Abstract
Background
Coronavirus disease 2019 (COVID-19) infection is associated with a coagulopathy with high incidence of venous thrombo-embolism. However, bleeding risk is also significant, causing difficulty in initiating and adjusting anticoagulation therapy in case of suspected thrombi. Cardiac masses can be challenging to be identified properly in the context of this disease. The use of bedside contrast echocardiography (CE) can be of a great value in this situation decreasing procedure-related risk and allowing proper diagnosis and management of a cardiac mass.
Cases summary
We present two cases who were admitted with severe COVID-19 infection. Both cases had additional risk factors for hypercoagulability. Un-enhanced echocardiography was performed and revealed right ventricular (RV) dysfunction with a suspected RV mass. The use of bedside CE could confirm a RV thrombus in the first case and exclude it in the second case. Hence, anticoagulation therapy could be adjusted accordingly in both patients.
Discussion
Coronavirus disease 2019 infection is associated with peripheral thrombo-embolism and cardiac thrombi. Given the critical condition of many patients affected by COVID-19, imaging for thrombo-embolic events is often restricted. With the use of bedside CE, cardiac masses may be correctly identified, aiding proper adjustment of anticoagulation therapy.
Keywords: Cardiac mass, Cardiac thrombi, Contrast echocardiography, COVID-19, Case series
Learning points
Coronavirus disease 2019 is associated with a coagulopathy and high rates of thrombo-embolic events.
When a thrombus is suspected, it can be confirmed or refuted easily using bedside boluses of pre-activated contrast agents, facilitating appropriate management of patients.
Introduction
The current report describes two cases of right ventricular (RV) masses in patients admitted with coronavirus disease 2019 (COVID-19). Coronavirus disease 2019 patients are known to have high thrombotic risk.1–6 An early and appropriate treatment of a suspected cardiac mass requires accurate diagnosis for implementing appropriate management. Thus, this report demonstrates the usefulness of contrast echocardiography (CE), a rapid bedside technique, as a part of the diagnostic workup in evaluating cardiac masses in COVID-19 patients.
Timeline Case 1
| Day 1 |
|
| Day 4 |
|
| Day 20 |
|
| Day 30 |
|
| Day 40 |
|
| Day 58 |
|
| Day 155 |
RV radial function normalized (fractional area change (FAC) = 38%), normal RV longitudinal systolic function, Apart from RV trabeculae, no mass or thrombi could be seen and confirmed by low MI contrast enhancement. |
Timeline Case 2
| Day 1 |
|
| Day 10 |
|
| Day 15 |
|
| Day 30 |
|
Case presentation
Case 1
A 35-year-old male with no medical co-morbidities [height: 178 cm, weight: 73 kg, body mass index (BMI): 23 kg/m2, peak D-dimer: 4310 mcg/mL, Troponins: 24 and 20 ngm/L-no significant change] was admitted to intensive care unit (ICU) with severe COVID-19 pneumonitis. On examination, he was febrile, tachycardic, tachypnoeic [respiratory rate (RR)]: 46/min, borderline blood pressure (BP): 93/60 mmHg, and O2 saturation: 82% on room air. Initial management with non-invasive ventilation progressed to invasive mechanical ventilation; however, his condition deteriorated and required his transfer for extra-corporeal membrane oxygenation (ECMO). Computed tomography with pulmonary angiography (CTPA) showed subsegmental pulmonary embolism with left lower lobe infarction. Initial echocardiography demonstrated RV dysfunction. Following his transfer back to our hospital for rehabilitation in the ICU, further unenhanced echocardiography was performed in the ICU due to persistent tachycardia. The echocardiographic images were compromised by patient’s immobility though left ventricular (LV) was well visualized. Left ventricular size and function were normal; [LV ejection fraction (LVEF) = 55–60%] with normal LV filling pressure. However, RV remained dilated (visually assessed in short axis) and impaired (fractional area shortening: 23%) with possible RV mass (Figure 1A1). Bedside CE using IV boluses of pre-activated Luminity of 0.3 mL (Perfluoropropane lipid microsphere; Bristol-Myers Squibb) (Philips EPIQ CVx scanner, using low mechanical index <0.14) showed a non-perfused RV mass (Figure 1A2). The differential diagnosis for poorly perfused cardiac masses includes stromal tumours and thrombi, with thrombus most likely in this clinical scenario. On this basis, anticoagulation therapy was extended for up to 6 months with request for a reassessment of the RV thrombus after patient’s discharge. Four months later, follow-up echocardiography was performed after about 3 months of patient’s discharge and revealed resolution of RV mass, confirmed by CE (Supplementary material online, Video S1) with normalization of RV function (fractional area shortening = 38%).
Figure 1.
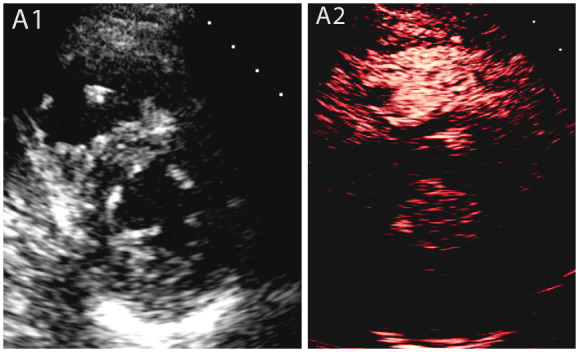
Case 1: A male coronavirus disease 2019 patient who had a previous pulmonary embolism with a suspected right ventricular thrombus in the non-enhanced study, which was subsequently confirmed by contrast echocardiography. (A1) Parasternal short axis view shows a small right ventricular mass, (A2) contrast echocardiography of the same view, showing a non-perfused right ventricular mass suggestive of thrombus.
Figure 2.
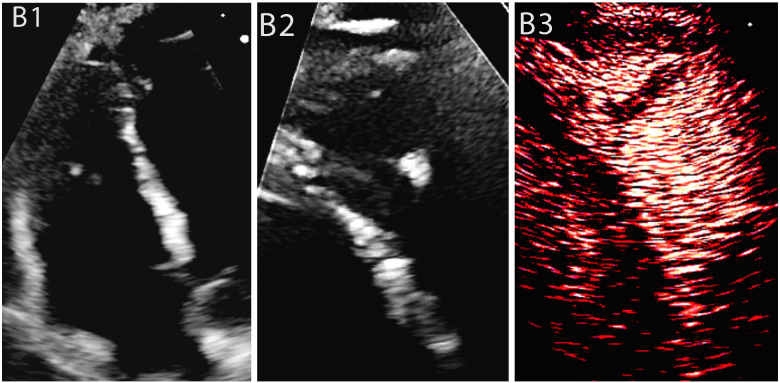
Case 2: A male COVID-19 patient with lymphoma on chemotherapy. Non-contrast study showed a dilated and dysfunctional RV with elevated PVR and a suspected RV thrombus which was identified as RV trabeculation on CE. (B1) Non-enhanced image of a dilated and dysfunctional RV, (B2) Non-enhanced image of RV modified view displays an apical mass, (B3) CE of RV modified view demonstrates no evidence of mass but a conglomerate of trabeculation with contrast noted between the trabeculae.
Case 2
A 55-year-old male with a history of chronic obstructive pulmonary disease (COPD), schizophrenia, epilepsy and progressive lymphoma on chemotherapy (height: 169 cm, weight: 52 kg, BMI: 18.2 kg/m2, peak D-Dimer: 1220 mcg/mL, Troponins: 22 and 25 ngm/L-no significant change) was admitted to the high dependency unit (HDU) with COVID-19 pneumonia, recurrent left-sided pleural effusion and type 2 respiratory failure. On examination, he was febrile, tachycardic, tachypnoeic [(RR): 28/min], O2 saturation: 62% on room air and his BP was 123/63 mmHg. He had left-sided chest drain inserted for his pleural effusion and required prolonged non-invasive ventilation. Persistent tachycardia and shortness of breath prompted an unenhanced echocardiogram in the HDU demonstrating, LV size and function were normal (LVEF: 60%) with normal LV filling pressure, significant RV dilatation (basal diameter: 4.5 cm) and dysfunction (fractional area shortening = 25%), high pulmonary vascular resistance and probable thrombus in the RV (Figure 1B1, B2, Supplementary material online, Videos S2–S4). However, bedside CE performed both to improve image quality which was poor and to characterize the RV mass revealed the mass to be a conglomeration of trabeculation with contrast moving between trabeculae-masquerading as a mass on unenhanced echocardiography. Therefore, unnecessary anticoagulation was avoided (Figure 1B3, Supplementary material online, Video S5). Interestingly, a CTPA was done later as a part of his progressive lymphoma workup and showed no evidence of pulmonary embolism.
Discussion
It is known that there is a significantly high incidence of both arterial and venous thrombotic events in critically ill patients with COVID-19.1–6 Thus, the threshold for the commencement of therapeutic anticoagulation is low in COVID-19. However, when a diagnosis of a thrombus is uncertain, over-treatment with therapeutic doses of anticoagulation may cause an unwanted increased risk of bleeding especially in septic patients, and under-treatment may result in catastrophic embolization. Therefore, it is important to establish a diagnosis of thrombus promptly in COVID-19 patients with suspected cardiac masses.
Two-dimensional echocardiography remains the imaging technique of choice in COVID-19 patients because it can be performed at the bedside and can assess cardiac structure and function rapidly. However, unenhanced echocardiography cannot distinguish thrombus from other cardiac masses. While the probability of thrombus is high in COVID-19 patients with suspected cardiac masses, especially with cardiac dysfunction, it is desirable to have an accurate diagnosis for appropriate management of patients. Echocardiographic contrast perfusion imaging which is performed at the bedside has been recommended to distinguish thrombus from other cardiac masses.7–9 Thrombus shows complete lack of perfusion. The first case showed that the suspected mass was indeed a thrombus as there was no contrast uptake. Another differential diagnosis for poorly perfused cardiac masses includes stromal tumours, but the clinical scenario of this patient made a diagnosis of thrombus most likely and allowed appropriate adjustment of the anticoagulation regimen. In the second case, CE refuted the diagnosis of a mass when contrast could be seen travelling between strands of tissues (the conglomerated trabeculae gave an appearance of a mass in unenhanced images). This prevented unnecessary institution of anticoagulation with high bleeding risk in COVID-19 patients. The first patient appeared to have a higher predisposition for thrombo-embolic phenomenon as he had more severe inflammatory response (approximately four folds higher D-dimer compared to the second case and was on ECMO).
The only contraindication of CE is known allergy against the contrast agent. If not present, CE has been found to be safe in the acute care setting including patients with acute respiratory distress syndrome.10 The CE needs to be performed and interpreted by the staff trained specifically in CE.
Conclusions
COVID-19 infection is associated with a hypercoagulable state with a high incidence of thrombo-embolic events. This report provides examples of the additive value of bedside CE in the investigation of suspected RV cardiac thrombi in COVID-19 patients, encouraging its wider use where appropriate.
Lead author biography

Christina Botrous MBBS, MSC, graduated in Medicine in Egypt in 2012. Currently, she is working as a cardiac research fellow in Northwick Park Hospital. She has special interest in clinical cardiology and non-invasive cardiovascular imaging.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: Patients have given a written consent for submission and publication of this case series including images and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: R.S. was given speaker fees by Bracco (Milan, Italy), Lantheus Medical Imaging (Boston, Massachusetts) and Philips Healthcare (Eindhoven, Holland). Other authors declare no conflicts of interest to disclose.
Funding: None declared.
Supplementary Material
References
- 1. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F. et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 2020;142:184–186. [DOI] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip M, Van Der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Nieuwkoop C. COVID-19 associated pulmonary thrombosis. Thromb Res 2020;191:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wise J. Covid-19 and thrombosis: what do we know about the risks and treatment? BMJ 2020;369: m2058. [DOI] [PubMed] [Google Scholar]
- 5. Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K. et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res 2020;191:76–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brüggemann R, Gietema H, Jallah B, ten Cate H, Stehouwer C, Spaetgens B.. Arterial and venous thromboembolic disease in a patient with COVID-19: a case report. Thromb Res 2020;191:153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandian NG. Clinical applications of contrast echocardiography. Eur J Echocardiogr 2005;5(SUPPL. 2):S3–S10. [DOI] [PubMed] [Google Scholar]
- 8. Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP. et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol 2004;43:1412–1419. [DOI] [PubMed] [Google Scholar]
- 9. Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, et al. Reviewers: This document was reviewed by members of the EACVI Scientific Documents Committee for 2014–16 and 2016–18. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging 2017;18:1205–1205af. [DOI] [PubMed] [Google Scholar]
- 10. Main ML, Hibberd MG, Ryan A, Lowe TJ, Miller P, Bhat G.. Acute mortality in critically ill patients undergoing echocardiography with or without an ultrasound contrast agent. JACC Cardiovasc Imaging 2014;7:40–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.