Abstract
Early identification of behavioral risk markers for anxiety is essential to optimize long-term outcomes in children with neurodevelopmental disorders. This study analyzed attentional avoidance and its relation to anxiety and autism spectrum disorder (ASD) symptomatology during social and nonsocial fear conditions in toddlers with fragile X syndrome (FXS) and Down syndrome (DS). Toddlers with FXS and DS exhibited increased nonsocial attentional avoidance relative to typically developing (TD) toddlers. Attentional avoidance was not related to anxiety symptom severity in any group; however, higher ASD symptom severity was related to more social attentional avoidance in the FXS and TD groups. Findings suggest that there may be different underlying mechanisms driving attentional avoidance across neurodevelopmental disorders.
Keywords: fragile X syndrome, Down syndrome, social anxiety, specific phobia, autism spectrum disorder
Anxiety disorders are among the most common pediatric mental health disorders, with 7–32% of children and adolescents diagnosed with at least one anxiety disorder and far more individuals exhibiting symptoms that do not reach the diagnostic threshold (Bandelow & Michaelis, 2015; Merikangas et al., 2010; Polanczyk et al., 2015). The etiology of anxiety is complex with many factors (e.g., temperament, genetics, neurobiology, environment) impacting the emergence and severity of symptoms. Anxiety is also conceptualized and studied in multiple forms, including anxiety as a single unitary construct that encompasses multiple disorders and as individual and discrete disorders with unique phenotypic profiles (Mian et al., 2012; Paulus et al., 2015, Roberts, Crawford, Will, et al., 2019; Shephard, et al., 2019, White et al., 2017).
Specific phobia and social anxiety have been identified as the most common anxiety disorders diagnosed in children with a prevalence of 10% and 11% respectively (Paulus et al., 2015). Despite the high prevalence and impairment associated with specific phobia and social anxiety in children, few studies have investigated the early behavioral manifestation of these anxiety disorders. Understanding the onset and developmental course of distinct anxiety disorders is complicated by the fact that some degree of transient fear and anxiety is a normative part of early development and specific types of fears and anxieties, as well as their severities, are subject to change across development (Beesdo et al., 2009). The individualistic and transient nature of anxiety in children across development makes confidently distinguishing normative anxiety from anxiety that may signal the presence of an anxiety disorder quite challenging.
Identifying the emergence of differential patterns of discrete anxiety disorders is further complicated in children with intellectual disability (ID) and/or neurodevelopmental disorders due to their limited cognitive and communication abilities. Research has shown that children with ID have higher rates of anxiety across development in comparison to typically developing (TD) children (Green et al., 2015). The severity of ID may be an important factor as several studies have shown that anxiety is much more prevalent in children with more severe ID, with 34–50% of children with severe ID experiencing clinically-significant levels of anxiety (Cormack et al., 2000; Munir, 2016; Whitney et al., 2019). In comparison to TD children with anxiety, children with ID and comorbid anxiety often experience a more significant and pervasive degree of impairment in their everyday life (Dekker & Koot, 2003; Green et al., 2015).
Previous studies have shown that individuals with neurodevelopmental disorders associated with ID, such as fragile X syndrome (FXS), are also at an elevated risk of anxiety in comparison to TD individuals (Crawford et al., 2017; Cordeiro et al., 2011; Royston et al., 2017). There are numerous neurobiological, cognitive, and behavioral characteristics shared between different neurodevelopmental disorders; however, there is also a considerable amount of variability between disorders (Moss et al., 2009; Tunnicliffe & Oliver, 2011). This variability can be seen in the prevalence, severity, and behavioral manifestation of distinct anxiety disorders within different neurodevelopmental disorders (Crawford et al., 2017; Crawford et al., 2020). Despite the prevalence of anxiety in children with neurodevelopmental disorders, few studies have compared the early behavioral risk markers of different anxiety disorders in young children with different neurodevelopmental disorders.
Anxiety in Fragile X Syndrome
FXS is a monogenic neurodevelopmental disorder that results from an expansion mutation of the CGG trinucleotide repeat on the fragile X mental retardation 1 (FMR1) gene on the long arm of the X chromosome. FXS, which affects approximately 1 in 2,500 individuals, is the most common inherited cause of ID and the leading known genetic cause of autism spectrum disorder (ASD), with a prevalence of approximately 60% (Abbeduto et al., 2019; Hogan et al., 2017; Hagerman, 2008; Roberts et al., 2020). Although FXS affects both males and females, males are typically more severely afflicted because, unlike females, they do not have the protective effect of an unaffected second X chromosome. Children with FXS often display heightened social avoidance in reaction to novel people, making social interactions difficult despite a general interest in social interaction (Tonnsen, Malone, et al., 2013; Roberts et al., 2007; Roberts, Crawford, Hogan, et al., 2019). Other defining characteristics of children with FXS include moderate to severe ID, language and motor deficits, atypical autonomic arousal, increased impulsivity, gaze aversion, and difficulties with emotional regulation (Chromik et al., 2019; Kidd et al., 2014; Klusek et al., 2015; McDuffie et al., 2015; Roberts et al., 2016).
Children with FXS are also at an increased risk of experiencing clinically significant levels of anxiety. Previous research has shown that 70–86% of individuals with FXS meet diagnostic criteria for at least one anxiety disorder and approximately 58% meet criteria for multiple anxiety disorders (Bailey et al., 2008; Cordeiro et al., 2011; Ezell et al., 2019). Among the most prevalent and impairing anxiety disorders diagnosed in children and adolescents with FXS are social anxiety, with a prevalence of 13–40%, and specific phobia, with a prevalence of 36–65% (Cordeiro et al., 2011; Ezell et al., 2019). However, the symptoms of anxiety disorders, ASD, and FXS can have a significant degree of overlap. For example, social avoidance, gaze aversion, and emotion dysregulation are common features of anxiety disorders, ASD, and FXS (Tonnsen, Malone, et al., 2013; Roberts et al., 2007). There is further overlap in symptomatology between anxiety and ASD, such as atypical behavioral and physiological reactivity, restricted interests, fear of change, and repetitive behaviors (Kerns et al., 2017; van Steensel et al., 2013). The symptoms of anxiety and ASD may exacerbate the core impairments associated with FXS; thus, it is important to disentangle this overlapping symptomatology to more precisely characterize the prevalence of anxiety and ASD in FXS as well as to determine the most effective treatment given that treatment approaches for anxiety and ASD can vastly differ.
Anxiety in Down Syndrome
Down syndrome (DS) is a genetic neurodevelopmental disorder that typically results from the presence of a third copy of chromosome 21. DS is the most common chromosomal disorder, affecting 1 in 700 individuals (Parker et al., 2010). DS is characterized by mild to severe ID and deficits in language and motor functioning, along with impulse control difficulties (Daunhauer & Fidler, 2011). Unlike children with FXS, children with DS are often characterized by a prosocial disposition and higher overall social competency, allowing children with DS to engage more readily in social contexts than many other children with ID (Daunhauer & Fidler, 2011; Fidler et al., 2008; Næss et al., 2017).
Although FXS and DS are both genetic neurodevelopmental disorders that result in ID, unlike children with FXS, individuals with DS appear less likely to experience comorbid anxiety. Existing studies indicate that approximately 20% of adolescents and young adults with DS are diagnosed with an anxiety disorder, which is associated with lower levels of functioning, including communication, community involvement (e.g., employment, social activities), and self-care skills (DiGuiseppi et al, 2010; Dykens et al., 2015; Määttä et al., 2011; Pikora et al., 2014). However, there is a significant paucity of research on the emergence of anxiety in young children with DS, including risk factors, protective factors, and phenotypic profiles. One study reported more temperamental fear in response to novel stimuli, which is known to predict anxiety in TD children, across the first year of life in 15 infants with DS relative to controls (Rothbart & Hanson, 1983). Another study of older children with DS found higher rates of animal fears and lower rates of social fear relative to children with ASD, but no differences in fears relative to TD children (Evans et al., 2005). Given the negative long-term impact of anxiety and the paucity of research on the prevalence and developmental course of anxiety in DS, more research in this area is greatly needed. A cross-syndrome comparison of prodromal anxiety features between FXS and DS provides a unique opportunity to investigate the manifestation of anxiety while inherently controlling for possible confounds, such as ID. Furthermore, investigating early risk markers of anxiety in young children across multiple neurodevelopmental disorders with well-known genetic etiologies, such as FXS and DS, can help elucidate the manifestation of specific anxiety disorders.
Anxiety and Attentional Bias
Attentional bias has been implicated as a potential early behavioral marker of anxiety symptomatology in children (Bar-Haim et al., 2007; Pérez-Edgar et al., 2010, 2011; Salum et al., 2013). Although the precise relationship between attention bias and anxiety has yet to be defined, numerous studies suggest that specific attentional bias patterns correspond with distinct anxiety profiles. For example, previous research has noted that attentional bias towards potentially threatening stimuli is a feature of distress disorders, such as generalized anxiety disorder, whereas attentional bias away from potentially threatening stimuli is a feature of fear disorders, such as specific phobia and social anxiety (Bar-Haim et al., 2007; Pérez-Edgar et al., 2010; Salum et al., 2013; Waters et al., 2014).
In addition to its relationship with anxiety, atypical attention is also commonly associated with ASD and ASD-related features (Chawarska et al., 2013; Keehn et a., 2013; Kleberg et al., 2017; Moriuchi et al., 2017). Given that approximately 60% of individuals with FXS and 20% of individuals with DS meet diagnostic criteria for ASD, it is essential that attention to novel and potentially threatening stimuli be understood to provide a more accurate developmental account on the emergence of anxiety or ASD in toddlers with neurodevelopmental disorders (Abbeduto et al., 2019; DiGuiseppi et al., 2010; Hogan et al., 2017; Moss & Howlin, 2009; Roberts et al., 2012; Roberts, Crawford, Will, et al., 2019; Roberts et al., 2020; Wheeler et al., 2015). This line of work disentangling anxiety from ASD is beginning to emerge, with a recent study demonstrating that reduced eye contact during social interactions with an unfamiliar person was associated with more severe ASD symptoms, but not anxiety symptoms, in infants and children with FXS (Roberts, Crawford, Will, et al., 2019).
Aims and Hypothesis
The present study examined attentional avoidance as an early risk marker of anxiety in toddlers across multiple neurodevelopmental disorders. The primary objective of this study was to identify patterns of visual attention elicited from conditions meant to evoke social fear (i.e., fear in response to a novel person) and nonsocial fear (i.e., fear in response to a novel object) in 24-month-old toddlers with FXS and DS contrasted to TD controls. Given evidence that individuals with FXS have a high prevalence of anxiety in adolescence and adulthood, we hypothesized that toddlers with FXS would exhibit more attentional avoidance than toddlers with DS or TD toddlers when exposed to novel and potentially frightening social and nonsocial fear targets. The present study also investigated whether anxiety symptom severity, ASD symptom severity, or developmental level were associated with attentional avoidance in toddlers with FXS, toddlers with DS, and TD toddlers. Based on previous findings that attentional avoidance is a common feature of anxiety disorders (Pérez-Edgar et al., 2010; Salum et al., 2013; Waters et al., 2014), we predicted that increased attentional avoidance in response to the social and nonsocial fear targets would be related to more severe anxiety symptom severity, but not ASD severity or developmental level, across all groups.
Method
Participants
Participants included 41 toddlers with FXS, 22 toddlers with DS, and 37 TD controls assessed at 24 months of age. Groups were not statistically equivalent on chronological age (F(2, 97) = 3.20, p = .045) in that toddlers with DS were slightly older than TD toddlers; however, toddlers with FXS did not significantly differ from toddlers with DS or TD toddlers. Data were drawn from larger longitudinal studies on the early development of children with FXS and DS. Participants with FXS and DS were recruited throughout the United States through research and medical sites as well as parent support groups, while TD controls were recruited locally through pediatrician’s offices and social media. The presence of FXS and DS were confirmed through genetic reports provided by the parents of the participants. TD participants were excluded from the study if they had a family history of ASD (i.e., first-degree relative) or if they were later diagnosed with ASD or developmental delay through the larger longitudinal study. Participants were excluded if they were born at <37 weeks gestation or were diagnosed with other genetic conditions besides FXS and DS (e.g., spina bifida), pre-existing medical conditions (e.g., cerebral palsy, seizure disorders), or hearing or vision conditions that would impede their ability to participate in the study. Detailed participant characteristics are included in Table 1.
Table 1.
Descriptive Characteristics
| FXS (n = 41) | DS (n = 22) | TD (n = 37) | |
|---|---|---|---|
| Chronological Age, months | 25.15 (1.43) 22.99–29.62 |
25.57 (2.64) 20.81–30.88 |
24.53 (0.63) 23.52–26.32 |
| Male Participants, n (%) | 28 (68.29%) | 19 (86.36%) | 29 (78.38%) |
| Participants with ASD, n (%) | 23 (56.10%) | 4 (18.18%) | 0 (0.00%) |
| CBCL Anxiety Problems M (SD) Range n |
4.11 (2.94) 0–12 37 |
1.41 (1.50) 0–4 17 |
1.74 (2.17) 0–10 34 |
| ADOS-2 Overall Raw Scores M (SD) Range n |
13.07 (7.96) 2–27 41 |
8.70 (4.41) 2–17 20 |
4.11 (3.25) 0–15 37 |
| ADOS CSS M (SD) Range n |
5.37 (3.18) 1–10 41 |
3.40 (1.64) 1–7 20 |
1.86 (1.16) 1–5 37 |
| Mullen ELC M (SD) Range n |
61.00 (14.36) 49–106 41 |
54.86 (8.52) 49–85 22 |
99.08 (15.22) 67–130 37 |
Observed Visual Attention
Social fear was represented using the Stranger Approach condition whereas nonsocial fear was represented using the Scary Spider condition of the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith & Rothbart, 1996). Before the start of either condition, the toddlers were ensured to be in a neutral emotional state and seated near or on a caregiver’s lap. For the Stranger Approach condition, a female examiner dressed in a standard manner approached the toddler using a standardized script. The Stranger Approach condition consisted of three phases: an approach phase (10–15 seconds); a kneel phase in which the stranger silently knelt in front of the toddler while maintaining a neutral expression (2 minutes); and a withdraw phase in which the stranger exited the room (10–15 seconds). All three phases were included in the analysis of the Stranger Approach condition. For the Scary Spider condition, the examiner presented the toddler with a fuzzy toy spider. The toddler was told that the toy spider was a soft, fuzzy spider that would not bite and was prompted to pet the spider up to four times. If the child’s hand came within approximately five centimeters of the spider, the examiner activated the spider to “jump.” The condition lasted approximately 1–1.5 minutes.
The Stranger Approach and the Scary Spider were videotaped and coded offline using Noldus Observer XT (version 10.5, Noldus Information Technology, Leesburg, VA, USA). For both conditions, the camera was positioned in front of the toddler and was focused on their whole face and body. If the toddler moved away, an examiner would follow the toddler with the camera. The toddler’s attentional avoidance was captured by coding the duration of looking away from the stranger or spider and included time spent looking at other individuals in the room, such as parents or examiners. Attentional avoidance was coded continuously with the onset and offset for each attention code marked across the entire duration of each task. The proportion of time engaged in attentional avoidance was calculated by dividing the total sum of attentional avoidance by the total duration of the task, excluding the duration of attention obscured (e.g., both eyes not visible because of camera angle). The proportion of time obscured was minimal during both the Stranger Approach (M = .08, SD = .11) and the Scary Spider condition (M = .04, SD = .06). Groups were similar in the proportion of time obscured during the Stranger Approach (F(2, 97) = 3.19, p = .046) and did not significantly differ during the Scary Spider condition (F(2, 97) = .05, p = .950). Behavioral coding of attention was completed by trained undergraduate research assistants, blind to the hypotheses of the current study, who established initial reliability with a master coder by obtaining 80% agreement with the master coder on three consecutive videos. Agreement, for both initial and ongoing reliability, was defined as both coders marking the onset and offset of the same attention variable (e.g., looking at the parent) within a defined tolerance window of one second (Jansen et al., 2003). To maintain reliability, 20% of the videos coded by research assistants were coded by the master coder, with Cohen’s kappa coefficients of .81 (Stranger Approach) and .80 (Scary Spider).
Anxiety Symptom Severity
Anxiety symptom severity was determined using the Child Behavior Checklist (CBCL) for ages 1 ½–5 years, a parent-report questionnaire designed to screen for a variety of problems, such as anxiety, depression, aggression, and hyperactivity (Achenbach & Rescorla, 2001). The CBCL Anxiety Problems subscale raw scores were used to assess symptoms associated with anxiety. A one-way analysis of variance with Bonferroni post-hoc comparisons revealed that toddlers with FXS were reported to have more anxiety symptoms in comparison to toddlers with DS (F(2, 85) = 11.27, p < .001) and TD toddlers (F(2, 85) = 11.27, p < .001) with 9.76% of toddlers with FXS at or above the clinical cutoff compared to 0% of toddlers with DS and 2.70% of TD toddlers.
ASD Symptom Severity
ASD symptom severity was evaluated using the Autism Diagnostic Observation Schedule-2 (ADOS-2) Toddler module (Lord et al., 2012). The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, and play, as well as restricted and/or repetitive behaviors. The ADOS-2 was administered by research reliable testers. The ADOS-2 overall raw scores were used to quantify ASD symptom severity.
Developmental Level
Cognitive abilities were assessed using the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The MSEL is a standardized developmental measure of cognitive, language, and motor abilities in young children. The MSEL is composed of five domains: Gross Motor, Visual Reception, Fine Motor, Expressive Language, and Receptive Language. An Early Learning Composite (ELC) score was derived from all domains except the Gross Motor domain. MSEL ELC scores significantly differed across group (F(2, 97) = 102.47, p < .001) in that toddlers with FXS and DS were significantly lower than TD toddlers; however, scores for toddlers with FXS and toddlers with DS did not significantly differ.
Procedures
All data were collected at the same comprehensive assessment that was part of a larger standardized protocol assessing temperament and development. Each participant was assessed at either the participant’s home or at the University of South Carolina research laboratory by at least two trained examiners. Written consent was obtained from the participant’s parent before data were collected. All study procedures were approved by the Institutional Review Board at the University of South Carolina.
Statistical Analysis
All analyses were conducted using SPSS 25. Between-group differences in the proportion of attentional avoidance away from the fear-inducing targets were tested with a repeated measures analysis of variance (RMANOVA) model. Results from assumptions testing indicated that the proportions of attentional avoidance met the assumptions of normality and sphericity. Main effects of condition (i.e., stranger, spider), diagnostic group, (i.e., FXS, DS, TD), and sex, as well as all interaction terms, were included in the model. Chronological age was not significantly correlated with the dependent variables and therefore was not included in further analyses.
To investigate the relationship between attentional avoidance and clinical-behavioral symptoms, correlational analyses were employed. Based on Shapiro-Wilk tests, the CBCL Anxiety Problems subscale raw scores, the ADOS-2 overall raw scores, and the MSEL ELC scores were not normally distributed; therefore, nonparametric correlations (Spearman) were employed. Spearman correlations were used to investigate the relationship between attentional avoidance, CBCL Anxiety Problems subscale raw scores, and ADOS-2 overall raw scores. Given that anxiety is more prevalent in individuals with more severe ID, a Spearman correlation was also conducted to determine the relationship between attentional avoidance and MSEL ELC scores.
Results
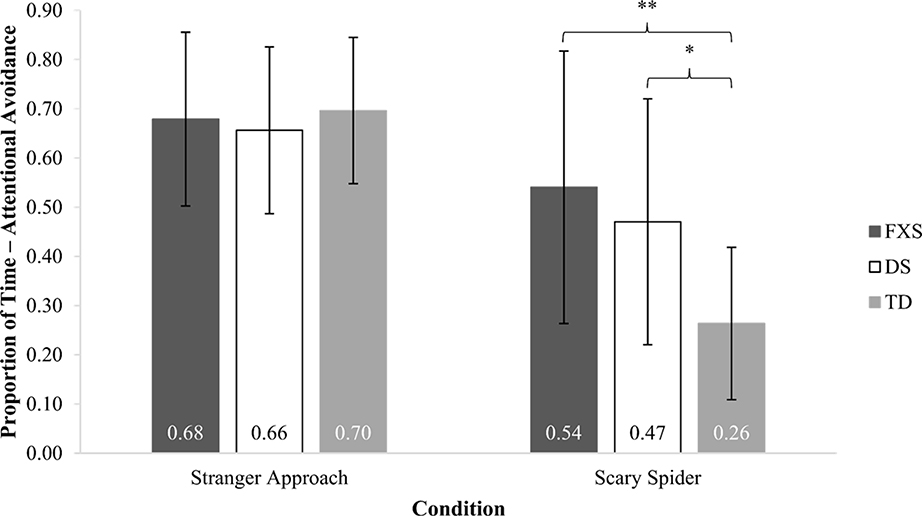
A RMANOVA was used to determine group differences in attentional avoidance away from the social fear target and the nonsocial fear target. The main effect of condition was significant (F(1, 94) = 30.19, p < .001), as was the main effect of group (F(2, 94) = 7.06, p < .001). The main effect of sex was not significant (F(1, 94) = 0.36, p = .548), nor was the interaction of sex-by-condition (F(1, 94) = 2.35, p = .128). A significant group-by-condition interaction did emerge (F(2, 94) = 4.65, p = .012). Bonferroni-corrected pairwise comparisons revealed that groups demonstrated similar patterns of attention during the social fear condition (all ps = 1.00). Group differences were observed for the nonsocial fear condition. Toddlers with FXS and toddlers with DS exhibited significantly more attentional avoidance than TD toddlers (ps ≤ .021) but did not differ from each other (p = 1.00). Figure 1 illustrates these findings.
Figure 1.
Proportion of Attentional Avoidance: Stranger Approach and Scary Spider
Note. * p < .05, ** p < .01. Error bars show standard errors. The stranger from the Stranger Approach condition and the spider from the Scary Spider condition represent novel and potentially fearful social and nonsocial fear targets, respectively.
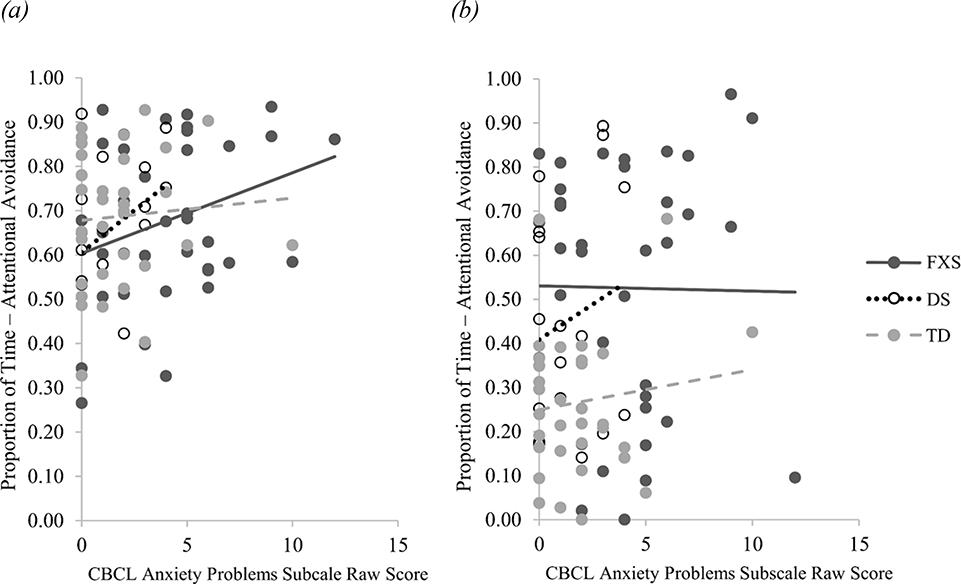
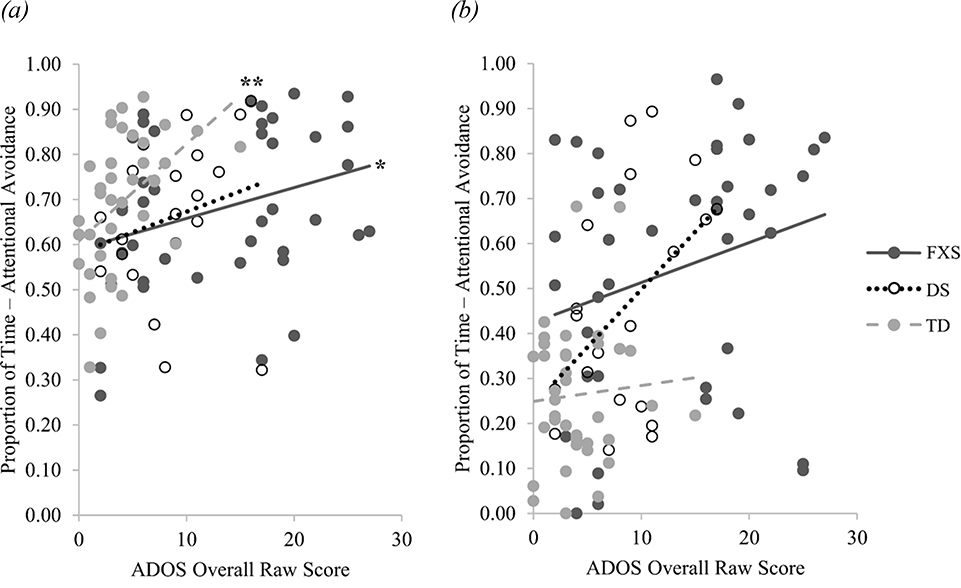
Spearman’s rank correlation coefficients were calculated to determine if attentional avoidance in either condition was related to parent-reported anxiety symptom severity, ASD symptom severity, or developmental level. Attentional avoidance away from the social fear target was not correlated with anxiety symptom severity for toddlers with FXS (rs(34) = .22, p = .191), toddlers with DS (rs(14) = . 39, p = .122), or TD toddlers (rs(31) = .04, p = .804). Similarly, attentional avoidance away from the nonsocial fear target was not correlated with anxiety symptom severity for toddlers with FXS (rs(34) = .02, p = .903), toddlers with DS (rs(14) = .11, p = .678), or TD toddlers, (rs(31) = −.04, p = .827; see Figure 2). Attentional avoidance away from the social fear target was not correlated with developmental level in toddlers with FXS (rs(38) = −.26, p = .095), toddlers with DS (rs(19) = −.28, p = .210), or TD toddlers (rs(34) = −.19, p = .262), nor was attentional avoidance away from the nonsocial fear target correlated with developmental level in toddlers with FXS (rs(38) = −.22, p = .171), toddlers with DS (rs(19) = .06, p = .776), or TD toddlers (rs(34) = −.07, p = .695 ; see Figure 3). However, more attentional avoidance away from the social fear target was significantly correlated with higher ADOS-2 overall raw scores in toddlers with FXS (rs(38) = .32, p = .039) and TD toddlers (rs(34) = .53, p = .001), but not in toddlers with DS (rs(17) = .37, p = 113). Attentional avoidance away from the nonsocial fear target was not significantly correlated with ADOS-2 overall raw scores in toddlers with FXS (rs(38) = .29, p = .067), toddler with DS (rs(17) = .37, p = .105), or TD toddlers (rs(34) = .03, p = .886; see Figure 4).
Figure 2.
Association between Anxiety Symptom Severity and Attentional Avoidance
Note. Association between anxiety symptom severity, indexed by the CBCL Anxiety Problems subscale raw scores, and proportion of attentional avoidance. (a) Correlation between anxiety symptom severity and proportion of attentional avoidance from the social fear target (i.e., stranger). (b) Correlation between anxiety symptom severity and proportion of attentional avoidance from the nonsocial fear target (i.e., spider).
Figure 3.
Association between Developmental Level and Attentional Avoidance
Note. Association between developmental level, indexed by the MSEL ELC scores, and proportion of attentional avoidance. Given that the lowest possible MSEL ELC score that can be obtained is 49, the x-axis starts at 40. (a) Correlation between developmental level and proportion of attentional avoidance from the social fear target (i.e., stranger). (b) Correlation between developmental level and proportion of attentional avoidance from the nonsocial fear target (i.e., spider).
Figure 4.
Association between ASD Symptom Severity and Attentional Avoidance
Note. Association between ASD symptom severity, indexed by the ADOS overall raw scores, and proportion of attentional avoidance. (a) Correlation between ASD symptom severity and proportion of attentional avoidance from the social fear target (i.e., stranger). (b) Correlation between ASD symptom severity and proportion of attentional avoidance from the nonsocial fear target (i.e., spider).
* p < .05, ** p < .01.
Discussion
Anxiety disorders are among the most common and impairing mental health disorders diagnosed in children. Due to the long-term impairment associated with childhood anxiety, understanding the early signs of anxiety in toddlers at elevated risk is essential for earlier detection, which facilitates early intervention and improvement of long-term outcomes. Attentional bias away from perceived sources of threat in toddlers has been identified as a potential behavioral risk marker for later diagnoses of anxiety in young children (Waters et al., 2014). The present study examined attentional avoidance as an early behavioral risk marker of anxiety in toddlers with FXS and DS contrasted to TD controls. This work is important given evidence that anxiety disorders are highly prevalent in FXS and the paucity of research on the emergence of anxiety disorders in DS.
In the present study, attentional avoidance in response to a social fear target and a nonsocial fear target was examined in FXS, DS, and TD toddlers. Our results indicated that all three groups displayed similar patterns of attentional avoidance during the social fear condition, spending over 65% of the condition directing their attention away from the social fear target (i.e., the stranger). This finding was inconsistent with our hypothesis that toddlers with FXS would exhibit greater attention bias away from the social fear target compared to the DS and TD groups. Toddlers with FXS displayed elevated anxiety as indexed by the CBCL, with approximately 10% at or above the clinical cutoff for Anxiety Problems; however, there was no discernable relationship between parent-reported anxiety and attentional avoidance. This result was surprising given previous work has shown that elevated attentional avoidance in response to social stimuli in early childhood has been linked to later social anxiety in TD children (Waters et al., 2014).
One explanation for the similar levels of attentional avoidance across groups is that toddlers with FXS and DS did not process the salience of the social fear condition given their lower cognitive levels. However, there was no relationship between developmental scores and attentional avoidance across any of the groups which fails to support that conclusion. Another explanation could be that social fear is so heightened at this age that it is difficult to tease apart group differences, which is somewhat supported by our finding that all groups spent the majority of the time (nearly 70%) averting their gaze from the stranger. It is also possible that the toddlers with FXS displayed normative levels of social fear at this stage in development. This hypothesis is supported by the lack of group differences as well as the lack of association between parent-reported anxiety and attentional avoidance across groups. Furthermore, this level of social fear may be developmentally appropriate and, over time, may subside in children with DS and TD children but remain stable or worsen in children with FXS. Previous research suggests that social avoidance increases in individuals with FXS over development, and the emergence of social anxiety might follow a similar developmental trajectory (Roberts, Crawford, Hogan, et al., 2019). A final explanation is that our social fear condition may not elicit social fear in these groups or at this age. This hypothesis is supported by the lack of group differences, no relationship between parent-reported anxiety and child behavior during the stranger condition, and earlier work showing physiological patterns suggesting interest rather than fear (Tonnsen, Shinkareva, et al., 2013). Although it is also possible that parent-reported and direct observational measures of anxiety are independent, it is difficult to know the discriminative value of either measure until the toddlers are older and have received an anxiety outcome.
However, attentional avoidance to a novel social target may be subserved by different mechanisms across distinct neurodevelopmental disorders. Along these lines, in toddlers with FXS, more attentional avoidance in response to the novel social target was significantly correlated with more severe ASD symptoms, but this correlation was not observed in the DS group. This is consistent with evidence from Roberts, Crawford, Will, et al. (2019), in which reduced eye contact during social interactions with an unfamiliar person was indicative of more severe ASD symptoms, but not anxiety, in young children with FXS. The lack of correlation between attentional avoidance to the social fear target and concurrent anxiety symptoms in any group provides further support that perhaps attentional avoidance to social fear targets is tapping more into reduced social interest than increased anxiety. These findings are an important reminder that even when behavioral patterns are similar across children with different neurodevelopmental disorders, such behavioral patterns may have different underlying mechanisms (e.g., social fear v. social interest) as well as different clinical implications (e.g., anxiety v. ASD) across disorders. The findings also highlight the complexity of distinguishing potential behavioral risk markers of social anxiety in toddlers with neurodevelopmental disorders as well as the difficulty of contributing attention to a singular function in groups with complex and often overlapping symptomatology.
In contrast to the social fear target, significant group differences in attentional avoidance were observed in response to the nonsocial fear target (i.e., spider). Specifically, toddlers with FXS and toddlers with DS showed significantly more avoidant attention than TD toddlers during the nonsocial fear condition. Interestingly, attentional avoidance to the nonsocial fear target was not correlated with anxiety symptom severity, ASD symptom severity, or developmental level in toddlers with FXS, toddlers with DS, or TD toddlers. Although significant group differences in attentional avoidance to the nonsocial fear target were observed, the lack of significant correlations suggest these differences are driven by other factors, such as lack of interest or inattentiveness. Attentional avoidance does not appear to be a behavioral marker of anxiety in toddlers with FXS, DS, or TD toddlers, further suggesting that attention cannot be used as an early behavioral marker of anxiety without further considerations.
Identifying early behavioral risk markers of anxiety is crucial for the diagnosis and treatment of specific anxiety disorders. Given the prevalence and impairment associated with anxiety, especially in children with neurodevelopmental disorders such as FXS and DS, understanding how anxiety manifests in at-risk populations is essential to providing an accurate diagnosis and initiating treatment. Discerning and describing the behavioral phenotype of anxiety in children with genetic neurodevelopmental disorders, such as FXS and DS, will help to disentangle the symptoms associated with anxiety disorders from the symptoms associated with other comorbid disorders, such as ASD. One such factor that has been used as a potential behavioral marker of anxiety in TD children is attention. However, in the present study, attentional avoidance was more strongly related to ASD symptoms than anxiety symptoms. The complexity in attentional patterns in children with neurodevelopmental disorders may be attributed to variability within syndromes, differing developmental trajectories, and the complex interplay between comorbid conditions. Although the use of attentional avoidance to potentially frightening novel stimuli could serve as a useful marker of atypical development and later-emerging anxiety, further research is needed to understand patterns of attentional avoidance in children with neurodevelopmental disorders.
Although these findings provide valuable insight into the use of attentional avoidance as an early behavioral risk marker, the present study has limitations. One limitation is the small sample size of our DS group, which may not be fully representative of the variability within the DS population. A larger sample size would increase the generalizability of these results, improve our power to detect subtle or nuanced differences, and further elucidate the emerging differential profiles of anxiety and ASD in children with FXS and DS. Additionally, while this study did account for both ID and ASD, we did not account for other common comorbid disorders, such as attention-deficit/hyperactivity disorder, which could affect the child’s allocation of attention. Furthermore, the study did not randomize the conditions across subjects; therefore, it is unclear whether the observed effect is influenced by the order of condition presentation. The study also did not perform repeated trials of the conditions at 24 months due to risk of habituation. Additionally, it is also worth noting that the CBCL has not been normed in children with ID; thus, the inclusion of additional or ID-specific anxiety measures may provide a more accurate depiction of anxiety symptomatology in toddlers with FXS and DS. Moreover, CBCL raw scores were also relatively restricted in our sample, particularly for the toddlers with DS and the TD toddlers. Anxiety symptomatology was not reflected in the present sample of toddlers with DS or TD toddlers, thus restricting our ability to accurately test the relationship between attentional avoidance and anxiety symptom severity. A similar restriction in range was observed for the Mullen ELC scores given that toddlers with FXS and DS have a lower IQ due to ID.
Future studies should investigate the development of early behavioral markers of anxiety, such as attentional avoidance, both prior to and beyond 24 months of age. A longitudinal design would provide critical insight into both the emergence and trajectory of anxiety and attentional bias in individuals with FXS and DS. The developmental trajectory of these early markers should also be correlated with diagnoses of anxiety and ASD later in childhood. Additionally, the incorporation of physiological markers should be used to establish a more comprehensive and holistic understanding of the emergence of anxiety and differentiation with ASD symptomatology by providing additional insight into the child’s emotional state and could either bolster or negate the direct behavioral observations.
Acknowledgements:
The authors sincerely thank the participants and their families for their time and support. This work was funded by the National Institute of Mental Health (R01MH107573 PI: Roberts and R01MH090194 PI: Roberts) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F32HD097877 PI: Will).
Footnotes
Declarations of Interest: None
Contributor Information
Kayla D. Smith, University of South Carolina, Department of Psychology, 1512 Pendleton St., Barnwell College Suite #220, Columbia, SC 29208, USA
Abigail L. Hogan, University of South Carolina, Department of Psychology, 1512 Pendleton St., Barnwell College Suite #220, Columbia, SC 29208, USA
Elizabeth A. Will, University of South Carolina, Department of Psychology, 1512 Pendleton St., Barnwell College Suite #220, Columbia, SC 29208, USA
Jane E. Roberts, University of South Carolina, Department of Psychology, 1512 Pendleton St., Barnwell College Suite #220, Columbia, SC 29208, USA.
References
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Brown WT, Harvey DJ, Adayev T, LaFauci G, Dobkins C, & Roberts JE (2019). ASD comorbidity in fragile X syndrome: Symptom profile and predictors of symptom severity in adolescent and young adult males. Journal of Autism and Developmental Disorders, 49(3), 960–977. 10.1007/s10803-018-3796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Bailey DB Jr., Raspa M, Olmsted M, & Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics, 146A(16), 2060–2069. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- Bandelow B & Michaelis S (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, & Pine DS (2009). Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. The Psychiatric Clinics of North America, 32(3), 483–524. 10.1016/j.psc.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, & Shic F (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203. 10.1016/j.biopsych.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromik LC, Quintin EM, Lepage JF, Hustyi KM, Lightbody AA, & Reiss AL (2019). The influence of hyperactivity, impulsivity, and attention problems on social functioning in adolescents and young adults with fragile X syndrome. Journal of Attention Disorders, 23(2), 181–188. 10.1177/1087054715571739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of neurodevelopmental disorders, 3(1), 57–67. 10.1007/s11689-010-9067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack KFM, Brown AC, & Hastings RP (2000). Behavioural and emotional difficulties in students attending schools for children and adolescents with severe intellectual disability. Journal of Intellectual Disability Research, 44(2), 124–129. 10.1046/j.1365-2788.2000.00251.x [DOI] [PubMed] [Google Scholar]
- Crawford H, Moss J, Groves L, Dowlen R, Nelson L, Reid D, & Oliver C (2020). A behavioural assessment of social anxiety and social motivation in fragile X, Cornelia de Lange and Rubinstein-Taybi syndromes. Journal of Autism and Developmental Disorders, 50(1), 127–144. 10.1007/s10803-019-04232-5 [DOI] [PubMed] [Google Scholar]
- Crawford H, Waite J, & Oliver C (2017). Diverse profiles of anxiety related disorders in fragile X, Cornelia de Lange and Rubinstein-Taybi Syndromes. Journal of Autism and Developmental Disorders, 47(12), 3728–3740. 10.1007/s10803-016-3015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhauer LA & Fidler DJ (2011). The Down syndrome behavioral phenotype: implications for practice and research in occupational therapy. Occupational Therapy in Health Care, 25(1), 7–25. 10.3109/07380577.2010.535601 [DOI] [PubMed] [Google Scholar]
- Dekker MC & Koot HM (2003). DSM-IV disorders in children with borderline to moderate intellectual disability. I: Prevalence and impact. Journal of the American Academy of Child & Adolescent Psychiatry, 42(8), 915–922. 10.1097/01.CHI.0000046892.27264.1A [DOI] [PubMed] [Google Scholar]
- DiGuiseppi C, Hepburn S, Davis JM, Fidler DJ, Hartway S, Lee NR, Miler L, Ruttenber M, & Robinson C (2010). Screening for autism spectrum disorders in children with Down syndrome: population prevalence and screening test characteristics. Journal of Developmental and Behavioral Pediatrics, 31(3), 181–191. 10.1097/DBP.0b013e3181d5aa6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens EM, Shah B, Davis B, Baker C, Fife T, & Fitzpatrick J (2015). Psychiatric disorders in adolescents and young adults with Down syndrome and other intellectual disabilities. Journal of Neurodevelopmental Disorders, 7(1), 9. 10.1186/s11689-015-9101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, & Taga K (2005). The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: Comparisons with developmentally and chronologically age matched children. Child Psychiatry and Human Development, 36(1), 3–26. 10.1007/s10578-004-3619-x [DOI] [PubMed] [Google Scholar]
- Ezell J, Hogan A, Fairchild A, Hills K, Klusek J, Abbeduto L, & Roberts J (2019). Prevalence and predictors of anxiety disorders in adolescent and adult males with autism spectrum disorder and fragile X syndrome. Journal of Autism and Developmental Disorders, 49(3), 1131–1141. 10.1007/s10803-018-3804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler DJ, Most DE, Booth-LaForce C, & Kelly JF (2008). Emerging social strengths in young children with Down syndrome. Infants & Young Children, 21(3), 207–220. 10.1097/01.IYC.0000324550.39446.1f [DOI] [Google Scholar]
- Goldsmith H, & Rothbart MK (1996). The Laboratory Temperament Assessment Battery (Lab-TAB): Locomotor Version 3.0 (Technical manual). Madison, WI: Department of Psychology, University of Wisconsin. [Google Scholar]
- Green SA, Berkovits LD, & Baker BL (2015). Symptoms and development of anxiety in children with or without intellectual disability. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 44(1), 137–144. 10.1080/15374416.2013.873979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ (2008). The fragile X prevalence paradox. Journal of Medical Genetics, 45(8), 498–499. 10.1136/jmg.2008.059055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AL, Caravella KE, Ezell J, Rague L, Hills K, & Roberts JE (2017). Autism spectrum disorder symptoms in infants with fragile X syndrome: A prospective case series. Journal of Autism and Developmental Disorders, 47(6), 1628–1644. 10.1007/s10803-017-3081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RG, Wiertz LF, Meyer ES, & Noldus LP (2003). Reliability analysis of observational data: Problems, solutions, and software implementation. Behavior Research Methods, Instruments, & Computers, 35(3), 391–399. 10.3758/BF03195516 [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller RA, & Townsend J (2013). Atypical attentional networks and the emergence of autism. Neuroscience & Biobehavioral Reviews, 37(2), 164–183. 10.1016/j.neubiorev.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Renno P, Kendall PC, Wood JJ, & Storch EA (2017). Anxiety disorders interview schedule-autism addendum: reliability and validity in children with autism spectrum disorder. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 46(1), 88–100. 10.1080/15374416.2016.1233501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D Visootsak J, & Berry-Kravis E (2014). Fragile X syndrome: A review of associated medical problems. Pediatrics, 134(5), 995–1005. 10.1542/peds.2013-4301 [DOI] [PubMed] [Google Scholar]
- Kleberg JL, Högström J, Nord M, Bölte S, Serlachius E, & Falck-Ytter T (2017). Autistic traits and symptoms of social anxiety are differentially related to attention to others’ eyes in social anxiety disorder. Journal of Autism and Developmental Disorders, 47(12), 3814–3821. 10.1007/s10803-016-2978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JE, & Losh M (2015). Cardiac autonomic regulation in autism and fragile X syndrome: a review. Psychiatric Bulletin, 141(1), 141–175. 10.1037/a0038237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Määttä T, Määttä J, Tervo- Määttä T, Taanila A, Kaski M, & Iivanainen M (2011). Healthcare and guidelines: A population-based survey of recorded medical problems and health surveillance for people with Down syndrome. Journal of Intellectual & Developmental Disability, 36(2) 118–126. 10.1080/13668250.2011.570253 [DOI] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, & Abbeduto L (2015). Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADI-R scores. Journal of Autism and Developmental Disorders, 45(7), 1925–1937. 10.1007/s10803-013-2013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, & Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian ND, Godoy L, Briggs-Gowan MJ, & Carter AS (2012). Patterns of anxiety symptoms in toddlers and preschool-age children: Evidence of early differentiation. Journal of Anxiety Disorders, 26(1), 102–110. 10.1016/j.janxdis.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi JM, Klin A & Jones W (2017). Mechanisms of diminished attention to eyes in autism. The American Journal of Psychiatry, 174(1), 26–35. 10.1176/appi.ajp.2016.15091222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J & Howlin P (2009). Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. Journal of Intellectual Disability Research, 53(10), 852–873. 10.1111/j.1365-2788.2009.01197.x [DOI] [PubMed] [Google Scholar]
- Moss J, Oliver C, Arron K, Burbidge C, & Berg K (2009). The prevalence and phenomenology of repetitive behavior in genetic syndromes. Journal of Autism and Developmental Disorders, 39(4), 572–588. 10.1007/s10803-008-0655 [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning: AGS Edition. Circle Pines, MN: American Guidance Services. [Google Scholar]
- Munir KM (2016). The co-occurrence of mental disorders in children and adolescents with intellectual disability/intellectual developmental disorder. Current Opinion in Psychiatry, 29(2), 95–102. 10.1097/YCO.0000000000000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Næss KB, Nygaard E, Ostad J, Dolva AS, & Lyster SH (2017). The profile of social functioning in children with Down syndrome. Disability and Rehabilitation, 39(13), 1320–1331. 10.1080/09638288.2016.1194901 [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, & Correa A (2010). Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research Part A: Clinical and Molecular Teratology, 88(12), 1008–1016. 10.1002/bdra.20735 [DOI] [PubMed] [Google Scholar]
- Paulus FW, Backes A, Sander CS, Weber M, & von Gontard A (2015). Anxiety disorders and behavioral inhibition in preschool children: A population-based study. Child Psychiatry & Human Development, 46(1), 150–157. 10.1007/s10578-014-0460-8 [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, & Fox NA (2010). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion, 10(3), 349–357. 10.1037/a0018486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, Hane AA, Pine DS, & Fox NA (2011). Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology, 39(6), 885–895. 10.1007/s10802-011-9495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikora TJ, Bourke J, Bathgate K, Foley KR, Lennox N, & Leonard H (2014). Health conditions and their impact among adolescents and young adults with Down syndrome. PloS One, 9(5), e96868. 10.1371/journal.pone.0096868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, & Rohde LA (2015). Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry, 56(3), 345–365. 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- Reardon TC, Gray KM, & Melvin GA (2015). Anxiety disorders in children and adolescents with intellectual disability: Prevalence and assessment. Research in Developmental Disabilities, 36, 175–190. 10.1016/j.ridd.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bradshaw J, Will E, Hogan AL, Mcquillin S, & Hills K (2020). Emergence and rate of autism in fragile X syndrome across the first years of life. Development and Psychopathology, 32(4), 1335–1352. 10.1017/s0954579420000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Crawford H, Hogan AL, Fairchild A, Tonnsen B, Brewe A, O’Conner S, Roberts D, & Abbeduto L (2019). Social avoidance emerges in infancy and persists into adulthood in fragile X syndrome. Journal of Autism and Developmental Disorders, 49(9), 3753–3766. 10.1007/s10803-019-04051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Crawford H, Will EA, Hogan AL, McQuillin S, Tonnsen BL, O’Connor S, Roberts DA & Brewe AM (2019). Infant social avoidance predicts autism but not anxiety in fragile X syndrome. Frontiers in Psychiatry, 10, 199. 10.3389/fpsyt.2019.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Bailey D, Long AC, Anello V, & Colombo J (2012). Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders, 42(6), 937–946. 10.1007/s10803-011-1316-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, McCary LM, Shinkareva SV, & Bailey DB Jr. (2016). Infant development in fragile X syndrome: Cross-syndrome comparisons. Journal of Autism and Developmental Disorders, 46(6), 2088–2099. 10.1007/s10803-016-2737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37(9), 1748–1760. 10.1007/s10803-006-0305-9 [DOI] [PubMed] [Google Scholar]
- Rothbart MK & Hanson MJ (1983). A caregiver report comparison of temperamental characteristics of Down syndrome and normal infants. Developmental Psychology, 19(5), 766. 10.1037/0012-1649.19.5.766. [DOI] [Google Scholar]
- Royston R, Howlin P, Waite J, & Oliver C (2017). Anxiety disorders in Williams syndrome contrasted with intellectual disability and the general population: A systemic review and meta-analysis. Journal of Autism and Developmental Disorders, 47(12), 3765–3777. 10.1007/s10803-016-2909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Gadelha A, Pan P, Tamanaha AC, Moriyama T, Graeff-Martins AS, Jarros RB, Polanczyk G, do Rosário MC, Leibenluft E, Rohde LA, Manfro GG, & Pine DS (2013). Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychological Medicine, 43(4), 733–745. 10.1017/S0033291712001651 [DOI] [PubMed] [Google Scholar]
- Shephard E, Bedford R, Milosavljevic B, Gliga T, Jones EJ, Pickles A, Johnson MH, Charman T, & BASIS Team (2019). Early developmental pathways to childhood symptoms of attention-deficit hyperactivity disorder, anxiety, and autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 60(9), 963–974. 10.1111/jcpp.12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, & Roberts JE (2013). Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology, 41(2), 267–280. 10.1007/s10802-012-9671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, & Roberts JE (2013). Biobehavioral indicators of social fear in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 118(6), 447–459. 10.1352/1944-7558-118.6.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliffe P & Oliver C (2011). Phenotype-environment interactions in genetic syndromes associated with severe or profound intellectual disability. Research in Developmental Disabilities, 32(2), 408–418. 10.1016/j.ridd.2010.12.008 [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, & Wood JJ (2013). Autism spectrum traits in children with anxiety disorders. Journal of Autism and Developmental Disorders, 43(2), 361–370. 10.1007/s10803-012-1575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Bradley BP, & Mogg K (2014). Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus ‘fear’ diagnostic categorization. Psychological Medicine, 44(3), 607–616. 10.1017/S0033291713000779 [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Mussey J, Villagomez A, Bishop E, Raspa M, Edwards A, Bodfish J, Bann C, & Bailey DB Jr. (2015). DSM-5 changes and the prevalence of parent-reported autism spectrum symptoms in fragile X syndrome. Journal of Autism and Developmental Disorders, 45(3), 816–829. 10.1007/s10803-014-2246-z [DOI] [PubMed] [Google Scholar]
- White LK, Degnan KA, Henderson HA, Pérez-Edgar K, Walker OL, Shechner T, Leibenluft E, Bar-Haim Y, Pine DS & Fox NA (2017). Developmental relations among behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development, 88(1), 141–155. 10.1111/cdev.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney DG, Shapiro DN, Peterson MD, & Warschausky SA (2019). Factors associated with depression and anxiety in children with intellectual disabilities. Journal of Intellectual Disability Research, 63(5), 408–417. 10.1111/jir.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]