Abstract
Objective:
To evaluate the descriptive epidemiology of pediatric cancers among Alaska Native (AN) people.
Study design:
We used data from the Alaska Native Tumor Registry, a population-based registry capturing cancer information among Alaska Native people 1969-present. Specifically, we examined all cases of cancer diagnosed among individuals ages 0-19 years. Cases were classified according to the International Classification of Childhood Cancers, 3rd edition (ICCC-3). We estimated incidence and distribution of cases by ICCC-3 cancer site, comparing between the time-periods 1969-1996, and 1997-2016. We assessed twelve month and five-year cause-specific survival, and examined differences over time-period, adjusted for age, sex, and ICCC-3 site.
Results:
Incidence rates of pediatric cancers increased between 1969-1996 (n = 134), and 1997-2016 (n = 186) among Alaska Native people, from 139.8/1,000,000 (95% Confidence Interval (CI): 116.99, 165.7) to 197.54/1,000,000 (95% CI: 170.1, 228.1). Distribution of ICCC-3 sites differed between time-periods (P<0.0001). Finally, cancer survival was high; 12-month survival probability from all ICCC-3 sites combined was 0.88 (95% CI: 0.84, 0.92) and five-year survival probability was 0.76 (0.70, 0.81) (1969-2016). After adjusting for age, sex, and ICCC-3 site, we observed a 57% reduction in risk of death when comparing AN pediatric cancer cases diagnosed in 1997-2016 to those diagnosed in 1969-1996.
Conclusions:
This information will be of value for our understanding of pediatric cancers among Indigenous peoples of the U.S., and will also be informative for clinicians providing care to this population.
Keywords: Native American, cancer surveillance, childhood cancers
Cancer is the leading cause of disease-related death in children nationwide, ranking only behind motor vehicle crash and firearm-related injury, and accounting for 9.1% of child and adolescent deaths nationwide (1). Mortality rates are higher for American Indian and Alaska Native (AIAN) children and adolescents compared with their U.S. white (USW) counterparts, and exhibit regional variation, with the highest rates observed among Alaska Native (AN) children living in Alaska (2). Furthermore, although the leading causes of death are similar among USW and AIAN children and adolescents (1, 2), data specific to AN children and adolescents living in Alaska show slight differences. Cancer is not among the ten leading causes of death (COD) for AN children aged 0-14 years, but is the fifth leading COD for AN individuals aged 15-24 years, behind suicide, unintentional injury, homicide, and heart disease (3). Differences in pediatric cancer incidence rates also exist. Rates are lower among all AIAN children and adolescents nationwide compared with all other racial groups in the US (4, 5); however, only one report has compared cancer incidence rates between AN people in Alaska and other ethnic groups (6). This report, published almost 20 years ago, indicated that rates among AN children were actually similar to those observed among USW, and higher than those observed among AIAN children living in New Mexico. This information agrees with what we know about variations in site-specific cancer incidence rates among AIAN adults nationwide; for example, rates of gastric, lung and colorectal cancers are known to be higher among AN people than their AIAN counterparts in the contiguous 48 states (7-9).
This study examines childhood and adolescent cancers among AIAN people living in Alaska (6). We used data from the Alaska Native Tumor Registry (ANTR), a population-based central cancer registry that records cancer information for AIAN people living in Alaska. The previous report of cancer among AN children and adolescents, which also used data from the ANTR, reported on cases diagnosed between 1969 and 1996; therefore, this report focuses primarily on cases diagnosed during the most recent 20 year period (1997-2016). However, we also present data from 1969-1996 for comparison. We present specific information on cancer frequency, incidence, and survival. We anticipate that these findings will be of interest to clinicians interested in childhood and adolescent cancers, particularly among Indigenous populations, as well as individuals with a broader interest in cancer among AIAN people.
METHODS
Data indicate that 147,752 AIAN people reside in Alaska, including 56,827 people aged under 18 years (10) (individuals reporting AIAN identity alone, or in combination with another racial identity). AIAN people comprise 19.5% of the Alaskan population, and almost 90% of AIAN people living in Alaska identify as Alaska Native (11); therefore, hereafter we will refer to all AIAN people resident in Alaska as “Alaska Native (AN) people”. Healthcare for AN people is provided by over 20 regional tribal health organizations, and the Alaska Native Tribal Health Consortium, a tribal health organization that provides statewide medical subspecialty and surgical services for all AN people. There is one tribally-managed tertiary healthcare facility in the state, located in Anchorage: the Alaska Native Medical Center (ANMC). However, almost all pediatric cancer care in Alaska is provided at other Anchorage-based, non-tribal clinics and hospitals in order to consolidate services and provide the highest quality care for the relatively small number of pediatric cancer cases that occur in Alaska. Unusual or challenging cancer cases may be treated out of state; usually by facilities in Seattle WA or Portland OR.
Data sources
Cancer data were collected by the ANTR, a population-based central cancer registry that records information on AIAN people who meet eligibility requirements for Indian Health Service benefits, who have been diagnosed with cancer in Alaska since 1969, and who resided in Alaska at the time of diagnosis. The ANTR has been collecting cancer information according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) standards since its inception and has been a full member of the SEER Program since 1999. According to ANTR standard case-finding practices, cases were ascertained through a variety of sources, including hospital discharge diagnoses for tribal and non-tribal health facilities in Alaska; tumor registry and pathology files of the ANMC and other in-state healthcare facilities; linkage to the Alaska Cancer Registry and the Washington State Cancer Registry; and death certificates (<1% cases were registered solely on the basis of information from a death certificate). Mortality data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics; additional death clearance procedures to determine vital status and cause of death included linkage with the Social Security Administration, and Centers for Medicare and Medicaid, as well as reviewing Alaska death certificate information in collaboration with the Alaska Cancer Registry. Information on treatment occurring outside of Alaska was obtained through linkage with other population-based cancer registries. For the purposes of this analysis, we report on cancers diagnosed between 01/01/1969 and 12/31/2016, with our primary focus on cases diagnosed in the last 20 years (1997-2016). Patient characteristics collected by the tumor registry and reviewed in this study include age at diagnosis, and sex. Clinical characteristics included histologic subtype, laterality, and cancer stage (SEER Historic Stage A: local vs. regional vs. distant/unknown) (12).
Case Definition
Cases were restricted to those diagnosed among children (aged 0-14 years) and adolescents (aged 15-19 years). Cases included only primary malignant neoplasms, and were classified into 12 major groups using the International Classification of Childhood Cancer, 3rd Edition(ICCC-3) (13).
Statistical Analyses
Differences in patient and clinical characteristics were assessed using the Chi-squared test for categorical variables, or the Fisher exact test for comparisons which contained cell sizes <5, and one-way ANOVA for continuous variables. Cancer incidence rates were expressed as average annual rates, expressed per 1,000,000 population and age-adjusted to the U.S. Census 2000 standard population using the direct method. Denominators for rate calculations were derived from population estimates from the U.S. Bureau of the Census and National Center for Health Statistics for AN people (bridged estimates) and USW, available from the NCI’s SEER Program (11). Where comparisons are made to data from U.S. whites, we used the SEER 13 Research Database, again available from the NCI’s SEER Program. 20-year limited duration prevalence counts were estimated using the SEER*Stat Software (National Cancer Institute, Surveillance Research Program, Bethesda MD). In accordance with prevailing standards (14), survival analyses were restricted to cases of known age, histologically confirmed and followed over time; cases that were identified solely on the basis of death certificates or autopsy reports were excluded from the survival analyses. We use cause-specific survival analyses, as these methods are more appropriate for use in populations where generic life-tables may not accurately represent the experience of the population (such as Indigenous peoples). Cause-specific survival is one method of assessing net survival, which provides information on the net effect of a cancer diagnosis in the absence of other causes of death. In a population-based setting, differences in net cancer survival reflect differences in survival due to the cancer rather than competing causes of death (15). Patients still alive on December 31, 2016, or who had died of other causes were censored from these analyses. We used Cox proportional hazards models to examine risk of death between time-periods, adjusted for age and sex.
All statistical tests were two-sided and were assessed at an alpha level of P < .05. Statistics were generated using the SEER*Stat Software version 8.3.5 (National Cancer Institute’s Surveillance Research Program, Bethesda, MD, USA) and SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). Incidence rates and case counts are not provided where cell sizes were <10, in order to protect individuals’ privacy and ensure stability of estimates presented. Institutional Review Board review was not required for this study, because it used publically-available surveillance data; tribal review and approval from the Alaska Native Tribal Health Consortium and Southcentral Foundation were obtained for publication of this study.
RESULTS
Over 47 years of surveillance, 320 cases of ICCC-3-classified cancer were diagnosed among AN people aged <20 years (Table I). Over one-half (58%) of these cases occurred in the most recent 20-year period (1997-2016). During this time-period (1997-2016), pediatric cancers accounted for 2.5% of cancers among AN people (n = 186). 20-year limited prevalence estimates indicated 132 (95% CI: 110, 157) pediatric cancer survivors, split between 0-14 years at prevalence (58 (95%CI: 44, 75)), 15-19 years (32 (95%CI: 22, 45)), and 20+ years (42 (95%CI: 30, 57)).
Table 1.
Case counts and incidence rates (per 1,000,000 population) for pediatric cancers by ICCC-3 site classification among Alaska Native children, diagnosed 1969-2016, stratified by time-period.a,b
| 1969-2016 | 1969-1996 | 1997-2016 | ||||
|---|---|---|---|---|---|---|
| All ages (00-19 years) | All ages (00-19 years) | All ages (00-19 years) | ||||
| Count | IR (95% CI) | Count | IR (95% CI) | Count | IR (95% CI) | |
| All ICCC Cancers | 320 | 167.5 (149.6, 186.9) | 134 | 139.8 (117.0, 165.7) | 186 | 197.5 (170.1, 228.1) |
| I Leukemias, myeloproliferative & myelodysplastic diseases | 97 | 49.6 (40.2, 60.5) | 36 | 36.6 (25.6, 50.8) | 61 | 64.1 (49.0, 82.3) |
| Acute lymphocytic leukemia | 60 | 30.3 (23.1, 39.0) | 24 | 24.5 (15.6, 36.5) | 36 | 37.4 (26.2, 51.8) |
| Acute myeloid leukemia | 20 | 10.5 (6.4, 16.2) | 14 | 14.9 (8.2, 25.0) | ||
| II Lymphomas and reticuloendothelial neoplasms | 44 | 23.2 (16.9, 31.2) | 14 | 14.8 (8.0, 24.8) | 30 | 32.3 (21.7, 45.9) |
| Hodgkin lymphomas | ||||||
| Non-Hodgkin lymphomas (except Burkitt lymphoma) | 17 | 9.2 (5.4, 14.8) | 13 | 14.1 (7.5, 24.1) | ||
| III CNS and misc intracranial and intraspinal neoplasms | 47 | 24.8 (18.2, 33.0) | 18 | 18.7 (11.0, 29.5) | 29 | 31.2 (20.9, 44.8) |
| IV Neuroblastoma and other peripheral nervous cell tumors | ||||||
| V Retinoblastoma | 10 | 4.9 (2.3, 9.0) | ||||
| VI Renal tumors | 17 | 8.4 (4.9 , 13.6) | ||||
| VII Hepatic tumors | 24 | 12.8 (8.2, 19.0) | 18 | 19.7 (11.7, 31.1) | ||
| VIII Malignant bone tumors | 12 | 6.4 (3.3, 11.2) | ||||
| IX Soft tissue and other extraosseous sarcomas | 23 | 12.3 (7.8, 18.4) | 15 | 16.2 (9.0, 26.6) | ||
| X Germ cell & trophoblastic tumors & neoplasms of gonads | 10 | 5.4 (2.6, 9.9) | ||||
| XI Other malignant epithelial neoplasms and melanomas | 27 | 15.4 (10.1, 22.3) | 18 | 19.8 (11.8, 31.3) | ||
| XII Other and unspecified malignant neoplasms | ||||||
Data not given where case count <10.
IR: Incidence rate; CI: Confidence interval.
Table 1 describes case counts and incidence rates for AN pediatric cancer cases, comparing the most recent 20 year period (1997-2016; hereafter “recent” time-period), to the 1969-1996 (hereafter “earlier” time-period; data previously reported by Lanier et al, but presented here for comparison).(6) Data are reported for all ages to maximize cell sizes. In the most recent time-period, approximately three quarters of AN pediatric cancer cases were diagnosed among children (0-14 years; 75%, n = 140), with the remaining 25% cases diagnosed among adolescents (15-19 years, n = 46). The most common cancer type among AN children was leukemia (38% in recent time-period), followed by central nervous system (CNS) malignancies (17%), and lymphomas (15%). Among AN adolescents, other malignant epithelial neoplasms and melanomas were the most common (30%), followed by leukemia and lymphomas, which were almost equally represented (18% and 20%, respectively).
We examined change in both ICC-3 site distribution and cancer incidence over time (Table 1, Figure 1, and Figure 2). Although not the primary focus of the current analysis, Table 2 (available at www.jpeds.com) presents incidence compared with USW. Site distribution differed between children and adolescents (p <0.001) in both time-periods (Figure 1). We also observed significant differences in the distribution of sites between the earlier and recent time-periods for both children (p = 0.037) and adolescents (p = 0.031). Specifically, hepatic tumors comprised a relatively large proportion of cancers in the earlier time-period (13%), whereas these cancers were relatively uncommon in the recent time-period (3%). Overall, the rate of childhood cancers among AN people aged <20 years during the recent time period was 197.5 (95% CI: 170.1, 228.1) per 1,000,000 population; this was slightly higher than in the earlier time-period (IR (95% CI): 139.8 (117.0, 197.5) per 1,000,000 population). Changes in 20-year average annual incidence rates varied by ICCC-3 site (Figure 2). Unfortunately, due to small case counts and wide confidence intervals, we were unable to detect any significant differences by ICCC-3 site between time-periods. However, we did observe decreases in incidence of retinoblastoma, hepatic tumors, malignant bone tumors, and germ cell tumors. In contrast, we observed increases in incidence of leukemia, lymphoma, CNS neoplasms, soft tissue and other extraosseous sarcomas, and other malignant epithelial neoplasms and melanomas. Despite an overall increase in incidence rates for leukemia among adolescents (15-19 years), we did observe a decrease in the incidence of acute lymphocytic leukemia for this group.
Figure 1.
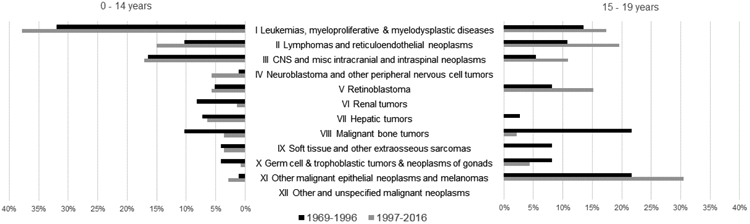
Distribution of pediatric cancer cases among ICCC-3 sites, by time period, stratified by age at diagnosis, among Alaska Native children, 1969-2016.
Figure 2.
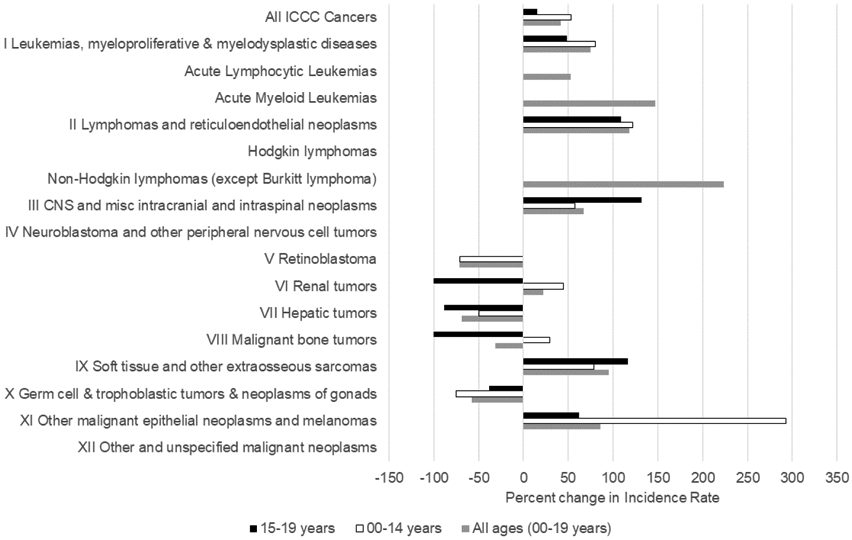
Change in incidence rate for childhood cancers, by age and ICCC-3 site, between time period (1969-1996 and 1997-2016).
a Data are not given where cell case counts <10.
b Differences were calculated as percent change between earlier time-period and recent time-period i.e., positive change indicates rate was higher in the recent time-period.
Table 2.
Case counts and twenty-five year average annual incidence rates (per 1,000,000 population) for pediatric cancers by ICCC-3 site classification among Alaska Native and U.S White children, diagnosed 1992-2016.a
| Alaska Native | U.S. White | |||
|---|---|---|---|---|
| All ages (00-19 years) | All ages (00-19 years) | |||
| Count | IR (95% CI) | Count | IR (95% CI) | |
| All ICCC-3 Cancers | 206 | 178.1 (154.5, 204.2) | 36628 | 180.5 (178.6, 182.3) |
| I Leukemias, myeloproliferative & myelodysplastic diseases | 69 | 58.3 (45.4, 73.9) | 10008 | 48.9 (48, 49.9) |
| Acute lymphocytic leukemia | 47 | 39.2 (28.8, 52.2) | 7836 | 38.3 (37.4, 39.1) |
| Acute myeloid leukemias | 16 | 13.9 (7.9, 22.6) | 1648 | 8.1 (7.7, 8.5) |
| II Lymphomas and reticuloendothelial neoplasms | 24 | 21 (13.5, 31.3) | 5113 | 25.6 (24.9, 26.3) |
| Hodgkin lymphomas | 2605 | 13.1 (12.6, 13.6) | ||
| Non-Hodgkin lymphomas (except Burkitt lymphoma) | 10 | 9 (4.3, 16.5) | 1774 | 8.9 (8.4, 9.3) |
| III CNS and misc intracranial and intraspinal neoplasms | 30 | 26.2 (17.7, 37.4) | 6260 | 30.8 (30.1, 31.6) |
| IV Neuroblastoma and other peripheral nervous cell tumors | 1734 | 8.2 (7.9, 8.6) | ||
| V Retinoblastoma | 692 | 3.3 (3, 3.5) | ||
| VI Renal tumors | 11 | 9.2 (4.6, 16.5) | 1355 | 6.5 (6.2, 6.9) |
| VII Hepatic tumors | 12 | 10.3 (5.3, 18) | 498 | 2.4 (2.2, 2.6) |
| VIII Malignant bone tumors | 1870 | 9.4 (9, 9.8) | ||
| IX Soft tissue and other extraosseous sarcomas | 17 | 15.1 (8.8, 24.2) | 2414 | 11.9 (11.5, 12.4) |
| X Germ cell & trophoblastic tumors & neoplasms of gonads | 2671 | 13.3 (12.8, 13.8) | ||
| XI Other malignant epithelial neoplasms and melanomas | 19 | 17.7 (10.6, 27.5) | 3900 | 19.6 (19, 20.2) |
| XII Other and unspecified malignant neoplasms | 113 | 0.6 (0.5, 0.7) | ||
Data not given where case count <10.
ICCC-3: International Classification of Childhood Cancers, 3rd edition; IR: Incidence rate; CI: Confidence interval.
Table 3 describes one and five-year cause-specific survival for AN pediatric cancers, stratified by ICCC-3 site classification, for both time-periods and all ages combined. Cause-specific survival probability from all cancers was high: 12-month survival probability was 0.88 (95% CI: 0.84, 0.92) and 5-year survival probability was 0.76 (0.70, 0.81). Survival probability varied by ICCC-3 site. For many sites for which there was sufficient data to calculate survival, 12-month survival probability was over 90%; exceptions to this were malignant bone tumors, and other malignant epithelial neoplasms and melanomas. Five-year survival probability was highest for lymphomas (0.90 (95% CI: 0.76, 0.96)) and renal tumors (0.94 (95% CI: 0.63, 0.99)), and lowest for malignant bone tumors (0.50 (95% CI: 0.21, 74)). Survival was higher in the recent period relative to the earlier period; a Kaplan-Meier plot is shown in Figure 3 (available at www.jpeds.com). In Cox proportional hazards models adjusted for age, sex, and ICCC-3 site, the risk of death was 57% lower in the recent period relative to the earlier period (HR (95% CI): 0.53 (0.32, 0.89)).
Table 3.
Cause-specific survival by ICCC-3 site for Alaska Native pediatric cancer cases diagnosed 1969-2016a, b
| ICCC-3 Site | N (Deaths) |
Months | Survival (95% CI) |
|---|---|---|---|
| I Leukemias, myeloproliferative & myelodysplastic diseases | 92 (23) | 12 | 0.88 (0.79, 0.93) |
| 60 | 0.72 (0.61, 0.81) | ||
| II Lymphomas and reticuloendothelial neoplasms | 43 (4) | 12 | 0.93 (0.8, 0.98) |
| 60 | 0.90 (0.76, 0.96) | ||
| III CNS and misc intracranial and intraspinal neoplasms | 30 (10) | 12 | 0.90 (0.71, 0.97) |
| 60 | 0.60 (0.38, 0.76) | ||
| IV Neuroblastoma and other peripheral nervous cell tumors | 12 | ||
| 60 | |||
| V Retinoblastoma | 12 | ||
| 60 | |||
| VI Renal tumors | 16 (2) | 12 | 0.94 (0.63, 0.99) |
| 60 | 0.94 (0.63, 0.99) | ||
| VII Hepatic tumors | 21 (8) | 12 | 0.95 (0.69, 0.99) |
| 60 | 0.74 (0.48, 0.88) | ||
| VIII Malignant bone tumors | 12 (7) | 12 | 0.67 (0.34, 0.86) |
| 60 | 0.50 (0.21, 0.74) | ||
| IX Soft tissue and other extraosseous sarcomas | 22 (4) | 12 | 0.95 (0.71, 0.99) |
| 60 | 0.80 (0.55, 0.92) | ||
| X Germ cell & trophoblastic tumors & neoplasms of gonads | 12 | ||
| 60 | |||
| XI Other malignant epithelial neoplasms and melanomas | 26 (9) | 12 | 0.77 (0.56, 0.89) |
| 60 | 0.73 (0.52, 0.86) | ||
| XII Other and unspecified malignant neoplasms | 12 | ||
| 60 |
Data not given where case count <10.
CNS: Central nervous system; ICCC-3: International Classification of Childhood Cancers, 3rd edition
Figure 3.
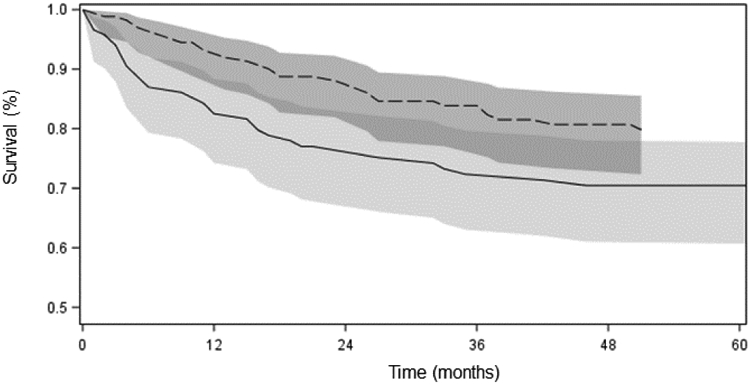
Survival probabilities among Alaska Native pediatric cancer cases (all sites, age <20 years); by period of diagnoses: 1969-1996 (solid line), 1997-2016 (dashed line) (shadings show 95% confidence intervals)
DISCUSSION
Despite representing a small proportion of total cancer cases (2.5%) diagnosed among AN people of all ages, AN pediatric cancers are important to understand to ensure that the Alaska Tribal Health System and the associated non-Tribal health systems both within and outside of Alaska are providing the highest quality cancer care to AN children. We present the most recent epidemiologic data (1997-2016), and compare it with data published by Lanier et al (1969-1996) in order to understand whether and how the epidemiology of pediatric cancers is changing in AN people.(6) Our results indicate a significant increase in the incidence rate of all cancers between the two time-periods, as well as an increase in incidence rates for several ICCC-3 cancer sites. Conversely, we observed a decrease in the risk of death between the two time-periods, indicating that survival has improved. This pattern reflects trends observed at the national level, where increased incidence has been accompanied by decreased mortality and improved survival for many pediatric cancer sites (5). These findings will be informative for those involved in the provision of healthcare services and clinical care to AN people, as well as those interested in cancer among Indigenous populations.
In contrast to many other cancer sites examined in this study, we saw a substantial decrease in hepatic tumors between the two time-periods. Lanier et al showed that AN children were at significantly greater risk of hepatocellular carcinoma relative to both US white, and New Mexico AIAN children (6). All children in that study were hepatitis B (HBV) antigen positive, and the authors demonstrated a significant decrease in incidence of these tumors between cohorts born before and after the implementation of a statewide HBV vaccination program began in 1982 (6). Our results confirm the continued success of this program for hepatic tumor prevention among AN children. This observation is paralleled by observations among AN adults, who have also seen a sharp decline in HBV-related cancers (16).
The increase in non-Hodgkin lymphoma observed between the two time-periods could be related to an increase in Epstein-Barr virus (EBV) prevalence in the AN population. EBV is an infectious agent linked to increased risk of developing non-Hodgkin’s lymphoma (17), and has also been shown to have a potential role in the development of adult nasopharyngeal cancers (18, 19), which are observed at 17 times higher rates among AN people than U.S. whites (20). Although there is some evidence from the 1980s to suggest a high prevalence of EBV infection among AN children (6), further research is necessary to understand EBV prevalence over time among AN children, and whether EBV may be linked to the increased incidence of non-Hodgkin lymphoma observed herein. Other risk factors for Non-Hodgkin’s lymphoma in children include immunodeficiency (including immunodeficiency syndromes, infection with human immunodeficiency virus, and organ transplant), radiation exposure, and possibly a family history of this disease (21).
Improvements in pediatric cancer survival nationally are thought to be linked to improvements in treatment as well as supportive care (5). The landscape of pediatric cancer care in Alaska has changed since cancer surveillance begun in 1969, with the development of local pediatric cancer resources and treatment. In 2005, the first pediatric oncology clinic opened in Alaska, giving AN children an option to remain in-state for most of their cancer care. Outcomes for these children have improved (Figure 3), and by remaining closer to home for therapy, disruptions to families and patient satisfaction is minimized, relative to when all children had to leave the state for oncology services [MH., LS. Pers. Comm]. For some harder to treat, or rare, cancers that are best treated in a large pediatric oncology facility, families may still need to travel out of state, usually to Washington or Oregon. It is unknown to what degree increased in-state healthcare access directly contributed to increased survival observed between the two time-periods; however, we know from patient surveys studies with Indigenous and non-Indigenous peoples that both individuals and families prefer to receive their care closer to home (22-24). Furthermore, access to culturally-appropriate, high quality healthcare is known to improve cancer outcomes (25). It is critical that AN children and their families continue to receive access to the highest quality cancer care and supportive services in Alaska for their continued health and wellbeing.
In considering access to cancer care services for AN children and adolescents, our results also support the need to consider ongoing follow-up care for cancer survivors. The number of pediatric survivors is increasing nationally, with an estimated 379,112 survivors alive in the U.S. as of January 1, 2010 (5). Although we estimate that the number of pediatric cancer survivors among AN people is small, due to the size of the AN population and the rarity of these cancers, this growing group is likely to have unique healthcare and cancer surveillance needs (26, 27). It is also critical to understand the needs of these individuals so that Tribal Health Organizations can ensure that these needs are being met. Yet, studies indicate that there is a dearth of childhood cancer survivorship research conducted among minority populations, not just AN people. We know of no published studies that examine the needs of AN cancer survivors, whether the cancer was diagnosed when the individual was a child or an adult.
The primary strength of this study was the use of high-quality population-based data from the ANTR, an NCI-supported SEER registry. In particular, the long history of cancer surveillance by this registry (almost 50 years), enabled us to examine trends in pediatric cancers over a long period of time. This was necessary, in part due to the small size of this population, and the resulting low case counts for pediatric cancers. The small sample size provided a challenge to examining trends, particularly in specific age, sex, or cancer site strata, and cautious interpretation of these data may be warranted. Yet, this does not diminish the importance of this work; several researchers have noted the importance of small population cancer research (28, 29). It is possible that data collected by the ANTR on pediatric cancer cases were incomplete. This may have occurred, for example, if an individual was diagnosed and treated out-of-state. Although the ANTR has long conducted routine linkages with both in- and out of state partners to ensure complete case ascertainment and follow-up, we know that racial misclassification is higher outside of Alaska, and in urban, non-IHS Purchased/Referred care delivery (PRCDA) counties (such as those where AN people might typically seek treatment) (30). Therefore, there is a possibility that a small number of cases may not have been captured in the registry. Finally, it is possible that the quality of cancer registration may have changed over time, which could impact interpretation of these data. However, we note that the Alaska Native Tumor Registry has followed the SEER Program standards since its inception in 1974; therefore, we do not perceive there to have been any systematic shifts in registration procedures that are likely to have greatly impacted these results.
We were able to demonstrate that although incidence of pediatric cancers is increasing in this population, survival has also improved markedly. These results support the need for ongoing provision of specialist pediatric oncology services in Alaska for AN people, as well as to understand the unique healthcare and support service needs of AN pediatric cancer survivors as they age. We anticipate that these results will be of interest to those who provide clinical care services to AN children, as well as those with an interest in pediatric cancers, and indigenous health issues.
Acknowledgments
S.N., G.Z., and the Alaska Native Tumor Registry are supported by the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results Program, NCI contract number HHSN26120130010I, Task Order HHSN26100005. M.H. is supported as a co-investigator by two grants, Diet and the CPT1A Arctic Variant: Impact on the Health of Alaska Native Children (NIH/NICHD R01HD089951-02) and the Clinical Sites for the IDeA States Pediatric Clinical Trials Network (NIH 1UG1HD090875-01). The funders had no role in the study design; collection, analysis or interpretation of data; the writing of the report; the decision to publish these data. The authors declare no conflicts of interest.
Abbreviations:
- AIAN
American Indian/Alaska Native
- AN
Alaska Native
- ANMC
Alaska Native Medical Center
- ANTR
Alaska Native Tumor Registry
- CI
Confidence Interval
- CNS
Central nervous system
- COD
Cause of Death
- HR
Hazard Ratio
- ICD-O-3
International Classification of Diseases for Oncology – Third Edition
- ICCC-3
International Classification of Childhood Cancers
- SEER
Surveillance, Epidemiology and End Results
- USW
U.S. white
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Cunningham RM, Walton MA, Carter PM. The Major Causes of Death in Children and Adolescents in the United States. New England Journal of Medicine. 2018;379:2468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CA, Gachupin FC, Holman RC, MacDorman MF, Cheek JE, Holve S, et al. American Indian and Alaska Native infant and pediatric mortality, United States, 1999-2009. Am J Public Health. 2014;104:S320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake I, Holck P, Provost EM Alaska Native Mortality Update: 2009-2013. Anchorage, AK: Alaska Native Epidemiology Center; 2016. [Google Scholar]
- 4.Li J, Thompson TD, Miller JW, Pollack LA, Stewart SL. Cancer incidence among children and adolescents in the United States, 2001-2003. Pediatrics. 2008;121(6):e1470–7. [DOI] [PubMed] [Google Scholar]
- 5.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 6.Lanier AP, Holck P, Ehrsam Day G, Key C. Childhood cancer among Alaska Natives. Pediatrics. 2003;112:e396. [DOI] [PubMed] [Google Scholar]
- 7.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104:S377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiggins CL, Espey DK, Wingo PA, Kaur JS, Wilson RT, Swan J, et al. Cancer among American Indians and Alaska Natives in the United States, 1999-2004. Cancer. 2008; 113:1142–52. [DOI] [PubMed] [Google Scholar]
- 9.Wiggins CL, Perdue DG, Henderson JA, Bruce MG, Lanier AP, Kelley JJ, et al. Gastric cancer among American Indians and Alaska Natives in the United States, 1999-2004. Cancer. 2008;113:1225–33. [DOI] [PubMed] [Google Scholar]
- 10.Alaska Department of Labor and Workforce Development. Alaska Population by Age, Sex, Race (Alone or in Combination) and Hispanic Origin, July 2015. ≤http://live.laborstats.alaska.gov/pop/index.cfm≥. 2015 [cited 3/23/2017].
- 11.U.S Census Bureau. 2010 Census Summary File 1. ≤https://factfmder/census.gov≥ 2010. [cited 6/24/20]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53:457–81. [Google Scholar]
- 13.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. 2005;103:1457–67. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariotto AB, Noone AM, Howlader N, Cho H, Keel GE, Garshell J, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;2014:145–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connelly M, Bruce MG, Bulkow L, Snowball M, McMahon BJ. The changing epidemiology and aetiology of hepatocellular carcinoma from 1969 through 2013 in Alaska Native people. Liver international : official journal of the International Association for the Study of the Liver. 2016;36:1829–35. [DOI] [PubMed] [Google Scholar]
- 17.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiology and Prevention Biomarkers. 2007;16:401–4. [DOI] [PubMed] [Google Scholar]
- 18.Lanier A, Bender T, Talbot M, Wilmeth S, Tschopp C, Henle W, et al. Nasopharyngeal carcinoma in alaskan eskimos, indians, and aleuts: A review of cases and study of epstein-barr virus, HLA, and environmental risk factors. Cancer. 1980;46:2100–6. [DOI] [PubMed] [Google Scholar]
- 19.Raab-Traub N, Flynn K, Pagano J, Pearson G, Huang A, Levine P, et al. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. International journal of cancer. 1987;39:25–9. [DOI] [PubMed] [Google Scholar]
- 20.Carmack A, Schade TL, Sallison I, Provost EM, Kelly JJ. Cancer in Alaska Native People: 1969-2013, The 45 Year Report. Anchorage, AK: Alaska Native Epidemiology Center, Alaska Native Tribal Health Consortium; 2015. [Google Scholar]
- 21.Allen CE KK, Bollard CM, Gross TG. Malignant non-Hodgkin lymphomas in children. In: Pizzo PA PD, editor. Principles and Practice of Pediatric Oncology, 7th Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2016. [Google Scholar]
- 22.Fitch MI, Gray RE, McGowan T, Brunskill I, Steggles S, Sellick S, et al. Travelling for radiation cancer treatment: patient perspectives. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2003;12:664–74. [DOI] [PubMed] [Google Scholar]
- 23.Nostedt MC, McKay AM, Hochman DJ, Wirtzfeld DA, Yaffe CS, Yip B, et al. The location of surgical care for rural patients with rectal cancer: patterns of treatment and patient perspectives. Canadian Journal of Surgery. 2014;57:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shalowitz DI, Nivasch E, Burger RA, Schapira MM. Are patients willing to travel for better ovarian cancer care? Gynecologic oncology. 2018;148:42–8. [DOI] [PubMed] [Google Scholar]
- 25.Surbone A Cultural aspects of communication in cancer care. Support Care Cancer. 2008;16:235–40. [DOI] [PubMed] [Google Scholar]
- 26.Aziz NM, Oeffmger KC, Brooks S, Turoff AJ. Comprehensive long-term follow-up programs for pediatric cancer survivors. Cancer. 2006;107:841–8. [DOI] [PubMed] [Google Scholar]
- 27.Group AAoPSoHOCsO. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan S, Moser RP, Willis G, Riley W, Alexander M, Berrigan D, et al. Small is essential: importance of subpopulation research in cancer control. Am J Public Health. 2015; 105: S371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etz KE, Arroyo JA. Small Sample Research: Considerations Beyond Statistical Power. Prevention science : the official journal of the Society for Prevention Research. 2015; 16:1033–6. [DOI] [PubMed] [Google Scholar]
- 30.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2008; 113:1120–30. [DOI] [PubMed] [Google Scholar]


