Abstract
Background:
Kangaroo Mother Care initiated after stabilization reduces mortality in infants with birthweight <2.0 kg, but the majority of deaths occur before stabilization. The safety and efficacy of Kangaroo Mother Care initiated soon after birth is uncertain.
Methods:
We conducted a randomized controlled trial in five hospitals in Ghana, India, Malawi, Nigeria, and Tanzania. Infants with birth weight between 1.0 and <1.8 kg were randomly assigned to immediate Kangaroo Mother Care (intervention) or to conventional care until stabilization, and Kangaroo Mother Care thereafter (control). The primary outcomes were deaths in the neonatal period (first 28 days of life) and in the first 72 hours of life. The study was stopped early on the recommendation of the DSMB owing to reduced neonatal mortality with the intervention.
Results:
A total of 3211 infants and their mothers were randomly allocated (1609 intervention, 1602 control group). The median daily duration of skin-to-skin contact in neonatal intensive care units was 16.9 hours (IQR 13.0–19.7) in the intervention and 1.5 hours (IQR 0.3–3.3) in control group. Neonatal death occurred in 191 infants (12.0%) and 249 (15.7%) infants, respectively (RR 0.75; 95% CI 0.64–0.89; p=0.001);death in the first 72 hours of life occurred in 74 infants (4.6%) and 92 infants (5.8%), respectively (RR 0.77, 95% CI 0.58–1.04; p=0.09).
Conclusion:
In infants with birthweight between 1.0 and <1.8 kg, immediate Kangaroo Mother Care (versus conventional care) resulted in a significant reduction in neonatal mortality, but not in mortality within the first 72 hours.
INTRODUCTION
Low birth weight (LBW) infants, born preterm and/or small for gestational age, constitute about 15% of neonates, but account for 70% of all neonatal deaths. Reducing deaths in LBW infants, particularly in low- and middle- income countries (LMICs) in Asia and Sub-Saharan Africa, is therefore key to the achievement of the Sustainable Development Goal target of reducing neonatal mortality to <12/1000 live births in each country by 2030.1–3
Kangaroo Mother Care, defined as continuous skin-to-skin contact of the baby with the mother’s chest and exclusive breastmilk feeding, is one of the most effective interventions for preventing mortality of LBW infants.4 World Health Organization (WHO) 5 guidelines currently recommend initiation of short intermittent Kangaroo Mother Care sessions when the infant’s condition begins to stabilize, and continuous Kangaroo Mother Care when fully stable. A Cochrane review reported a 40% reduction in mortality in LBW infants given Kangaroo Mother Care after stabilization compared to conventional care in hospitals (3.2% versus 5.3%; risk ratio (RR) 0.60, 95% confidence interval (CI) 0.39 to 0.92; eight trials, 1736 infants).6 This review also showed fewer infections, higher exclusive breastfeeding and better weight gain in infants who received Kangaroo Mother Care. In studies included in the review, the mean age at randomization (when infants were considered stable) ranged from 10 hours to 24.5 days of life. About 45% of neonatal deaths occur within 24 hours of birth and 80% within the first week of life; 7 thus the majority of deaths among LBW infants occur before Kangaroo Mother Care can be initiated.
Two randomized controlled trials (RCTs) have evaluated the effect of initiating Kangaroo Mother Care immediately after birth on physiological stabilization. In South Africa 8 and Vietnam9, skin-to-skin contact started soon after birth in LBW infants resulted in earlier stabilization than conventional care.
There is a critical knowledge gap regarding the effect of initiating continuous Kangaroo Mother Care soon after birth before stabilization on mortality in LBW infants. We conducted this large RCT to evaluate the safety and efficacy of continuous Kangaroo Mother Care initiated immediately after birth in infants with a birthweight of 1.0 to <1.8 kg.
METHODS
The details of the study methods have been published previously and are briefly summarized here.10
Study design and participants
This multi-center, non-blinded RCT was undertaken in five tertiary-level hospitals in Ghana, India, Malawi, Nigeria and Tanzania. All liveborn infants in the participating hospitals, with birthweight between 1.0 to <1.8kg regardless of their gestational age, mode of delivery or singleton/twin status, were eligible for inclusion. Mother-infant(s) pairs were excluded if the mother was <15 years old, unable or unwilling to provide consent, had triplets or more, was sick and unlikely to be able to provide Kangaroo Mother Care within the first 3 days after birth, could not be enrolled within 2 hours of childbirth or resided outside the study area. Infants who were unable to breathe spontaneously by one hour of age or had a major congenital malformation were also excluded.
This trial was approved by Ethics Review Committees at WHO and at each site. The study was overseen by a steering committee and a Data and Safety Monitoring Board (DSMB). RB, SR, SY and NM vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Study procedures
Three independent teams, trained in study standard operating procedures, were responsible for (1) screening and enrolment (2) Kangaroo Mother Care support and (3) outcome measurement at each site.
Pre-screening of all pregnant women admitted for childbirth was conducted to identify women at high risk of delivering a LBW infant and consent for study participation was sought. All infants born in the hospital were weighed and screened for eligibility. If the mother and infant were eligible, consent was confirmed if it had already been obtained before birth. If consent could not be obtained before birth, it was obtained after birth. At enrolment, mothers were asked to identify one or two adult women who could act as their surrogates for providing Kangaroo Mother Care; only women are permitted to stay in postnatal areas in all study hospitals.
Randomization was performed using a computer-generated block list, with variable block size, stratified by site and birthweight 1.0 to <1.5kg and 1.5 to <1.8kg. The random allocation was sealed in serially numbered, opaque envelopes prepared at the WHO and delivered to the sites. A research assistant conducted randomization by opening the next numbered envelope. Twins were allocated to the same group. The nature of the intervention prevented blinding, but outcome assessment was done by an independent team not involved in intervention delivery.
System changes in obstetric and neonatal care as well as structural changes to the neonatal intensive care unit (NICU) were necessary. Mother-NICU, which included mothers’ beds and reclining chairs, were built or converted from an existing NICU. All equipment, staff and care provision in the Mother-NICU remained the same as the NICU. The infant was secured firmly to the mother’s chest with a binder that ensured a patent airway.11 All care to the mother and infant were provided while in skin-to-skin contact if possible, all interruptions were documented. Obstetricians supervised essential postpartum care provided to mothers in the Mother-NICU.
Infants allocated to control group were transferred to the NICU without the mother, following the standard care practices. The mother provided expressed breast milk, and brief sessions of Kangaroo Mother Care when the infant started to recover and was at least 24 hours old.
Hospital staff provided care for all enrolled infants according to the WHO minimum care package for small infants.12 In both intervention and control groups, once clinically stable based on pre-specified criteria10 for 24 hours, the infant was shifted from Mother-NICU or NICU to Kangaroo Mother Care ward where continuous Kangaroo Mother Care was provided until discharge.
Outcomes and their measurement
The primary outcomes were mortality from enrolment to 28 days of age and mortality from enrolment to 72 hours of age. Secondary outcomes included hypothermia (any axillary temperature < 36°C), hypoglycemia (any blood glucose level < 45 mg/dl, measured when clinically indicated), suspected sepsis, time to clinical stabilization, fully breastfed (only by suckling) at the time of discharge, exclusive breastfeeding at the end of neonatal period, maternal satisfaction with care and maternal depression (supplementary Table S1).10 Additionally, mortality from birth to 72 hours in non-enrolled infants 1.0 to <1.8 kg was documented. The only serious adverse event assessed according to the protocol was death. Outcome data were collected using identical methods and procedures for all enrolled infants. Clinical monitoring was done every 6 hours for all infants while they were in Mother-NICU or NICU. Information on duration of skin-to-skin contact and duration of hospital stay was collected by research assistants. A home visit was performed on day 29 for data on survival, breastfeeding and maternal depression.
Statistical analysis
We estimated that 4200 infants were needed to detect a 20% relative mortality reduction at 28 days (16.8% mortality in intervention group compared with 21.1% in control group), with 95% confidence level and 90% power and 10% loss to follow-up. The DSMB conducted interim analyses at 50% and 75% enrolment. After the second interim analysis, the DSMB recommended stopping enrolment in the trial because of a clear benefit in neonatal survival. (See supplementary appendix.)
Intention-to-treat analyses were performed for primary and secondary outcomes.10 Risk ratios and 95% CI were calculated for the outcomes. Adjusted risk ratios were estimated using log-binomial regression controlled for clustering due to multiple births and other important baseline characteristics which could be potential confounders. Hazard ratios were calculated using multivariable Cox survival analysis to compare time to clinical stabilization between groups. We used marginal mean imputation for missing values in continuous covariates and the most frequent response to impute categorical variables. No imputation was made for the primary outcomes.
Pre-specified subgroup analyses were performed to explore modification of effect of immediate Kangaroo Mother Care on primary outcomes by birthweight (1.0 to <1.2kg, 1.2 to <1.5 kg, 1.5 to <1.8 kg), gestational age (<31, 31 to <34, 34 to <37, ≥37 weeks), mode of delivery (vaginal birth, Caesarean section), singleton/twin gestation, and size for gestational age (small for gestational age, not small for gestational age).10 Subgroup analyses by site was conducted post-hoc. In the intervention group, we examined the primary outcomes in subgroups by compliance to Kangaroo Mother Care (skin-to-skin contact for ≥20 hours, 10–19 hours, and <10 hours per day). To address reverse causality in this analysis, we excluded infants with any sign of severe illness in the first 6 hours of life. Causes of death were assigned by investigators based on clinical information for hospital deaths and by verbal autopsy for deaths at home after discharge.
Post hoc analyses were conducted to explore the effect of the intervention on breastfeeding during hospital stay, including proportion of newborns by group who had initiated breastmilk feeds within 24 hours, were put to breast in the first 72 hours, reached full breastmilk feeds within 7 days, and were discharged on exclusive breastmilk feeding.
RESULTS
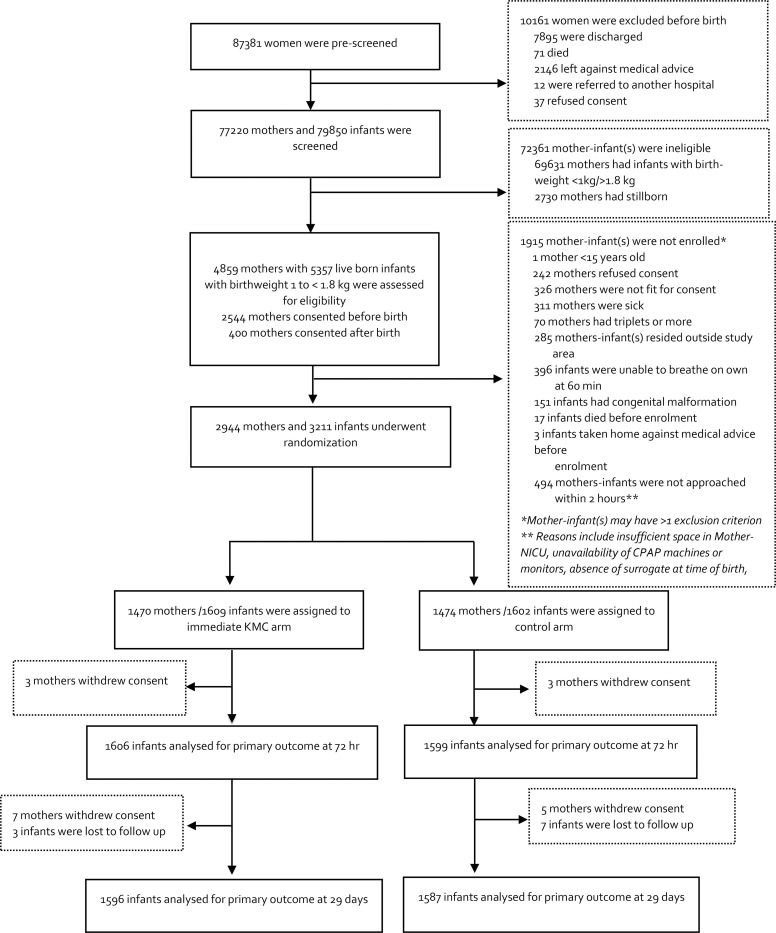
A total of 87,381 pregnant women were pre-screened and 79,850 infants were screened for eligibility between 30 November 2017 and 20 January 2020, of which 5357 infants (from 4859 mothers) met the weight criteria for enrolment. Of them, 3211 infants (2944 mothers) were randomly allocated - 1609 infants (1470 mothers) to the intervention group and 1602 infants (1474 mothers) to the control group (Figure 1).
Figure 1.
Participants flowchart
Table 1 and Table S2 show the baseline characteristics of randomized infants, their mothers and their families. Socio-demographic, newborn and maternal characteristics were similar in both groups. The mean gestation was 32.6 weeks and mean birthweight was 1.5 kg in both groups. Only 0.3% randomized infants had missing observations for covariates, except family income which was missing for 8%.
Table 1.
Baseline characteristics of randomized infants, mothers and households
| Immediate Kangaroo Mother Care | Control | |
|---|---|---|
| Infant’s characteristics # | N=1609 | N=1602 |
| Age at randomization in minutes (median, IQR) | 35 (20,55) | 33 (20,54) |
| Birth weight in kg, mean (SD) | 1.5 (0.2) | 1.5 (0.2) |
| Gestational age at birth, mean (SD)*¥ | 32.6 (3.0) | 32.6 (2.8) |
| Male, n (%) | 752 (46.7) | 748 (46.7) |
| Infants born as twin, n (%) | 430 (26.7) | 430 (26.8) |
| Delivery by C-section n (%) | 559 (34.7) | 614 (38.3) |
| Site, n (%) | ||
| Ghana | 205 (12.7) | 205 (12.8) |
| India | 695 (43.2) | 682 (42.6) |
| Malawi | 217 (13.5) | 222 (13.9) |
| Nigeria | 108 (6.7) | 107 (6.7) |
| Tanzania | 384 (23.9) | 386 (24.1) |
| Mother and household’s characteristics # | N=1470 | N=1474 |
| Mother’s age in years, mean (SD) | 26.7 (5.8) | 26.7 (5.8) |
| Mother's years of schooling, median (IQR) § | 10 (7,12) | 10 (7,12) |
| Family income in US dollars, median (IQR) | 168 (110,285) | 176 (110,280) |
| Piped water as main source of drinking water,n (%) | 934 (63.5) | 953 (64.7) |
| Households with a toilet in the house, n (%) § | 1288 (87.9) | 1343 (91.3) |
There were 534 infants (from 267 mothers) who were born from a multiple pregnancy and both were eligible and enrolled (278 infants in the intervention and 256 infants in the control).
In addition, there were 325 mothers with multiple pregnancies in whom only one of the infants was eligible and the other one was ineligible (152 infants in the intervention group and 173 in the control).
Gestational age based on ultrasound in first or second trimester, and if not available then based on LMP, and if both USG and LMP not available, then based on Ballard score (assessing measures of maturity on examination)21
Gestational age at birth missing for 27 infants in intervention and 18 infants in control group
2 households in intervention and 2 in control group have missing data on mother’s education
5 households in intervention and 3 in control group have missing data on availability of toilet Additional baseline characteristics are provided in Table S1
The median age of initiation of skin-to-skin contact was 1.3 hours (IQR 0.8–2.7) in intervention group, and 53.6 hours (IQR 33.8 – 101.4) in control group. The duration of NICU stay was similar in intervention and control groups (median 6.4 days in both groups). During NICU stay, median daily duration of skin-to-skin contact was 16.9 hours (and 1.5 hours, respectively. The daily duration on each day in the first two weeks is given in Table S3. The main reasons for not being in skin-to-skin contact in the intervention group were medical procedures, infant care and routine activities of mother. The median daily duration of skin-to-skin contact in the Kangaroo Mother Care ward was similar in both groups (20.2 hours vs 19.0 hours) (Table 2).
Table 2.
Initiation and duration of skin -to-skin contact in randomized infants
| Immediate Kangaroo Mother Care N=1609 | Control N=1602 | |
|---|---|---|
| Time to initiation of skin-to-skin contact in hours* median (IQR) | 1.3 (0.8–2.7) | 53.6 (33.8–101.4) |
| Time to initiation of skin-to-skin contact by category, n (%) | ||
| <2 hours | 1098 (68.2%) | 4 (0.2%) |
| 2 to ≤6 hours | 306 (19.0%) | 14 (0.9%) |
| 6 to ≤12 hours | 94 (5.8%) | 14 (0.9%) |
| 12 to ≤24 hours | 62 (3.9%) | 74 (4.6%) |
| 24 to ≤168 hours | 32 (2.0%) | 1176 (73.4%) |
| >168 hours to end of neonatal period | 1 (0.1%) | 142 (8.9%) |
| Never initiated | 16 (1.0%) | 178 (11.1%) |
| Skin-to-skin contact while in NICU, hours per day, median (IQR) | 1609 | 1602 |
| Overall | 16.9 (13.0–19.7) | 1.5 (0.3–3.3) |
| With mother | 12.3 (6.8–16.5) | 1.5 (0.2–3.2) |
| With surrogate | 2.3 (0.1–6.5) | 0 (0–0) |
| Skin-to-skin contact while in Kangaroo Mother Care ward, hours per day, median (IQR) | 1300 | 1224 |
| Overall | 20.2 (18.6–21.3) | 19.0 (16.3–20.4) |
| With mother | 19.4 (14.8–20.6) | 18.0 (14.1–19.9) |
| With surrogate | 0 (0–0.85) | 0 (0–0) |
If the infant never initiated skin-to-skin contact and: (i) died: censored at the time of death (ii) taken home against medical advice or refused consent: censored at time of leaving the hospital or refusing consent, respectively; (iii) was discharged: censored at time of discharge; (iv) was still in hospital at the end of the neonatal period: censored at day 28.
From enrolment to 28 days of age, 191 infants (12.0%) in the intervention group and 249 (15.7%) in the control group died (RR 0.75, 95% CI 0.64–0.89; p=0.001). The number needed to treat was 27 (95% CI 17–77) to prevent one death. From enrolment to 72 hours of age, 74 infants (4.6%) in intervention and 92 infants (5.8%) in control group died (RR 0.77, 95% CI 0.58–1.04; p=0.09) (Table 3).
Table 3.
Primary and secondary outcomes in randomized infants
| Immediate Kangaroo Mother Care (1609 assigned) | Control (1602 assigned) | Adjusted RR (95%CI)* | P | |
|---|---|---|---|---|
| Primary outcomes | ||||
| Death between enrolment and 28 days of age, n (%) | 191/1596 (12.0%) | 249/1587 (15.7%) | 0.75 (0.64–0.89) | 0.001 |
| Death between enrolment and 72hr of age, n (%) | 74/1606 (4.6%) | 92/1599 (5.8%) | 0.77 (0.58–1.04) | 0.09 |
| Secondary outcomes † | ||||
| Exclusive breastfeeding at the end of neonatal period, n (%) | 1208/1401 (86.2%) | 1140/1336 (85.3%) | 1.01 (0.98–1.05) | |
| Fully breastfed (only by suckling) at hospital discharge, n (%) | 62/1435 (4.3%) | 55/1376 (4.0%) | 1.06 (0.73–1.53) | |
| Hypothermia, n (%)1 | 90/1609 (5.6%) | 133/1602 (8.3%) | 0.65 (0.51–0.83) | |
| Time to clinical stabilization in hr, median (IQR) 2 | 73.8 (26.8;138.5) (n=1609) | 74.8 (25.3;140.6) (n=1602) | 0.98 (0.90; 1.07) § | |
| Suspected sepsis, n (%) 3 | 361/1575 (22.9%) | 434/1561 (27.8%) | 0.82 (0.73–0.93) | |
| Hypoglycemia at any time between 0–36h of age, n (%)4 | 82/799 (10.3%) | 66/651 (10.1%) | 1.15 (0.85–1.56) | |
| Duration of hospital stay in days, mean (SD)5 | 14.9 (0.2) (n=1609) | 15.2 (0.2) (n=1602) | 1.07 (0.99;1.16) § | |
| Maternal satisfaction with health care in the hospital, mean (SD)6 | 9.2 (1.0) (n=1282) | 9.1 (1.2) (n=1233) | 0.11 (0.03–0.19) ¥ | |
| Maternal depression, n (%)7 | 2/1276 (0.2%) | 7/1231 (0.6%) | 0.23 (0.05–1.14) |
Any instance of axillary temperature <36ºC at any time from 2 hours after randomization until discharge from hospital.
First time at which the infant had all signs of clinical stability: no need for CPAP, no episodes of apnea, SpO2>90, Respiratory rate 40 to < 60, Heart rate 80 to < 180 bpm, Temperature between 36 to 37.4ºC, and no need for IV fluids.
Suspected sepsis defined as one or more of the following signs/symptoms: temperature<35.5ºC or > 38ºC, no movement or movement only on stimulation, chest indrawing, convulsions. For all the signs/symptoms, we removed the first 24 hours, after that time the child should have been well for at least 24 hours before becoming sick. Denominator excludes infants that died, LAMA or were discharged before 48 hours of age.
Hypoglycemia defined as blood sugar < 45 m/dl or < 2.6 mmol/L measured when clinically indicated
Duration of hospital stay was a pre-specified process outcome
Maternal satisfaction with health care in the hospital was collected at discharge on a score of 1 to 10. Higher score implies higher satisfaction
Maternal depression defined as a score of >15 points on Patient Health Questionnaire 9
adjusted for clustering due to multiple births, site, delivery mode, multiple pregnancy, age at randomization, infant’s sex, infant’s weight, mother's years of schooling, maternal age, households with toilet in the house, and family income.
The 95% confidence intervals for secondary outcomes are not adjusted for multiplicity and should not be used to infer definitive intervention effects.
Hazard ratio
Mean difference
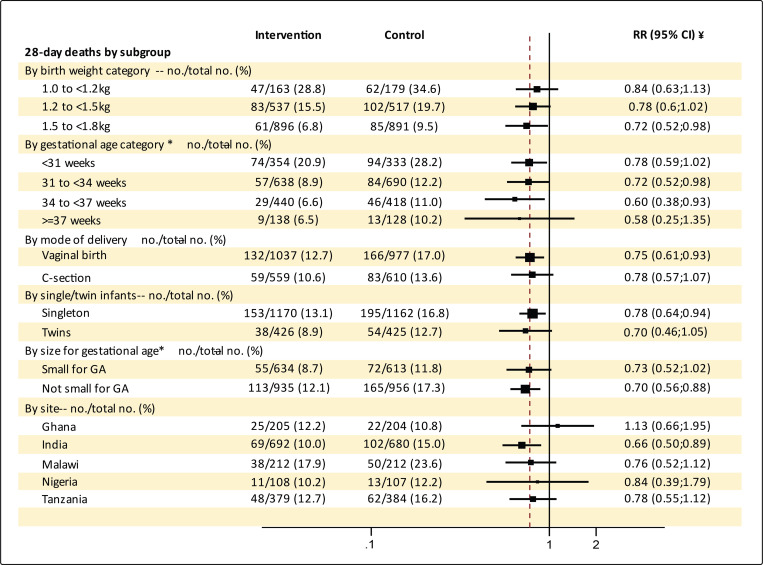
The intervention had similar effects across birthweights, gestation and weight for gestational age categories, different modes of delivery, and singletons or twins. (Figure 2 and Figure S1). All sites showed benefit in their point estimates except Ghana. In the intervention group, the risk of death was lower in infants who received more hours of skin-to-skin contact per day (Table S4). Most deaths were caused by sepsis and preterm birth complications. Sepsis-associated mortality was 4.4% in the intervention group and 6.9% in the control group (RR 0.64, 95% CI 0.48 to 0.86). (Table S5).
Figure 2.
Subgroup analyses of primary outcomes by birthweight, gestational age, multiple pregnancy, mode of delivery and size for gestational age
* 26 infants in the intervention and 18 infants in the control group have their gestational age at birth missing. Size for gestational age could not be calculated for one additional infant that was born with indeterminate sex.
¥ adjusted by site and clustering due to multiple births. For the subgroup analysis by site, adjustment was only done for clustering due to multiple births.
**The widths of the confidence intervals were not adjusted for multiplicity, so the intervals should not be used to infer definitive intervention effects. The size of squares representing the point estimates is proportional to the weight assigned to the subgroup.
Secondary outcome results are presented in Table 3. The proportion of infants with suspected sepsis was 22.9% in intervention group and 27.8% in control group (adjusted RR 0.82, 95% CI 0.73–0.93); hypothermia was documented in 5.6% and 8.3%, respectively (adjusted RR 0.65, 95%CI 0.51–0.83). The time to stabilization and incidences of hypoglycemia, feeding fully by suckling at the time of discharge, and exclusive breastfeeding at the end of neonatal period were similar in both groups. In post-hoc analyses, breastmilk feeding was initiated within the first 24 hours in 58.5% vs 45.5% of infants, and full breastmilk feeding within 7 days was achieved in 78.4% vs 69.0%, respectively (Table S6). Of 2146 infants with birthweight between 1.0 and <1.8 kg who were not enrolled in the trial, 340 (15.8%) died by 72 hours.
DISCUSSION
This multicenter trial demonstrated that initiation of continuous Kangaroo Mother Care soon after birth for infants with birthweight between 1.0 to <1.8 kg improved neonatal survival by 25%, compared with Kangaroo Mother Care initiated after stabilization, as is currently recommended. The intervention would need to be provided to 27 infants (95% CI 17–77) to prevent one neonatal death. Implementation of the intervention required the mother, or a surrogate, to be with the baby all the time which required establishment of Mother-NICUs. The lower observed rates of hypothermia and suspected sepsis, though not adjusted for multiplicity, are consistent with results for the primary outcome and may at least in part explain the mortality benefits of immediate Kangaroo Mother Care.
Findings for the primary outcome and for infection and hypothermia were similar to those reported in earlier trials of the use of Kangaroo Mother Care in clinically stable infants.6 However, we did not find differences between the intervention and control groups in the two pre-specified feeding outcomes—being fully breastfed by suckling at discharge and exclusive breastfeeding at the end of the neonatal period, despite post-hoc analyses suggesting higher rates of initiation of breast-milk feeding within 24 hours, putting the baby to the breast within 72 hours and reaching full breast-milk feeding within 7 days of birth in the intervention group. We also did not find a material difference between groups in the time to stabilization, unlike two previous RCTs of a similar intervention. 8,9 As compared to the studies that achieved intermittent Kangaroo Mother Care in the Cochrane review 6, we achieved high compliance with the intervention, i.e., about 17 hours of skin-to-skin contact per day.
There are several possible mechanisms by which immediate Kangaroo Mother Care might confer benefit. As the mother and baby are in close contact from birth, the baby is more likely to be colonized by the mother’s protective microbiome, more likely to receive early breastmilk feeding and there is less handling of the baby, thus reducing the risk of infection.13–19 Constant monitoring of the infant by the mother, more frequent glucose monitoring, and decreased stress20 of mother-infant separation could also contribute to reduced mortality. Further studies in well-resourced settings could help to determine to what extent these enhanced survival results from LMIC settings are relevant to low-mortality settings with intensive infant monitoring. We observed that the risk of death was lower in infants who received more hours of skin-to-skin contact per day. However, this association is subject to confounding by medical issues in the infant that may have precluded prolonged skin-to-skin contact.
The results of this study are generalizable to most hospitals in low-resource settings where immediate-Kangaroo Mother Care can be implemented as described here. Challenges in scaling up of this intervention would include multiple stakeholders’ involvement, establishment of Mother-NICUs, a strong collaboration between obstetric and neonatal departments, and policy changes allowing surrogates to provide Kangaroo Mother Care.
Some limitations merit discussion. The nature of the intervention made blinding impossible. However, ensuring allocation concealment until completion of enrolment, rigorous adherence to predefined protocol and choice of mortality as a primary outcome minimize measurement bias. The open label design may have resulted in measurement bias in some of the secondary outcomes, which were more subjective, but would not affect our primary mortality outcomes. There was heterogeneity in the infrastructure, staff, practices and possible differences in patient profile across sites; however, this should increase the generalizability of our findings. It is not possible to partition the beneficial effect of the intervention between immediate initiation of Kangaroo Mother Care and simply the presence of the mother with her baby, because both are integral part of the intervention. Finally, approximately 20% of infants 1.0 to <1.8 kg born in study hospitals were not enrolled because mother or newborn was too sick for this intervention, which needs to be considered in estimating the potential public health impact of the intervention.
In summary, in this large, multi-site, multi-country study, conducted in low resource hospitals, continuous Kangaroo Mother Care initiated immediately after birth in infants with a birthweight of 1.0 to <1.8 kg resulted in a significantly lower risk of neonatal death, although not of death within 72 hours, compared to the current WHO recommendation of initiating Kangaroo Mother Care after stabilization.
Supplementary Material
ACKNOWLEDGEMENTS
The WHO Immediate KMC study group would like to thank the women, infants, and families that have participated in the trial. All staff members in all participating sites are acknowledged for their dedication. The authors appreciate the independent oversight provided by the DSMB members, including Prof. Betty Kirkwood (Chair), Prof. Elizabeth Molyneux, Prof. Ravindra Mohan Pandey (statistician), Prof. Siddarth Ramji, Prof. Esther Mwaikambo, Prof. Olugbenga Mokuolu, and Ms. Charlotte Tawiah.
Funding:
This trial was funded by the Bill and Melinda Gates Foundation through a grant given to the World Health Organization (grant number OPP1151718)
(Funded by Bill and Melinda Gates Foundation through a grant to the World Health Organization; Australia and New Zealand Clinical Trials Registry number ACTRN12618001880235; Clinical Trials Registry-India number, CTRI/2018/08/01536)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Ethics approval and consent to participate: The study has been reviewed and approved by the WHO Ethics Review Committee and institutional review boards in the five sites: Ghana’s School of Medical Science/Komfo Anokye Teaching Hospital ethics committee, India’s VMMC & Safdarjung Hospital ethics committee, Malawi’s College of Medicine Research and Ethics Committee (COMREC), Nigeria’s OAUTHC Ethics and Research Committee and Tanzania’s National Institute for Medical Research ethics committee.
Writing Committee: Sugandha Arya, M.D.; Helga Naburi, M.D., M.Med., M.P.H., Ph.D.; Kondwani Kawaza, M.B.B.S., F.C.Paeds; Sam Newton, M.B.Ch.B., M.P.H., Ph.D.; Chineme Henry Anyabolu, M.B.B.S., F.W.A.C.P.; Nils Bergman, M.B.Ch.B.,M.P.H., Ph.D.; Suman P.N. Rao, M.D., D.M.; Pratima Mittal, M.S.; Evelyne Assenga, M.D., M.P.H., M.Med.; Luis Gadama, F.C.O.G.; Roderick Larsen- Reindorf, M.B.Ch.B.; Oluwafemi Kuti, F.W.A.C.S., F.M.C.O.G., F.R.C.O.G., M.D.; Agnes Linnér, M.D.; Sachiyo Yoshida, Ph.D.; Nidhi Chopra, M.D.; Matilda Ngarina, M.D., Ph.D.; Ausbert Thoko Msusa, M.B.B.S.,F.C.O.G.; Adwoa Boakye-Yiadom, M.B.Ch.B.; Bankole Peter Kuti, M.B.Ch.B., F.W.A.C.P., F.M.C.Paed.; Barak Morgan, M.B.B.Ch., Ph.D.; Nicole Minckas, M.Sc.; Jyotsna Suri, M.S.; Robert Moshiro, M.D.,M.Med.,Ph.D.; Vincent Samuel, M.Sc.; Naana Wireko-Brobby, M.B.Ch.B.; Siren Rettedal, M.D., Ph.D.; Harsh Vardhan Jaiswal, B.Tech.; M. Jeeva Sankar, M.D., D.M.; Isaac Nyanor, M.P.H.; Hiresh Tiwary; Pratima Anand, M.D., D.M.; Alexander Ansah Manu, M.B.Ch.B., M.Sc., Ph.D.; Kashika Nagpal, M.S.; Daniel Ansong, M.Sc., M.B.Ch.B.; Isha Saini, M.D.; Kailash C. Aggarwal, M.D.; Nitya Wadhwa, M.D.; Rajiv Bahl, M.D., Ph.D.; Bjorn Westrup, M.D., Ph.D.; Ebunoluwa Aderonke Adejuyigbe, B.Sc., M.B.Ch.B. F.M.C.Paed., M.D.; Gyikua Plange-Rhule, B.Sc., M.B.Ch.B., M.W.A.C.P., F.G.C.P.; Queen Dube, Ph.D.; Harish Chellani, M.D.; Augustine Massawe, M.D.
The affiliations of the members of the writing committee are as follows: Department of Maternal, Newborn, Child and Adolescent Health and Ageing, World Health Organization, Geneva, Switzerland (S.P.N.R., S.Y., N.M., H.V.J., H.T., R.B.); Vardhaman Mahavir Medical College and Safdarjung Hospital, New Delhi, India (S.A., P.M., N.C., J.S., P.A., K.N., I.S., K.C.A., H.C.); All India Institute of Medical Sciences, New Delhi, India (M.J.S.); Translational Health Science and Technology Institute, Faridabad, India (N.W.); Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania (H.N., E.A., A.M.); Muhimbili National Hospital, Dar es Salaam, Tanzania (M.N., R.M.); University Of Malawi, College Of Medicine, Blantyre, Malawi (K.K., L.G., A.T.M., V.S., Q.D.); Obafemi Awolowo University, Ile- Ife, Nigeria (C.H.A., O.K., B.P.K., E.A.A.); Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (S.N., R.L.R., D.A., G.P.R.); Komfo Anokye Teaching Hospital, Kumasi, Ghana (A.B.Y., N.W.B., I.N.); School of Public Health, University Of Ghana (A.A.M.); Karolinska Institute, Stockholm, Sweden (N.B., A.L., B.W.); Institute for Safety Governance and Criminology, University of Cape Town. South Africa (B.M.); Stavanger University, Stavanger, Norway (S.R.).
REFERENCES
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO/UNICEF. Low birthweight : country, regional and global estimates [Internet]. Available from: https://apps.who.int/iris/handle/10665/43184
- 3.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 Million neonatal deaths: When? Where? Why? Lancet. 2005;365(9462):891–900. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost?. Lancet 2014;384(9940):347–70. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Kangaroo Mother Care: a practical guide [Internet]. WHO. 2003. [cited 2020 Jul 7];Available from: http://www.who.int/maternal_child_adolescent/documents/9241590351/en/ [Google Scholar]
- 6.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo Mother Care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016; 8: CD002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alliance for Maternal and Newborn Health Improvement (AMANHI) mortality study group. Population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa: a multi-country prospective cohort study. Lancet Glob Health. 2018. Dec;6(12):e1297–e1308.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman N, Linley L, Fawcus SR. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr. 2004;93(6):779–85. [DOI] [PubMed] [Google Scholar]
- 9.Chi Luong K, Long Nguyen T, Huynh Thi DH, Carrara HP, Bergman NJ. Newly born low birthweight infants stabilise better in skin-to-skin contact than when separated from their mothers: A randomised controlled trial. Acta Paediatr. 2016;105(4):381–90. [DOI] [PubMed] [Google Scholar]
- 10.WHO immediate KMC Study Group. Impact of continuous Kangaroo Mother Care initiated immediately after birth (iKMC) on survival of newborns with birth weight between 1.0 to < 1.8 kg: Study protocol for a randomized controlled trial. Trials 2020;21(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linnér A, Westrup B, Lode-Kolz K, et al. Immediate parent-infant skin-to-skin study (IPISTOSS): study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open 2020;10:e038938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Standards for maternal and newborn health. WHO; [Internet] 2007. [cited 2020 Jul 7];Available from: http://www.who.int/maternal_child_adolescent/documents/a91272/en/ [Google Scholar]
- 13.Boo NY, Jamli FM. Short duration of skin-to-skin contact: Effects on growth and breastfeeding. J Paediatr Child Health 2007;43(12):831–6. [DOI] [PubMed] [Google Scholar]
- 14.Charpak N, Ruiz-Peláez JG, Figueroa De C Z, Charpak Y. Kangaroo mother versus traditional care for newborn infants: A randomized, controlled trial. Pediatrics 1997;100(4):682–8. [DOI] [PubMed] [Google Scholar]
- 15.Pratiwi IGAP, Soetjiningsih S, Kardana IM. Effect of kangaroo method on the risk of hypothermia and duration of birth weight regain in low birth weight infants: A randomized controlled trial. Paediatr Indones 2009;49(5):253. [Google Scholar]
- 16.Ghavane S, Murki S, Subramanian S, Gaddam P, Kandraju H, Thumalla S. Kangaroo Mother Care in Kangaroo ward for improving the growth and breastfeeding outcomes when reaching term gestational age in very low birth weight infants. Acta Paediatr. 2012; 101(12): e545–9. [DOI] [PubMed] [Google Scholar]
- 17.Sloan NL, Camacho LWL, Rojas EP, Stern C. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Maternidad Isidro Ayora Study Team. Lancet 1994;344(8925):782–5. [DOI] [PubMed] [Google Scholar]
- 18.Suman RP, Udani R, Nanavati R. Kangaroo Mother Care for Low Birth Weight Infants: A Randomized Controlled Trial. Indian Pediatr. 2008;45(1):17–23. [PubMed] [Google Scholar]
- 19.Whitelaw A, Heisterkamp G, Sleath K, Acolet D, Richards M. Skin to skin contact for very low birthweight infants and their mothers. Arch Dis Child 1988;63(11):1377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forde D, Deming DD, Tan JC, et al. Oxidative stress biomarker decreased in preterm neonates treated with Kangaroo Mother Care. Biol Res Nurs 2020; 22(2): 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.