Abstract
Background:
Mutations in the KRAS gene are the most common driver oncogenes present in lung adenocarcinomas. We analyzed the largest multi-institutional database available containing patients with metastatic KRAS mutant lung adenocarcinomas.
Methods:
The Lung Cancer Mutation Consortium (LCMC) is a multi-institutional collaboration to study the genomic characteristics of lung adenocarcinomas, treat them with genomically directed therapeutic approaches, and assess their outcomes. Since its inception in 2009, the LCMC has enrolled over 1900 patients and has performed pretreatment, multiplexed, molecular characterization along with collecting clinical data. We evaluated the characteristics of patients with KRAS mutation in the LCMC and the association with overall survival (OS).
Results:
Data from 1655 patients with metastatic lung adenocarcinomas were analyzed. 450 (27%) patients had a KRAS mutation, 58% female, 93% smokers, and median age of 65 years. Main KRAS subtypes were: G12C 39%; G12D and G12V at 18% each. Among patients with KRAS mutation, G12D had a higher proportion of never smokers (22%, P<0.001). Patients with KRAS mutant tumors had a trend toward shorter median survival compared to all others in the series (1.96 vs. 2.22; P=0.08) and lower 2-year survival rate (49% (95% CI: 44-54%) and 55% (95% CI: 52-58%), respectively.
Conclusions:
In the LCMC study, 27% of lung adenocarcinomas patients harbored a KRAS mutation and up to third of them had another oncogenic driver. Patients with both KRAS and STK11 mutations had a significantly inferior clinical outcome.
1. Introduction
Non-small cell lung cancers (NSCLC) have two major subtypes, adenocarcinoma and squamous cell carcinomas each with unique and shared clinico-pathological characteristics1. They account for nearly 85% of all lung cancers and include a number of distinct molecular subsets based on their genomic characteristics. Many of these genomic aberrations can be effectively targeted including EGFR2 sensitizing mutations, ALK3 and ROS14 rearrangements, and BRAF V600E5 mutations. Treatment with specific targeted therapies improves survival for patients in these molecular cohorts. KRAS was one of the first oncogenes found to be mutated in human cancers including in lung, colorectal and pancreatic cancers6. This somatic mutation is the most frequently found in non-Asian patients with lung adenocarcinoma than Asian patients7 with an incidence rate of 25-35%.
In a 500 patients’ cohort with lung adenocarcinomas8, KRAS mutations were present in 22%. While tranversion mutations (G→T or G→C) were more common in ever-smokers, transition mutations (G→A) were more common in never-smokers.
KRAS mutations have been linked with a poor prongosis9-12, though this observation has not been consistent across studies13-15. The majority of KRAS mutations occur at codon 12 and 13; all three common G12 mutations (G12C, G12V, and G12R) have been associated with poor outcomes10. In particular, point mutations in G12C and G12V are associated with worst survival compared to other KRAS mutant subtypes16. It is thought that all KRAS mutations lead to tumor development and growth by activating a complex set of downstream signaling pathways including mitogen-activated protein kinase. Targeting KRAS mutations has proven extremely challenging and current drug development is focused on inhibition of downstream activated pathways17-20. In addition, cells harboring these mutations create an immunosuppressive tumor microenvironment, thus allowing them to evade the immune system20.
To better understand the clinical significance of KRAS mutations in lung adenocarcinomas, we analyzed the findings of patients with KRAS mutant lung adenocarcinomas from the Lung Cancer Mutation Consortium (LCMC). The goals of the LCMC were: to conduct molecular tests (LCMC1) on consecutive patients with advanced lung adenocarcinomas across 11 academic medical centers; and to enroll patients with driver mutations on targeted therapy clinical trials to improve patient outcomes (2009-2012)21, 22. Oncogenic driver events were detected in 64% of lung adenocarcinomas; approximately half of those drivers (30%) were felt to be actionable for therapy21. In the second phase (LCMC2), the consortium adopted an expanded testing panel to cover 16 molecular alterations and included 16 academic centers (2012-2015)23. Patients with an oncogenic driver alteration who received a matched targeted therapy experienced the most favorable overall survival23. These findings supported the use of next generation sequencing (NGS) panels for lung adenocarcinomas to make treatment decisions24.
Here, we describe the characteristics of patients with KRAS mutant lung adenocarcinomas in the entire LCMC cohort and the association of this mutation with their survival.
2. Materials and methods
We analyzed data and interpreted results of patients who consented to LCMC between 2009-2015 and had known KRAS mutation status, complete dates of birth, distant metastasis and last follow up. LCMC1 and LCMC2 data were combined for analysis. We obtained patient’s baseline characteristics (age, gender, race, smoking history, performance status, and treatment history), KRAS mutation status, subtype and codon, and other associated mutations (co-mutations). We evaluated patients’ characteristics and the association of their KRAS status with overall survival (OS). In addition, we evaluated for the presence of co-mutations, and their impact on OS. AKT1, BRAF V600E, BRAF non-V600E, ERBB2, MAP2K1, NRAS, PIK3CA, sensitizing EGFR, non-sensitizing EGFR, and ALK, were checked in LCMC1 and LCMC2. During enrollment in LCMC2, most institutions switched from focused testing to NGS which enabled simultaneous analysis of non-targetable mutations in several other genes in lung cancer (specifically tumor suppressor genes, TP53 and STK11). In addition, amplification of MET (ampMET), ROS1 and RET rearrangements, and PTEN loss of expression, were also tested in the LCMC2. The molecular testing methods used for detection of mutations have been described previously21-23.
Data were presented as frequency (percentage,%) for categorical variables and median (interquartile range, IQR) for continuous variables. Associations between variables were examined with either Wilcoxon rank-sum test, Kruskal-Wallis test, chi-square test or Fisher’s exact test as appropriate. Survival functions were estimated by the Kaplan- Meier method and compared using a log-rank test25. Univariate and multivariable survival analyses were carried out using a Cox proportional hazards model26. The proportional hazards assumption was assessed with scaled Schoenfeld residuals27. Variable selections were carried out by a stepwise procedure based on Akaike Information Criterion28 and the possibility of multicollinearity was assessed by tolerance and the variance inflation factor. Statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) with two-sided tests and a significance level of 0.05.
3. Results
3.1. Clinical characteristics of patients with KRAS mutant lung adenocarcinomas
Data from all patients (N=1918) with lung adenocarcinomas who consented to the LCMC between 2009 and 2015 were available. Out of these, 263(13.71%) patients were excluded from this analysis: 190(9.9%) patients had an incomplete date of birth, date of consent, or date of distant metastasis; 73(3.8%) patients had unknown KRAS status. KRAS mutation was present in 450(27%) of 1655 patients; 260(58%) were female; 401(94%) were white, and 416(93%) were smokers. The median age was 65 years and 59% of the patients had performance status of 1 as assessed by the ECOG (Eastern Cooperative Oncology Group) scale (Tables 1A, 1B, and 1C). There were significant differences between KRAS mutant (no associated co-mutation) and KRAS wildtype (no associated co-mutation) patients in median age, race, and smoking history (Table 3). Patients with KRAS mutations (no associated co-mutation) (33;8.31%) were more likely to have received targeted therapy than patient with KRAS wildtype (no associated co-mutation) lung adenocarcinomas (21;3.87%) (P=0.004). This is likely due to the fact that EGFR inhibitors were available for routine clinical use for an unselected patient population during the LCMC study.
Table 1A.
Characteristics of patients with KRAS mutant and wild type lung adenocarcinomas.
| Variable | N= 1655 |
|---|---|
| KRAS mutation | |
| Mutation | 450 (27.19) |
| Wildtype | 1205 (72.81) |
| KRAS associated co-mutation | |
| Yes | 509 (30.83) |
| No | 1142 (69.17) |
| Missing | 4 |
| KRAS with/without associated Co-mutation | |
| KRAS mutation/Co-mutation | 14 (0.85) |
| KRAS mutation/No co-mutation | 436 (26.41) |
| KRAS wildtype/Co-mutation | 495 (29.98) |
| KRAS wildtype/No co-mutation | 706 (42.76) |
| Missing | 4 |
| Age (Years) | |
| Median (IQR) | 63 (56 - 70) |
| Missing | 6 |
| Gender | |
| Male | 713 (43.08) |
| Female | 942 (56.92) |
| Race | |
| African American | 100 (6.46) |
| Asian/Other | 74 (4.78) |
| White | 1374 (88.76) |
| Missing | 107 |
| Smoking history | |
| Current Smoker | 165 (10.05) |
| Former smoker | 1014 (61.75) |
| Never smoker | 463 (28.2) |
| Missing | 13 |
| ECOG | |
| Asymptomatic | 529 (32.2) |
| Symptomatic, fully ambulatory | 969 (58.98) |
| Symptomatic, in bed less than 50% of day | 145 (8.83) |
| Missing | 12 |
| Surgery history for lung cancer treatment | |
| Yes | 779 (49.3) |
| No | 801 (50.7) |
| Missing | 75 |
| Radiation treatment history for lung cancer | |
| Yes | 592 (36.39) |
| No | 1035 (63.61) |
| Missing | 28 |
| Chemotherapy history for lung cancer | |
| Yes | 454 (54.18) |
| No | 384 (45.82) |
| Missing | 817 |
| Targeted therapy given | |
| Yes | 384 (26.85) |
| No | 1046 (73.15) |
| Missing | 225 |
Data are presented as number of patients (%) or median (IQR, interquartile range).
Table 1B.
Number of patients tested for a given marker in all patients (N= 1655)
| Variable | N= 1655 |
|---|---|
| AKT1 tested | |
| No | 164 (19.32) |
| Yes | 685 (80.68) |
| Missing | 806 |
| BRAF V600E tested | |
| No | 93 (10.95) |
| Yes | 756 (89.05) |
| Missing | 806 |
| oBRAF tested | |
| No | 93 (10.95) |
| Yes | 756 (89.05) |
| Missing | 806 |
| ERBB2 tested | |
| No | 304 (35.81) |
| Yes | 545 (64.19) |
| Missing | 806 |
| KRAS tested | |
| Yes | 849 (100) |
| Missing | 806 |
| MAP2K1 tested | |
| No | 133 (15.67) |
| Yes | 716 (84.33) |
| Missing | 806 |
| NRAS tested | |
| No | 93 (10.95) |
| Yes | 756 (89.05) |
| Missing | 806 |
| PIK3CA tested | |
| No | 93 (10.95) |
| Yes | 756 (89.05) |
| Missing | 806 |
| sEGFR tested | |
| No | 91 (10.72) |
| Yes | 758 (89.28) |
| Missing | 806 |
| oEGFR tested | |
| No | 91 (10.72) |
| Yes | 758 (89.28) |
| Missing | 806 |
| ALK tested | |
| No | 49 (5.77) |
| Yes | 800 (94.23) |
| Missing | 806 |
| ampMET tested | |
| No | 190 (22.38) |
| Yes | 659 (77.62) |
| Missing | 806 |
| ROS1 tested | |
| No | 54 (6.36) |
| Yes | 795 (93.64) |
| Missing | 806 |
| RET tested | |
| No | 69 (8.13) |
| Yes | 780 (91.87) |
| Missing | 806 |
| mutPTEN tested | |
| No | 502 (59.13) |
| Yes | 347 (40.87) |
| Missing | 806 |
| TP53 tested | |
| No | 469 (55.24) |
| Yes | 380 (44.76) |
| Missing | 806 |
| STK11 tested | |
| No | 470 (55.36) |
| Yes | 379 (44.64) |
| Missing | 806 |
Data are presented as number of patients (%).
oBRAF: BRAF other than V600E; sEGFR: sensitizing EGFR; oEGFR: non-sensitizing EGFR.
Table 1C.
Characteristics of patients with KRAS mutant lung adenocarcinomas.
| Variable | N= 450 |
|---|---|
| KRAS mutation | |
| With associated co-mutation | 14 (3.11%) |
| Without associated co-mutation | 436 (96.89%) |
| Age (years) | |
| Median (IQR) | 65 (58 – 71) |
| Missing | 1 |
| Gender | |
| Male | 190 (42.22%) |
| Female | 260 (57.78%) |
| Race | |
| White | 401 (93.91%) |
| Non-White | 26 (6.09%) |
| Missing | 23 |
| Smoking history | |
| Ever Smoker | 416 (92.86%) |
| Never Smoker | 32 (7.14%) |
| Missing | 2 |
| ECOG | |
| 0 | 134 (29.84%) |
| 1 | 267 (59.47%) |
| 2 | 48 (10.69%) |
| Missing | 1 |
| KRAS subtype | |
| KRAS_c.34G.T (G12C) | 176 (39.11%) |
| KRAS_c.35G.A (G12D) | 83 (18.44%) |
| KRAS_c.35G.T (G12V) | 80 (17.78%) |
| KRAS codon | |
| Codon 12 | 389 (86.44%) |
| Codon 13 | 32 (7.11%) |
| Codon 61 | 29 (6.44%) |
| Surgery history for lung cancer treatment | |
| Yes | 227 (52.42%) |
| No | 206 (47.58%) |
| Missing | 17 |
| Radiation treatment history for lung cancer | |
| Yes | 156 (35.14%) |
| No | 288 (64.86%) |
| Missing | 6 |
| Chemotherapy history for lung cancer | |
| Yes | 117 (50.87%) |
| No | 113 (49.13%) |
| Missing | 220 |
| Targeted therapy given | |
| Yes | 37 (9.02%) |
| No | 373 (90.98%) |
| Missing | 40 |
Data are presented as number of patients (%, percentage) or median (IQR, interquartile range).
Table 3.
Univariate association of KRAS mutation vs. wildtype with covariates in patients with lung adenocarcinomas.
| Variable |
KRAS mutation/No associated co- mutation (N= 436) |
KRAS wildtype/No associated co-mutation (N= 706) |
P |
|---|---|---|---|
| Age (years), median (IQR) | 65 (56 - 70) | 64 (56 - 70) | 0.031 |
| Male | 185 (42.43) | 339 (48.02) | 0.066 |
| White | 390 (94.2) | 595 (88.94) | 0.003 |
| Ever smoker | 403 (92.86) | 528 (75.86) | <.001 |
| ECOG | 0.470 | ||
| 0 | 132 (30.34) | 207 (29.49) | |
| 1 | 256 (58.85) | 433 (61.68) | |
| 2 | 47 (10.8) | 62 (8.83) | |
| Surgery history for lung cancer treatment | 221 (52.74) | 354 (52.29) | 0.883 |
| Radiation treatment history for lung cancer | 154 (35.81) | 271 (39.11) | 0.269 |
| Chemotherapy history for lung cancer | 111 (51.15) | 245 (61.56) | 0.013 |
Data are presented as number of patients (%) or median (IQR, interquartile range).
P-value is calculated by Wilcoxon rank-sum test for age, and chi-square test or Fisher’s exact test for categorical variables, where appropriate.
3.2. KRAS mutation subtypes
The most common nucleotide change in tumor specimens was guanine to thymidine (34_G>T or G12C) seen in 176(39.11%) patients; 35_G>A (or G12D) was present in 83(18.44%) patients. The 35_G>T (or G12V) was present in 80(17.78%) patients. The most common codons of KRAS mutations were: codon 12 (389 patients;86.44%), codon 13 (32 patients;7.11%), and codon 61 (29 patients;6.44%) (Table 1C).
Representation of never-smokers was more common in the G12D subtype than the G12V or G12C subtypes (22%vs.5%vs.1.7%, respectively;P<0.001); there was no significant difference between different mutation subtypes in term of median age, gender, race, ECOG score, incidence of co-mutations, and treatment history (Table 4). There was no association between these covariates and the main three codons of KRAS mutation (Table 5).
Table 4.
Univariate association of KRAS mutations subtypes with covariates in patients with KRAS mutant lung adenocarcinomas.
| Variable |
KRAS_c.34G.T (G12C) (N= 176) |
KRAS_c.35G.A (G12D) (N= 83) |
KRAS_c.35G.T (G12V) (N= 80) |
P |
|---|---|---|---|---|
| Age (years), median (IQR) | 64 (58 – 71) | 65 (58 – 71) | 66 (58 – 71) | 0.925 |
| Male | 74 (42.05) | 37 (44.58) | 35 (43.75) | 0.920 |
| White | 157 (95.73) | 77 (96.25) | 67 (90.54) | 0.230 |
| Ever smoker | 173 (98.3) | 65 (78.31) | 75 (94.94) | <0.001 |
| ECOG | 0.984 | |||
| 0 | 52 (29.71) | 24 (28.92) | 22 (27.5) | |
| 1 | 106 (60.57) | 50 (60.24) | 51 (63.75) | |
| 2 | 17 (9.71) | 9 (10.84) | 7 (8.75) | |
| Co-mutations | 4 (2.27) | 4 (4.82) | 1 (1.25) | 0.397 |
| Surgery history for lung cancer treatment | 89 (52.66) | 42 (52.5) | 37 (48.05) | 0.783 |
| Radiation treatment history for lung cancer | 63 (36.42) | 24 (29.27) | 28 (35.44) | 0.520 |
| Chemotherapy history for lung cancer | 43 (48.31) | 23 (67.65) | 21 (44.68) | 0.092 |
| Targeted therapy given | 9 (5.63) | 9 (12.16) | 8 (10.96) | 0.169 |
Data are presented as number of patients (%) or median (IQR, interquartile range).
P-value is calculated by Kruskal-Wallis test for age, and chi-square test or Fisher’s exact test for categorical variables, where appropriate.
Table 5.
Univariate association of KRAS mutation subtypes with covariates in KRAS mutant patients with lung adenocarcinomas.
| Variable | Codon 12 (N= 389) | Codon 13 (N= 32) | Codon 61 (N= 29) | P |
|---|---|---|---|---|
| Age (years), median (IQR) | 65 (58 – 71) | 61.5 (58 – 71) | 65 (58 – 71) | 0.285 |
| Male | 164 (42.16) | 16 (50) | 10 (34.48) | 0.471 |
| White | 346 (94.28) | 27 (87.1) | 28 (96.55) | 0.236 |
| Ever smoker | 360 (92.78) | 30 (96.77) | 26 (89.66) | 0.544 |
| ECOG | 0.105 | |||
| 0 | 109 (28.09) | 13 (40.63) | 12 (41.38) | |
| 1 | 240 (61.86) | 14 (43.75) | 13 (44.83) | |
| 2 | 39 (10.05) | 5 (15.63) | 4 (13.79) | |
| Co-mutations | 11 (2.83) | 1 (3.13) | 2 (6.9) | 0.284 |
| Surgery history for lung cancer treatment | 195 (51.86) | 14 (50) | 18 (62.07) | 0.550 |
| Radiation treatment history for lung cancer | 131 (34.11) | 15 (48.39) | 10 (34.48) | 0.277 |
| Chemotherapy history for lung cancer | 102 (51.52) | 7 (43.75) | 8 (50) | 0.834 |
| Targeted therapy given | 31 (8.78) | 3 (9.68) | 3 (11.54) | 0.805 |
Data are presented as number of patients (%) or median (IQR, interquartile range).
P-value is calculated by Kruskal-Wallis test for age, and chi-square test or Fisher’s exact test for categorical variables, where appropriate.
3.3. Other mutations associated with KRAS mutation
Of 450 patients with KRAS mutant lung adenocarcinomas, co-mutation of TP53 (48(52%) of 93patients studied) was the most frequent, followed by STK11 (17(18%) of 92patients). As mentioned earlier, the adoption of NGS occurred during the course of the study period and hence only a subset of patients were tested for TP53 and STK11. 14(3.11%) patients had a driver co-mutation or a molecular aberration (AKT1, BRAF V600E, BRAF non-V600E, ERBB2, MAP2K1, NRAS, PIK3CA, sensitizing EGFR (sEGFR), non-sensitizing EGFR (oEGFR), or ALK) (Table 1). The incidence of specific mutations associated with KRAS mutations (referred to KRAS co-mutation) among the 16 molecular aberrations checked is summarized in Table 2. 93(40%) of 232 KRAS mutant patients were tested for TP53 and 92(39.66%) of 232 were tested for STK11. The incidence of targetable co-mutations were less frequent; concurrent sEGFR mutation was found in 3(1.3%) of 232 patients. No concurrent ALK or ROS1 rearrangements were found. There was no significant difference between patients with KRAS mutant lung adenocarcinomas with and without any associated co-mutation in terms of median age, gender, race, smoking history, and ECOG score. However, patients with KRAS mutant lung adenocarcinomas with co-mutation were more likely to receive targeted therapy (30.77%vs.8.31%;P<0.001;data not shown).
Table 2.
Incidence of associated co-mutations in KRAS mutant patients with lung adenocarcinomas.
| Associated Co-mutation | Number of patients tested for a specific co- mutation |
Number of patients with a specific co-mutation (%) |
|---|---|---|
| TP53 | 93 | 48 (52%) |
| STK11 | 92 | 17 (18%) |
| ampMET | 174 | 7 (4%) |
| PIK3CA | 231 | 9 (3.9%) |
| sEGFR | 232 | 3 (1.3%) |
| BRAF V600E | 231 | 1 (0.4%) |
| NRAS | 231 | 1 (0.4%) |
| AKT1 | 207 | 0 (0%) |
| oBRAF | 231 | 0 (0%) |
| ERBB2 | 151 | 0 (0%) |
| MAP2K1 | 221 | 0 (0%) |
| oEGFR | 232 | 0 (0%) |
| ALK | 218 | 0 (0%) |
| ROS1 | 213 | 0 (0%) |
| RET | 207 | 0 (0%) |
| mutPTEN | 91 | 0 (0%) |
3.4. KRAS mutation and overall survival
The median follow-up for all study patients was 2.15 years (95%CI:2.01-2.27) with median OS of 2.15 years (95%CI:2.02-2.30) and 2-year OS rate of 53.38%(95%CI:50.50-56.18). 888(53.7%) of patients were alive at the time of the analyses. Patients with KRAS mutations (with or without co-mutation; N=450) had a shorter overall survival compared to KRAS wildtype patients (with or without co-mutation; N=1205) (HR:1.22;95%CI:1.05-1.43;P=0.011) with estimated 2-year OS rates of 49.1%(95%CI:43.6-54.3%) and 55% (95%CI:51.6-58.3%), respectively. In multivariable analysis stratified by chemotherapy history for lung cancer, there was a trend towards inferior survival for these patients with KRAS mutation compared to KRAS wildtype (HR:1.24;95%CI:0.97-1.58;P=0.081) after adjusting for gender, smoking history, performance status, number of co-mutations, and surgical intervention. In multivariable analysis stratified by chemotherapy for lung cancer, KRAS mutant patients (with no associated co-mutation) had worse survival than KRAS wildtype patients (with no associated co-mutation) after adjusting for gender, performance status, and surgical intervention (HR: 1.32; 95% CI: 1.03-1.70; P=0.028).
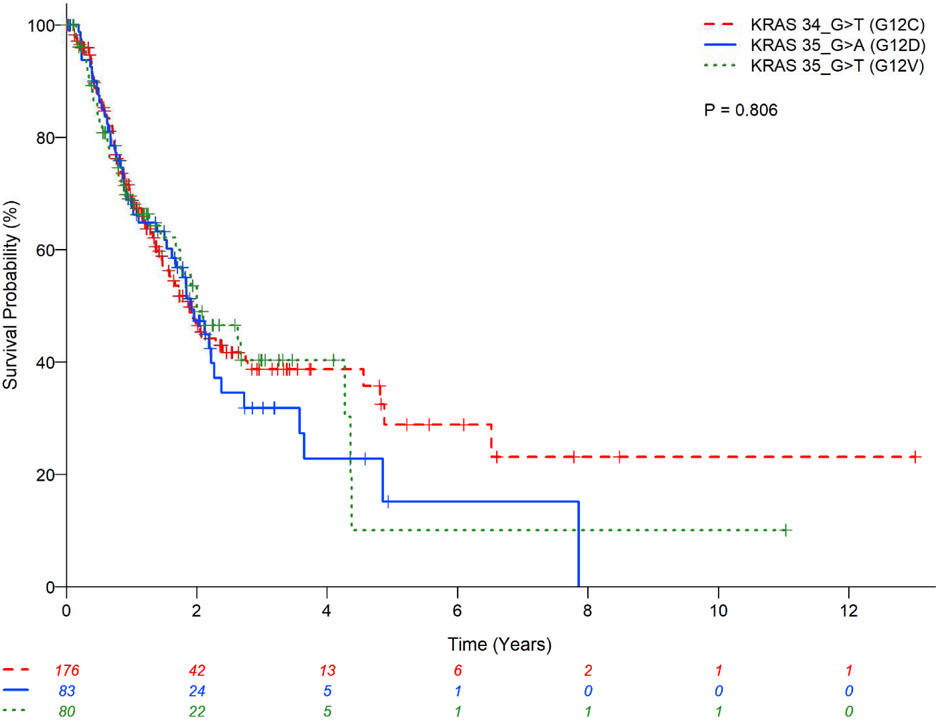
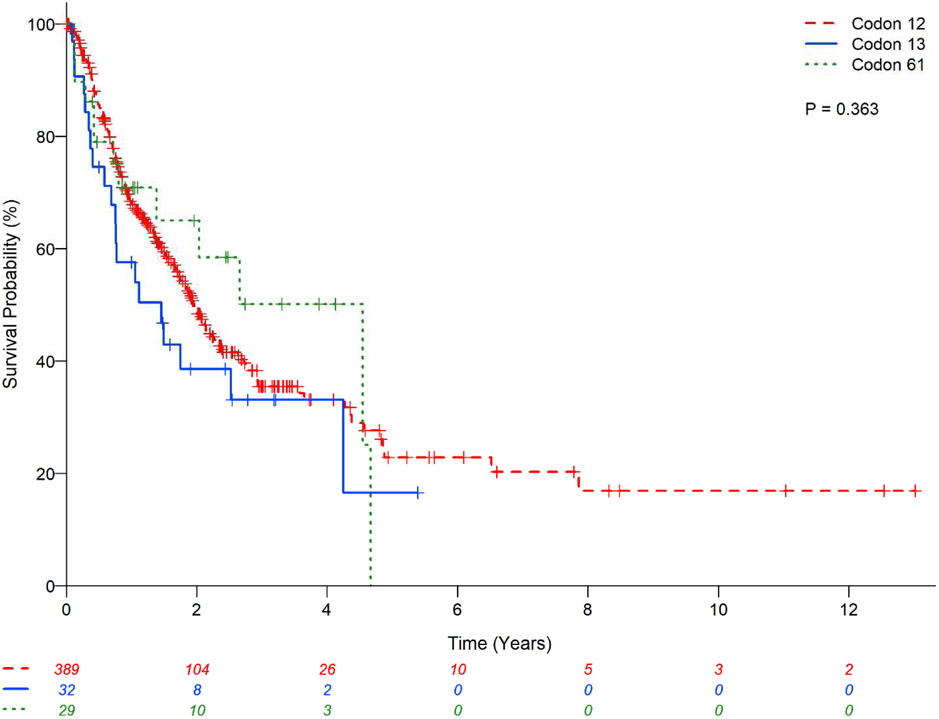
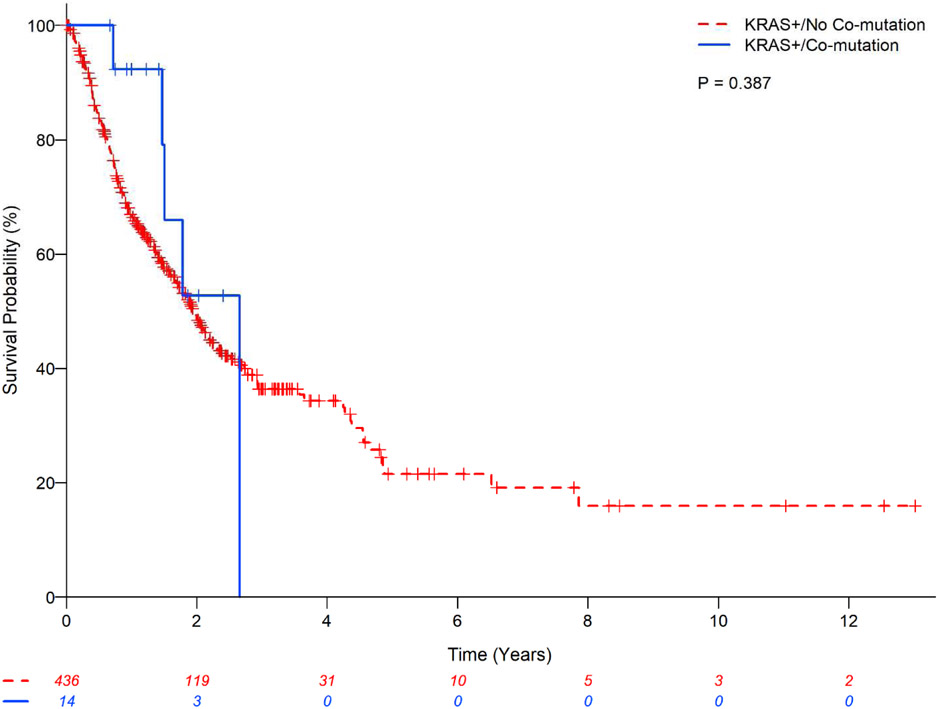
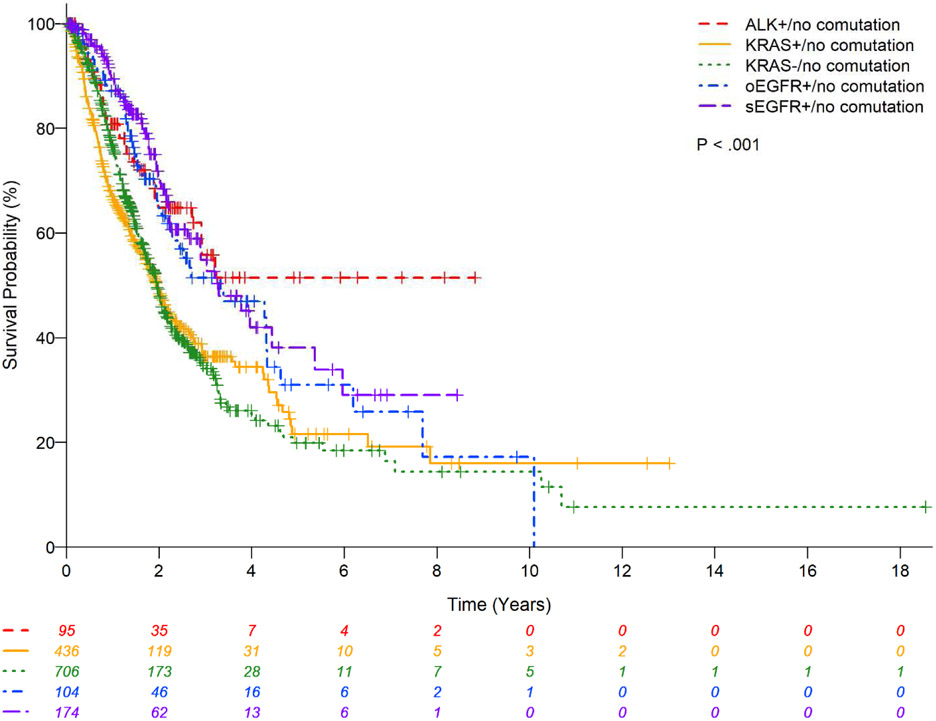
There was no statistically significant difference in OS between the main three subtypes (G12C vs. G12D vs. G12V) (P=0.81;Figure 2) and codons (12 vs. 13 vs. 61) (P=0.36;Figure 3) of KRAS mutations; codon 13 had a lower estimated 2-year OS rate of 38.6% (95%CI:21.0-55.9) and codon 61 had relatively higher 2-year OS rate of 65.0%(95%CI:42.5-80.5). The presence or absence of co-mutation did not affect OS in patients with KRAS mutant in univariate analysis (HR:0.68;95% CI:0.28-1.65;P=0.39;Table 7) with estimated 2-year OS rates of 52.75%(95%CI: 16.59-79.63) vs. 48.83%(95%CI:43.32-54.10), respectively (Figure 1). However, in multivariable analysis, the presence of any associated co-mutation had an improved OS after adjusting for gender, performance status, history of surgical resection for lung cancer treatment, and chemotherapy for lung cancer (HR:0.35;95%CI:0.13-0.97;P=0.044;Table 7). Patients with EGFR mutation or ALK rearrangement had a better OS than KRAS mutant (no co-mutations) patients (P<0.01;Figure 4) with estimated 2-year OS rates of 64.7%(95%CI:53.7-73.7) for non-sensitizing EGFR mutation (oEGFR), 70.7%(95%CI:61.5-78.0) for sensitizing EGFR mutation (sEGFR), 64.8%(95%CI:52.3-74.8) for ALK vs. 48.8%(95%CI:43.3-54.1) for KRAS mutation. Patients with sEGFR or ALK rearrangement were more likely to be younger, never smoker, with better performance status and receive targeted therapy compared to patients with KRAS mutation (no co-mutation) (Table 6).
Figure 2.
Kaplan-Meier estimates for KRAS mutant patients with lung adenocarcinomas stratified by main 3 subtypes of KRAS mutation.
Figure 3.
Kaplan-Meier estimates for KRAS mutant patients with lung adenocarcinomas stratified by codon of KRAS mutation.
Table 7.
Univariate and multivariable analyses with KRAS mutation with and without co-mutation, KRAS subtypes, KRAS codon on overall survival in Patients with KRAS mutation.
| Variable | Multivariable analysis with | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | KRAS co-mutation | KRAS subtypes | KRAS codon | ||||||
| N | Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | |
| KRAS mutation/co-mutation | |||||||||
| KRAS mutation/co-mutation | 14 | 0.68 (0.28-1.65) | 0.390 | 0.35 (0.13-0.97) | 0.044 | Not included | Not included | ||
| KRAS mutation/No co-mutation | 436 | 1 (Reference) | 1 (Reference) | ||||||
| KRAS main subtypes | 0.806* | 0.112* | |||||||
| KRAS_c.34G.T (G12C) | 176 | 0.96 (0.65-1.41) | 0.836 | Not included | 0.95 (0.54-1.68) | 0.866 | Not included | ||
| KRAS_c.35G.A (G12D) | 83 | 1.08 (0.70-1.67) | 0.720 | 1.68 (0.88-3.23) | 0.116 | ||||
| KRAS_c.35G.T (G12V) | 80 | 1 (Reference) | 1 (Reference) | ||||||
| KRAS codon | 0.367* | Not included | Not included | 0.574* | |||||
| Codon 12 | 389 | 1.12 (0.64-1.97) | 0.689 | 1.09 (0.48-2.52) | 0.833 | ||||
| Codon 13 | 32 | 1.53 (0.76-3.08) | 0.232 | 1.61 (0.55-4.74) | 0.389 | ||||
| Codon 61 | 29 | 1 (Reference) | 1 (Reference) | ||||||
| Age (Years) | 449 | 1.00 (0.98-1.01) | 0.531 | † | † | † | |||
| Gender | |||||||||
| Male | 190 | 1.29 (0.99-1.67) | 0.057 | 1.37 (0.93-2.01) | 0.109 | 1.54 (0.98-2.43) | 0.061 | 1.43 (0.97-2.11) | 0.068 |
| Female | 260 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Race | |||||||||
| Non-White | 26 | 0.78 (0.42-1.42) | 0.412 | † | † | † | |||
| White | 401 | 1 (Reference) | |||||||
| Smoking history | |||||||||
| Ever Smoker | 416 | 0.76 (0.45-1.29) | 0.313 | † | † | † | |||
| Never smoker | 32 | 1 (Reference) | |||||||
| ECOG | <0.001* | <0.001* | <0.001* | <0.001* | |||||
| 1 | 267 | 1.96 (1.42-2.72) | <0.001 | 2.46 (1.44-4.20) | <0.001 | 2.37 (1.23-4.57) | 0.010 | 2.42 (1.41-4.14) | 0.001 |
| 2 | 48 | 3.77 (2.45-5.80) | <0.001 | 4.76 (2.33-9.71) | <0.001 | 5.82 (2.58-13.14) | <0.001 | 4.77 (2.31-9.83) | <0.001 |
| 0 | 134 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Surgery history for lung cancer treatment | |||||||||
| Yes | 227 | 0.44 (0.33-0.58) | <0.001 | 0.62 (0.42-0.92) | 0.017 | † | 0.65 (0.44-0.97) | 0.035 | |
| No | 206 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||
| Radiation treatment history for lung cancer | |||||||||
| Yes | 156 | 0.67 (0.51-0.89) | 0.006 | † | † | † | |||
| No | 288 | 1 (Reference) | |||||||
| Chemotherapy history for lung cancer | |||||||||
| Yes | 117 | 0.58 (0.39-0.85) | 0.005 | 0.60 (0.40-0.90) | 0.013 | 0.64 (0.39-1.04) | 0.070 | 0.63 (0.42-0.94) | 0.024 |
| No | 113 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| Targeted therapy given | |||||||||
| Yes | 37 | 0.71 (0.44-1.14) | 0.157 | † | † | † | |||
| No | 373 | 1 (Reference) | |||||||
230 observations were used in the multivariable models with KRAS co-mutation and with KRAS codon.
170 observations were used in the multivariable mode with KRAS main subtypes.
Overall P-value for variables with more than 2 categories.
Dropped out of the final multivariable model.
Figure 1.
Kaplan-Meier estimates for KRAS mutant patients with lung adenocarcinomas with and without co-mutation.
Figure 4.
Kaplan-Meier estimates for patients with lung adenocarcinomas stratified by molecular status.
Table 6.
Comparison of covariates in patients with lung adenocarcinomas with different molecular status.
| Variable |
KRAS mutation/ no associated co-mutation (N= 436) |
sEGFR mutation/ no associated co-mutation (N= 174) |
oEGFR mutation/ no associated co-mutation (N= 104) |
ALK rearrangemen/ no associated co-mutation (N= 95) |
KRAS wildtype/ no associated co-mutation (N= 706) |
P |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 65 (56 - 70) | 61 (56 - 70) | 63 (56 - 70) | 54.5 (46 - 63) | 64 (56 - 70) | <0.001 |
| White | 390 (94.2) | 124 (79.49) | 78 (81.25) | 79 (92.94) | 595 (88.94) | <0.001 |
| Ever smoker | 403 (92.86) | 75 (43.1) | 42 (40.38) | 41 (43.16) | 528 (75.86) | <0.001 |
| ECOG | ||||||
| 0 | 132 (30.34) | 68 (39.53) | 41 (40.59) | 38 (40.43) | 207 (29.49) | 0.023 |
| 1 | 256 (58.85) | 96 (55.81) | 51 (50.5) | 49 (52.13) | 433 (61.68) | |
| 2 | 47 (10.8) | 8 (4.65) | 9 (8.91) | 7 (7.45) | 62 (8.83) | |
| Surgery history for lung cancer treatment | 221 (52.74) | 52 (31.9) | 49 (51.58) | 45 (48.39) | 354 (52.29) | <0.001 |
| Radiation treatment history for lung cancer | 154 (35.81) | 49 (28.82) | 44 (43.14) | 39 (41.49) | 271 (39.11) | 0.067 |
| Chemotherapy history for lung cancer | 111 (51.15) | 39 (41.49) | 9 (52.94) | 12 (35.29) | 245 (61.56) | <0.001 |
| Targeted therapy given | 33 (8.31) | 151 (88.82) | 70 (70.71) | 65 (69.15) | 21 (3.87) | <0.001 |
Data are presented as number of patients (%) or median (IQR, interquartile range).
P-value is calculated by Kruskal-Wallis test for age, and chi-square test or Fisher’s exact test for categorical variables, where appropriate.
3.5. Univariate association and multivariable analysis (Tables 3 and 7)
When compared to patients with KRAS wild type (no co-mutation) lung adenocarcinomas, patients with KRAS mutation (no co-mutation) lung adenocarcinomas were more likely to be older, white, smoker, and received targeted therapy (P<0.001). KRAS co-mutation vs. no co-mutation, KRAS main subtypes (G12C vs. G12D vs. G12V), and codons (12 vs. 13 vs. 61) were not associated with OS.
3.6. Co-mutation STK11
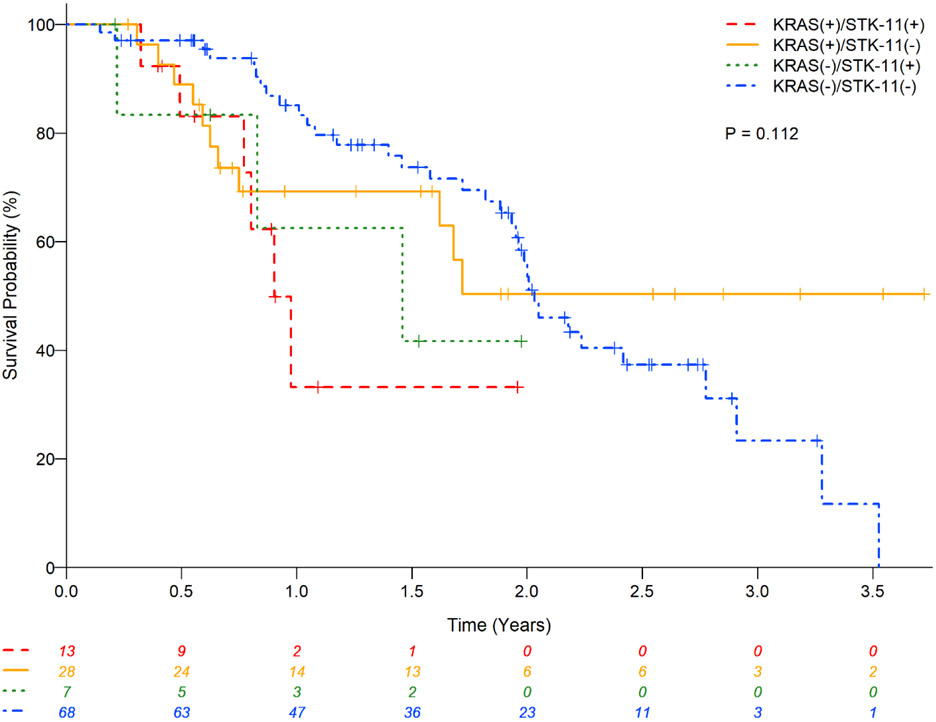
KRAS and associated STK11 mutations were reported in 17(18%) of 92 (Table 2). These patients had were younger than the remaining 75 KRAS mutant patients without associated STK11 mutation (median,IQR): 61(58-71) vs. 67(58-71) years;P=0.08); no difference in other characteristics were observed (gender,race,smoking history,performance status,prior therapy) (Table 8). Co-mutation with STK11 was associated with poor OS in univariate analyses (HR:2.66;95%CI:1.07-6.60;P=0.035). In addition, STK11 mutation worsened OS of patients with either KRAS mutation (0.9year vs. not reached) or KRAS wildtype (1.46vs.2.03years) and who did not have any other associated co-mutation (Table 9 and Figure 5).
Table 8.
Univariate Association of STK11 co-mutation vs. No co-mutation with Covariates in Patients with KRAS mutation from LCMC 2 (N=232).
| Variable |
STK11 mutation (N= 17) |
STK11 No mutation (N= 75) |
P |
|---|---|---|---|
| Age (Years) | |||
| Median (IQR) | 61 (58 - 71) | 67 (58 - 71) | 0.081 |
| Gender | |||
| Male | 9 (52.94) | 30 (40) | 0.330 |
| Female | 8 (47.06) | 45 (60) | |
| Race | |||
| Non-White | 1 (5.88) | 3 (4) | 0.322 |
| Unknown | 0 (0) | 10 (13.33) | |
| White | 16 (94.12) | 62 (82.67) | |
| Smoking history | |||
| Current Smoker | 2 (11.76) | 11 (14.86) | 1.000 |
| Former smoker | 14 (82.35) | 58 (78.38) | |
| Never smoker | 1 (5.88) | 5 (6.76) | |
| ECOG | |||
| 0 | 5 (29.41) | 12 (16) | 0.442 |
| 1 | 10 (58.82) | 51 (68) | |
| 2 | 2 (11.76) | 12 (16) | |
| Surgery history for lung cancer | |||
| Yes | 7 (41.18) | 30 (40) | 0.929 |
| No | 10 (58.82) | 45 (60) | |
| Radiation treatment history for lung cancer | |||
| Yes | 3 (17.65) | 26 (34.67) | 0.173 |
| No | 14 (82.35) | 49 (65.33) | |
| Chemotherapy history for lung cancer | |||
| Yes | 8 (47.06) | 40 (54.05) | 0.602 |
| No | 9 (52.94) | 34 (45.95) | |
| Targeted therapy given | |||
| Yes | 1 (6.67) | 3 (4.55) | 0.567 |
| No | 14 (93.33) | 63 (95.45) |
Data are presented as number of patients (column %) or median (IQR, interquartile range). P-value is calculated by Wilcoxon rank-sum test for age; and chi-square or Fisher’s exact test for categorical variables, where appropriate.
Table 9.
Univariate and multivariable analyses with presence or absence of mutation STK11 on overall survival in patients with lung adenocarcinomas.
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| N | Median OS years (95% CI) |
Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | |
| KRAS/STK11/No other comutations | 0.135* | 0.158* | ||||
| KRAS (+) / STK11 (+) | 13 | 0.9 (0.49 – n/a) | 2.66 (1.07-6.60) | 0.035 | 2.22 (0.88-5.61) | 0.093 |
| KRAS (+) / STK11 (−) | 28 | NA (0.75 – n/a) | 0.90 (0.45-1.80) | 0.762 | 0.84 (0.42-1.68) | 0.625 |
| KRAS (−) / STK11 (+) | 7 | 1.46 (0.22 – n/a) | 1.85 (0.56-6.13) | 0.312 | 2.38 (0.70-8.05) | 0.164 |
| KRAS (−) / STK11 (−) | 68 | 2.03 (1.93 - 2.78) | 1 (Reference) | 1 (Reference) | ||
Overall P-value.
116 observations were used in the multivariable model. Multivariable model was adjusted for chemotherapy history.
Figure 5.
Kaplan-Meier estimates for patients with lung adenocarcinomas stratified by KRAS and STK11 molecular status.
4. Discussion
KRAS mutation is a common event in lung adenocarcinomas; there is increasing knowledge that KRAS mutations include a heterogeneous group of patients defined by mutation subtype and presence of co-mutations. Despite being a common molecular event in lung adenocarcinomas, few studies have thoroughly analyzed the biological impact of various KRAS mutation subtypes and their impact of disease behavior and clinical outcomes. In a large series of 677 KRAS mutated patients from a single-institution study10, certain differences were noted between the mutation sub-type and patient survival; however outcomes with conventional therapies appears to be similar.
Our analysis allowed for studying KRAS mutations in a multi-institutional setting and with robust associated clinical findings. Among the three main KRAS mutation subtypes, KRAS transition mutation (35 G→A; known as KRAS G12D) was more commonly seen in never smoker patients (21.69%; P<0.001) compared to G12C and G12V subtypes. Despite our findings of KRAS mutation’s association to smoking history, our study suggest that these mutations can occur in never-smokers (7%) and these patients may have a distinct mutation subtype. Our analyses showed that never smoker lung cancer patients with KRAS mutation and no associated co-mutation (N=31) had a shorter OS compared to never smoker lung cancer patients (N=168) with KRAS wildtype and no associated co-mutation with and estimated 2-year OS rates of 43.3%(21.4%,63.5%) vs. 61.42%(51.5%,69.9%) (P=0.005), respectively.
We noted that KRAS mutation with only STK11 tumor suppressor loss had a tendency to occur in younger patients (median age, 61years). These patients had the worst overall survival (0.9year) compared to patients with KRAS mutation without STK11 or other co-mutation. In addition, we were able to define the impact of baseline patient characteristics with various KRAS mutation sub-types and the overall outcome. These findings add to the growing knowledge about the differences between various KRAS mutation sub-types and the presence of co-mutations.
We observed that KRAS mutation and sEGFR were not entirely mutually exclusive: 3(4.11%) of 73 patients had both mutations. In a prior study by Yu et at10, none of the 677 patients with KRAS mutant lung cancers had a concurrent EGFR mutation. KRAS mutations have been linked to resistance to anti-EGFR therapy; therefore, our observation could represent either an acquired resistance through KRAS or a de novo phenomenon. Our study showed that KRAS mutation and ALK rearrangements in lung adenocarcinomas were mutually exclusive.
There are limitations to our analysis. First, the molecular analysis was limited to testing for 10 mutations in LCMC1 and 16 mutations in LCMC2. In addition, some of the patients did not have all these mutations tested probably because of lack of tissue. Second, molecular testing was not uniform among different sites. In LCMC1, FISH technique was used. In LCMC2, most sites used NGS but with different technologies which can lead to different target and different results, particularly for non-hotspot mutations (as are more common in tumor suppressor genes like TP53 and STK11). Third, LCMC1 and LCMC2 findings were not homogeneous: EGFR and ALK were seen less in LCMC2 than LCMC1 probably because patients were treated in the community with erlotinib or crizotinib instead of being referred to the study. We were limited to make conclusions regarding rare subsets of KRAS mutation and duration of therapy.
LCMC did not test for mutations in KEAP1/NFE2L2. Arbour et al29 found that KRAS and KEAP1/NFE2L2 co-mutations were associated with shorter overall survival and duration of response. This further emphasizes the notion that KRAS mutant NSCLC represents a heterogeneous group of patients with co-mutations playing a key role in the biological behavior of the cancer.
Presently, targeted therapy options for patients with KRAS mutation are being explored in first-in-human clinical trials30-32. Systemic chemotherapy remains as the main treatment modality. The combination of docetaxel and MEK inhibitor was associated with promising outcomes for these patients. Notably, the objective response rate was higher for patients with KRAS G12C and G12V mutation in this trial19. Though this regimen was not developed further due to failure to confirm this observation in phase 3 trial, the implications of varying sensitivity to MEK inhibition based on mutation sub-type provide an important lesson for future trial design to not group KRAS mutations as one entity.
In recent years, immunotherapy has emerged as an effective treatment approach for NSCLC. Smokers and patients with high tumor mutation burden are more likely to respond to immune checkpoint inhibitors (ICI); it is notable that these characteristics enrich for KRAS mutations. Conversely, never-smokers and patients with EGFR and ALK gene abnormalities are less likely to respond to ICI. Presently, there is no firm evidence to indicate varying clinical outcomes with ICI based on the presence or absence of KRAS mutation. However, patients with KRAS and STK11 co-mutation do not derive durable clinical benefit with ICI33. This has been attributed to a ‘cold’ immune microenvironment in patients harboring the co-mutation. The LCMC is moving forward with ICI as neoadjuvant and adjuvant treatment with surgical resection34. Defining the role of KRAS mutation and subtypes, drug response phenotypes and effect on immune system are important for designing future trials in this heterogeneous population.
We are expecting adoption of NGS and a shift away from targeted testing for treatable molecular abnormalities with the recent FDA approval of NGS use for molecular testing. Consequently, the impact of KRAS mutation sub-types on disease biology and outcomes with various standard treatment approaches will become increasingly evident. ICI and systemic chemotherapy use will remain the mainstay of treatment of patients with KRAS mutations while research for effective novel and combined targeted approaches continues35-37.
Acknowledgements:
- Research reported in this publication was supported in part by the Winship Research Informatics Shared Resource, a core supported by the Winship Cancer Institute of Emory University
- Supported by NIH NCI [Grant 1RC2CA148394-010], [Grant 1U10CA180950-01], and Free to Breathe, Madison, WI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Pillai reports grants from Bristol Myers Squibb, grants and other from AstraZeneca, grants from Genentech, grants from Jannsen, grants from Bavarian Nordic, outside the submitted work.
Dr. Owonikoko reports grants from Pfizer, grants from Regeneron/Sanofi, grants from BMS, grants from AstraZeneca, grants from G1 Therapeutics, grants from Novartis, grants from Amgen, grants from AbbVie, grants from Corvus, grants from Adaptimmune, during the conduct of the study; other from AbbVie, other from Amgen, other from AstraZeneca, other from BMS, other from Lilly/Armo, other from Pharmamar, other from Boehringer Ingelheim, other from EMD Serono, other from Roche/Genentech, other from Cambium Oncology, outside the submitted work.
Outside the submitted work, Dr. Kris reports personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Regeneron. Mark G Kris has received honoraria for participation in education programs from WebMD, OncLive, Physicians Education Resources, Prime Oncology, Intellispher, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, AstraZeneca, and Research to Practice. Mark G Kris is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from The Lung Cancer Research Foundation, Genentech Roche, and PUMA Biotechnology for trials conducted by Mark G Kris. Memorial Sloan Kettering has a collaboration for the development of Watson for Oncology with IBM and receives royalties from IBM for this activity.
Dr. Johnson reports grants from Novartis, grants from Toshiba, outside the submitted work; In addition, Dr. Johnson has a patent EGFR Mutation Testing with royalties paid to Dr. Johnson.
Dr. Lynette reports fees from Foghorn Therapeutics, personal fees from AstraZeneca Pharmaceuticals, personal fees from LOXO Oncology, outside the submitted work.
Dr. Aisner reports personal fees from Bayer Oncology, personal fees from Genentech, personal fees from AbbVie, personal fees from Bristol Myers Squibb, outside the submitted work.
Dr. Bunn reports grants from Lung Cancer Mutation Consortium, during the conduct of the study.
Dr. Ramalingam reports grants from Merck, grants from Tesaro, during the conduct of the study; personal fees from Amgen, grants from AstraZeneca, personal fees from Abbvie, personal fees from BMS, personal fees from Lilly, personal fees from Genentech, personal fees from Takeda, personal fees from Loxo, outside the submitted work.
References.
- 1.Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 2016;48:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riely GJ, Ladanyi M. KRAS mutations: an old oncogene becomes a new predictive biomarker. J Mol Diagn 2008;10:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito M, Shiraishi K, Kunitoh H, et al. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci 2016;107:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201–205. [DOI] [PubMed] [Google Scholar]
- 9.Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med 1990;323:561–565. [DOI] [PubMed] [Google Scholar]
- 10.Yu HA, Sima CS, Shen R, et al. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J Thorac Oncol 2015;10:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer 2013;119:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900–5909. [DOI] [PubMed] [Google Scholar]
- 14.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res 2007;13:2890–2896. [DOI] [PubMed] [Google Scholar]
- 15.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744–752. [DOI] [PubMed] [Google Scholar]
- 16.Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst 2012;104:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 2007;6:2209–2219. [DOI] [PubMed] [Google Scholar]
- 18.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38–47. [DOI] [PubMed] [Google Scholar]
- 19.Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA 2017;317:1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells. Cancer Immunol Res 2016;4:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aisner DL, Sholl LM, Berry LD, et al. The Impact of Smoking and TP53 Mutations in Lung Adenocarcinoma Patients with Targetable Mutations-The Lung Cancer Mutation Consortium (LCMC2). Clin Cancer Res 2018;24:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Network NCC. Non-Small Cell Lung Cancer. 2018.
- 25.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- 26.Cox DR. Regression Models and Life Tables. J Royal Stat Society 1972;34:187–220. [Google Scholar]
- 27.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 28.Yamashita T, Yamashita K, Kamimura RA Stepwise AIC Method for Variable Selection in Linear Regression. Communications In Statistics - Theory And Methods 2007;36:2395–2403. [Google Scholar]
- 29.Arbour KC, Jordan E, Kim HR, et al. Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2018;24:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MRTX849 in Patients With Cancer Having a KRAS G12C Mutation. Available at https://clinicaltrials.gov/ct2/show/NCT03785249?term=NCT03785249&rank=1.
- 31.Dose Escalation of RMC-4630 Monotherapy in Relapsed/Refractory Solid Tumors. Available at https://clinicaltrials.gov/ct2/show/NCT03634982?term=NCT03634982&rank=1.
- 32.A Phase 1, Study Evaluating the Safety, Tolerability, PK, and Efficacy of AMG 510 in Subjects With Solid Tumors With a Specific KRAS Mutation. Available at https://clinicaltrials.gov/ct2/show/NCT03600883?term=NCT03600883&rank=1.
- 33.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaft JE, Forde PM, Smith KN, et al. Neoadjuvant nivolumab in early-stage, resectable non-small cell lung cancers. Journal of Clinical Oncology 2017;35. [Google Scholar]
- 35.Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503:548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchado E, Weissmueller S, Morris JPt, et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature 2016;534:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Hu K, Guo J, et al. Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat Commun 2016;7:11363. [DOI] [PMC free article] [PubMed] [Google Scholar]