To the Editor,
Rapid and reliable detection of SARS-CoV-2 is instrumental to control and ultimately contain the COVID-19 pandemic. Nucleic acid based testing (NAT) remains the gold standard for SARS-CoV-2 detection [1]. However, NAT is usually restricted to diagnostic laboratories with specialized equipment and professional personnel. This limits their application for point of care testing (POCT).
Recently, rapid antigen tests (RAT) entered the market for POCT use and proved their principle in different studies [2], [3], [4]. We aimed to evaluate the clinical performance of the Sofia antigen test in comparison to SARS-CoV-2 NAT in a real-life setting in a large tertiary care center.
We prospectively enrolled all patients admitted to the emergency department at Bielefeld Hospital, a tertiary care hospital with 1300 beds in northern Germany from 1 November to 30 November 2020. Trained medical personnel took two nasopharyngeal swab samples per patient. One sample was immediately tested on site using the Sofia antigen fluorescent immunoassay (FIA) (Quidel, Kornwestheim, Germany) according to the manufacturer's instructions. The Sofia FIA is a sandwich-based lateral flow assay and provides automated and user-independent read out using the Sofia 2 FIA analyzer. The second sample was tested using NAT in two different laboratories. NAT assays included the VIASURE SARS-CoV-2 RT-PCR (CerTest Biotec S.L.), the RIDA®GENE SARS-CoV-2 (r-biopharm, Darmstadt, Germany) and Xpert®Xpress SARS-CoV-2 (Cepheid, Frankfurt, Germany). Ct-values were recorded for each positive NAT sample. As a limitation, we did not compare Ct-values with the FIA analyzer result individually. Ethical approval was obtained (Az 2020–870-f-S; AeKWL/WWU Muenster).
A total of 1404 patients were enrolled, median age was 68 years (range: 4–102 years), and 767 (54.6%) were females. Of these, 91/1404 (6.5%) tested positive for SARS-CoV-2 using NAT. We retrieved clinical data for all 91 PCR-positive cases from the hospital based information system. Of these, 65/91 (71%) showed at least one of four COVID-19 associated symptoms, i. e. cough, dyspnea, loss of smell/taste, and/or fever. The cycle threshold (Ct) values (E-gene or N-gene) of the 91 NAT-positive samples had a median of 26.2 (Ct range: 13–41).
The antigen FIA detected 52/91 NAT-positive samples, corresponding to an overall sensitivity of 57.1% (Table 1 ).
Table 1.
Comparison of the clinical performance of the Sofia antigen FIA with NAT.
| Results Sofia antigen assay |
|||||
|---|---|---|---|---|---|
| Positive, n= | Negative, n= | Sensitivity (%) | Specificity (%) | ||
| Results NAT | Positive (n = 91) | 52 | 39 | 57.1 | |
| Negative (n = 1300) | 9 | 1291 | 99.3 | ||
Nine samples showed a positive antigen FIA result, but were negative using NAT (8 of the 9 patients were re-tested with NAT within 2 days and remained NAT negative, one was not re-tested). This indicates a specificity of 99.3%. Thirteen (0.9%) antigen FIA showed invalid results. Of note, this low rate compares very well to previous findings [5].
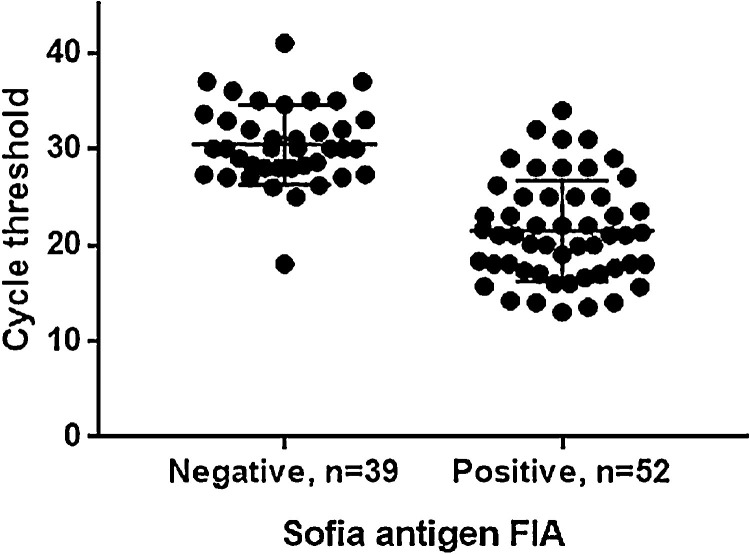
Positive and negative predictive value and diagnostic test accuracy were 85.2%, 97.1% and 96.5%, respectively. The mean Ct value in concordant NAT/antigen FIA positive samples was significantly lower than in discordant NAT positive/antigen FIA negative samples (Fig. 1 ).
Fig. 1.
Scatter dot plot of threshold (Ct) values for Sofia antigen FIA negative and positive results in 91 NAT positive samples.
To appreciate the performance in samples with different viral loads a delimitation was made along a Ct value of ≤25, which likely indicates the threshold to isolate infectious virus. The detection rate of the antigen FIA for Ct values ≤25 was 95.3% (41/43 NAT positive samples).
In conclusion, we demonstrated an overall low sensitivity of 57.1% of the Sofia antigen POCT in a real-life setting. Sensitivity increased to 95% in samples with Ct values ≤25. This matches very well with previous data [4, 6]. Technically, the FIA analyzer allows user-independent interpretation of test results and electronic connectivity to the laboratory-information system, which also facilitates documentation of test results. However, the use of technical equipment is associated with slightly higher costs including e.g. maintenance. Our findings support the notion that RAT are able to identify infectious patients, who can potentially transmit virus to others. Importantly, their applicability for POCT and inexpensiveness may prove beneficial for frequent on-site testing in settings beyond hospitals like long-term care facilities and schools.
Funding
This work was in part supported by the German Federal Ministry of Education and Research (CEO-sys; TF-1/Z24; Fkz. 01KX2021)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We kindly acknowledge expert literature search by Alexey Fomenko.
References
- 1.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linares M., Perez-Tanoira R., Carrero A., Romanyk J., Perez-Garcia F., Gomez-Herruz P., et al. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindner A.K., Nikolai O., Kausch F., Wintel M., Hommes F., Gertler M., et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2020 doi: 10.1183/13993003.03961-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., et al. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mak G.C., Lau S.S., Wong K.K., Chow N.L., Lau C.S., Lam E.T., et al. Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pray I.W., Ford L., Cole D., Lee C., Bigouette J.P., Abedi G.R., et al. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]