decline. Secondly, a visual rating of EEGs might not be sensitive enough and a more detailed quantitative analyses could be better at finding (small) differences between groups. Thirdly, APOE ε4 allele carriage is associated with an increased risk of late‐onset AD but only in combination with a positive family history in early onset. 44 , 45 Moreover, although APOE ε4 is associated with an increased risk of dementia due to AD, it is not associated with a different rate of cognitive decline compared to non‐carrier patients with dementia due to AD. 46 Hence, the reason that APOE ε4 allele carriage did not differ between groups could be due to independent effects on cognitive decline, the combination of the heterogeneity of our sample, which contains dementia stage AD patients with mixed ages of onset, the relative small effect APOE ε4 has on EEG recordings, and that APOE ε4 only has influence on the incidence but not progression of the disease.
Our results showed a clinical difference between patients with AD dementia and visually normal or abnormal EEGs and it would be most interesting to understand what causes the occurrence of the normal EEGs. EEG directly measures synaptic activity, which reflects the (cognitive) function of the brain. It is, therefore, to be expected for the EEG to change in concordance with the cognitive performance of the patient. Previous research has indeed shown a diffuse slowing of the posterior dominant rhythm, 4 yet a good explanation of normal EEGs in a subset of patients with clinically manifest AD is still lacking. Literature has suggested different protective factors for AD such as cognitive reserve, socioeconomic factors, lifestyle factors, the APOE ε2 allele, and network resistance or resilience. 47 , 48 , 49 The cognitive reserve theory does, however, not fit our normal EEG group. Patients with a high cognitive reserve should stay cognitively normal longer but decline faster when a threshold of neurodegeneration is reached. Furthermore, the protective effect of the APOE ε2 allele did not explain the difference in cognitive decline because the incidence did not differ between groups. One explanation could be a natural variation between patients before the onset of AD pathology. A relative decrease in EEG properties could go unnoticed when the normal healthy situation is above the normal population average. Additionally, a visual inspection might not capture all changes that could have been captured with quantitative EEG using spectral, functional connectivity, and network measures. The spatial resolution and range of 21 electrode EEG recordings are other limiting factors. Deep, basal, and medial signals are difficult to estimate. A different potential lead is that our results suggest that patients with favorable oscillatory properties endure a milder disease course. This might be due to a more resilient or resistant underlying network. Multiple studies have been done to investigate vulnerabilities in functional networks and have found evidence that specific and regional network deterioration correlate with syndrome‐specific neurodegeneration. 48 , 50 , 51 Instead of investigating risk factors for deterioration, it would be interesting for future studies to focus on what network features are protective or predict a slower decline.
The current study design has several strengths. A large sample was available and a large subsample had follow‐up data available. All included AD patients had gone through an extensive clinical assessment for diagnosis and all patients had positive amyloid and tau CSF biomarkers. A potential limitation is the use of a visual rating of the EEG instead of using quantitative measures. In our final analyses, we have not included the types of abnormalities and the scale ignores characteristics such as benign temporal theta of the elderly which might not actually be benign in these patients. Quantitative measures could give more detailed descriptions of EEG metrics and are not influenced by interobserver variability. In contrast, in the clinical routine, quantitative assessment of EEGs has limited value and provides many different obstacles such as choices in epoch selection, epoch processing, and the time that is required to go through these steps. Our research question was aimed at what clinical consequences EEGs visually regarded as normal had. This question requires the use of the visual assessment as the predictor of the clinical outcome. Our results indicate that clinically a patient with dementia due to AD with a visually normal EEG has an overall relatively better cognition and slower cognitive decline compared to patients with an abnormal EEG. These findings can help in determining clinical prognosis and can be used in clinical trials. Heterogeneous treatment effects can be expected when including both patient groups. For example, knowing that the rate of decline is higher in the abnormal EEG group, a treatment effect might be larger or spotted earlier.
Conclusion
Although EEG recordings of patients with dementia due to AD typically show diffuse oscillatory slowing, a considerable proportion has a visually normal EEG. The results of this study show that patients with a normal EEG are overall cognitively less affected and show a less rapid progression compared to patients with an abnormal EEG. This can be informative for clinicians or clinical trials in terms of expected disease progression and expected treatment effects. Future studies could investigate what causes this relative protective state.
Conflict of Interest
Authors C.T. Briels and C.J. Stam declare no conflict of interest. No funding was received for the current study. P. Scheltens has received consultancy/speaker fees (paid to the institution) from Biogen, People Bio, Roche (Diagnostics), and Novartis Cardiology. He is PI of studies with Vivoryon, EIP Pharma, IONIS, CogRx, AC Immune, and Toyama. A.A. Gouw has received past (2014–2018) research support from Probiodrug and Boehringer Ingelheim via the VUmc Alzheimer Center.
Supporting information
Figure S1. Comparison of cognitive tests between groups. Z‐scores of the individual cognitive tests between EEG groups (normal, mild, and abnormal).
Table S1. Distribution of types of EEG abnormalities in the abnormal EEG group.
Table S2. Follow‐up data availability.
Table S3. Baseline values of demographic variables, CSF profile, MRI markers, and cognitive tests.
Table S4. Prediction of cognitive decline over time for three EEG groups.
Acknowledgments
We thank Dr. N. Beker for her help constructing the statistical models and we thank all the patients and personnel of the Alzheimer Center Amsterdam for their work and for participating in the Amsterdam Dementia Cohort.
References
- 1. Liedorp M, van der Flier WM, Hoogervorst EL, et al. Associations between patterns of EEG abnormalities and diagnosis in a large memory clinic cohort. Dement Geriatr Cogn Disord 2009;27:18–23. [DOI] [PubMed] [Google Scholar]
- 2. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pijnenburg YAL, Strijers RLM, Made YV, et al. Investigation of resting‐state EEG functional connectivity in frontotemporal lobar degeneration. Clin Neurophysiol 2008;119:1732–1738. [DOI] [PubMed] [Google Scholar]
- 4. Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol 2004;115:1490–1505. [DOI] [PubMed] [Google Scholar]
- 5. de Waal H, Stam CJ, Blankenstein MA, et al. EEG abnormalities in early and late onset Alzheimer's disease: understanding heterogeneity. J Neurol Neurosurg Psychiatry 2011;82:67–71. [DOI] [PubMed] [Google Scholar]
- 6. Smits LL, Liedorp M, Koene T, et al. EEG abnormalities are associated with different cognitive profiles in Alzheimer's disease. Dement Geriatr Cogn Disord 2011;31:1–6. [DOI] [PubMed] [Google Scholar]
- 7. Koch G, Di Lorenzo F, Del Olmo MF, et al. Reversal of LTP‐like cortical plasticity in Alzheimer's disease patients with tau‐related faster clinical progression. J Alzheimers Dis 2016;50:605–616. [DOI] [PubMed] [Google Scholar]
- 8. Mendez MF. Early‐onset Alzheimer disease. Neurol Clin 2017;35:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Waal H, Stam CJ, de Haan W, et al. Young Alzheimer patients show distinct regional changes of oscillatory brain dynamics. Neurobiol Aging 2012;33:1008.e25–1008.e31. [DOI] [PubMed] [Google Scholar]
- 10. van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early‐onset versus late‐onset Alzheimer's disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol 2011;10:280–288. [DOI] [PubMed] [Google Scholar]
- 11. Veitch DP, Weiner MW, Aisen PS, et al. Understanding disease progression and improving Alzheimer's disease clinical trials: recent highlights from the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement 2019;15:106–152. [DOI] [PubMed] [Google Scholar]
- 12. van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimer's Dis 2018;62:1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Zande JJ, Gouw AA, van Steenoven I, et al. EEG characteristics of dementia with Lewy bodies, Alzheimer's disease and mixed pathology. Front Aging Neurosci 2018;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kane N, Acharya J, Benickzy S, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract 2017;2:170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam AD, Deck G, Goldman A, et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med 2017;23:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horváth A, Szucs A, Barcs G, et al. Epileptic seizures in Alzheimer disease: a review. Alzheimer Dis Assoc Disord 2016;30:186–192. [DOI] [PubMed] [Google Scholar]
- 19. Vossel KA, Beagle AJ, Rabinovici GD, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 2013;70:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liedorp M, Stam CJ, van der Flier WM, et al. Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord 2010;29:432–437. [DOI] [PubMed] [Google Scholar]
- 21. Tijms BM, Willemse EAJ, Zwan MD, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid‐beta 1–42 analysis results. Clin Chem 2018;64:576–585. [DOI] [PubMed] [Google Scholar]
- 22. Willemse EAJ, van Maurik IS, Tijms BM, et al. Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer's disease biomarkers in a nonacademic, multicenter memory clinic cohort: the ABIDE project. Alzheimer's Dement (Amsterdam, Netherlands) 2018;10:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allaire J. RStudio: integrated development environment for R. Boston, MA. 2012;537:538. [Google Scholar]
- 24. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. 2015. 2015 2015‐10‐07;67:48.
- 25. Benjamini Y, Hochberg Y. Controlling the false discovery rate ‐ a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B‐Stat Methodol 1995;57:289–300. [Google Scholar]
- 26. Field A. Discovering statistics using IBM SPSS statistics. London, UK: Sage; 2013. [Google Scholar]
- 27. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Selected papers of hirotugu akaike. pp. 199–213. New York, NY: Springer; 1998. [Google Scholar]
- 28. Briels CT, Eertink JJ, Stam CJ, et al. Profound regional spectral, connectivity, and network changes reflect visual deficits in posterior cortical atrophy: an EEG study. Neurobiol Aging 2020;96:1–11. [DOI] [PubMed] [Google Scholar]
- 29. Luckhaus C, Grass‐Kapanke B, Blaeser I, et al. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): results of a 1‐year follow‐up study. Int J Geriatr Psychiatry 2008;23:1148–1155. [DOI] [PubMed] [Google Scholar]
- 30. Jelic V, Johansson S, Almkvist O, et al. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging 2000;21:533–540. [DOI] [PubMed] [Google Scholar]
- 31. Huang C, Wahlund L, Dierks T, et al. Discrimination of Alzheimer's disease and mild cognitive impairment by equivalent EEG sources: a cross‐sectional and longitudinal study. Clin Neurophysiol 2000;111:1961–1967. [DOI] [PubMed] [Google Scholar]
- 32. Gouw AA, Alsema AM, Tijms BM, et al. EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiol Aging. 2017;57:133–142. [DOI] [PubMed] [Google Scholar]
- 33. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maestú F, Cuesta P, Hasan O, et al. The importance of the validation of M/EEG with current biomarkers in Alzheimer's disease. Front Hum Neurosci 2019;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babiloni C, Lizio R, Del Percio C, et al. Cortical sources of resting state EEG rhythms are sensitive to the progression of early stage Alzheimer's disease. J Alzheimers Dis 2013;34:1015–1035. [DOI] [PubMed] [Google Scholar]
- 36. Knyazeva MG, Carmeli C, Khadivi A, et al. Evolution of source EEG synchronization in early Alzheimer's disease. Neurobiol Aging 2013;34:694–705. [DOI] [PubMed] [Google Scholar]
- 37. Seppälä TT, Koivisto AM, Hartikainen P, et al. Longitudinal changes of CSF biomarkers in Alzheimer's disease. J Alzheimers Dis 2011;25:583–594. [DOI] [PubMed] [Google Scholar]
- 38. Brys M, Pirraglia E, Rich K, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging 2009;30:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wallin ÅK, Blennow K, Zetterberg H, et al. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 2010;74:1531–1537. [DOI] [PubMed] [Google Scholar]
- 40. Lehtovirta M, Partanen J, Könönen M, et al. A longitudinal quantitative EEG study of Alzheimer’s disease: relation to apolipoprotein E polymorphism. Dement Geriatr Cogn Disord 2000;11:29–35. [DOI] [PubMed] [Google Scholar]
- 41. Babiloni C, Benussi L, Binetti G, et al. Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: a multicentric electroencephalogram study. Ann Neurol 2006;59:323–334. [DOI] [PubMed] [Google Scholar]
- 42. Kramer G, van der Flier WM, de Langen C, et al. EEG functional connectivity and ApoE genotype in Alzheimer's disease and controls. Clin Neurophysiol 2008;119:2727–2732. [DOI] [PubMed] [Google Scholar]
- 43. Jelic V, Julin P, Shigeta M, et al. Apolipoprotein E ε4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. J Neurol Neurosurg Psychiatry 1997;63:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early‐onset Alzheimer's disease revisited. Alzheimer's Dement 2016;12:733–748. [DOI] [PubMed] [Google Scholar]
- 45. van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community‐based cohort study. Lancet Neurol 2018;17:434–444. [DOI] [PubMed] [Google Scholar]
- 46. Suzuki K, Hirakawa A, Ihara R, et al. Effect of apolipoprotein E ε4 allele on the progression of cognitive decline in the early stage of Alzheimer's disease. Alzheimer's Dement 2020;6:e12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012;11(11):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lehmann M, Madison CM, Ghosh PM, et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. Proc Natl Acad Sci USA 2013;110:11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu C‐C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou J, Gennatas ED, Kramer JH, et al. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012;22:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ranasinghe KG, Hinkley LB, Beagle AJ, et al. Regional functional connectivity predicts distinct cognitive impairments in Alzheimer's disease spectrum. Neuroimage Clin 2014;5:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of cognitive tests between groups. Z‐scores of the individual cognitive tests between EEG groups (normal, mild, and abnormal).
Table S1. Distribution of types of EEG abnormalities in the abnormal EEG group.
Table S2. Follow‐up data availability.
Table S3. Baseline values of demographic variables, CSF profile, MRI markers, and cognitive tests.
Table S4. Prediction of cognitive decline over time for three EEG groups.


