Abstract
BACKGROUND
While there are reports of acute ischemic stroke (AIS) in coronavirus disease 2019 (COVID-19) patients, the overall incidence of AIS and clinical characteristics of large vessel occlusion (LVO) remain unclear.
OBJECTIVE
To attempt to establish incidence of AIS in COVID-19 patients in an international cohort.
METHODS
A cross-sectional retrospective, multicenter study of consecutive patients admitted with AIS and COVID-19 was undertaken from March 1 to May 1, 2020 at 12 stroke centers from 4 countries. Out of those 12 centers, 9 centers admitted all types of strokes and data from those were used to calculate the incidence rate of AIS. Three centers exclusively transferred LVO stroke (LVOs) patients and were excluded only for the purposes of calculating the incidence of AIS. Detailed data were collected on consecutive LVOs in hospitalized patients who underwent mechanical thrombectomy (MT) across all 12 centers.
RESULTS
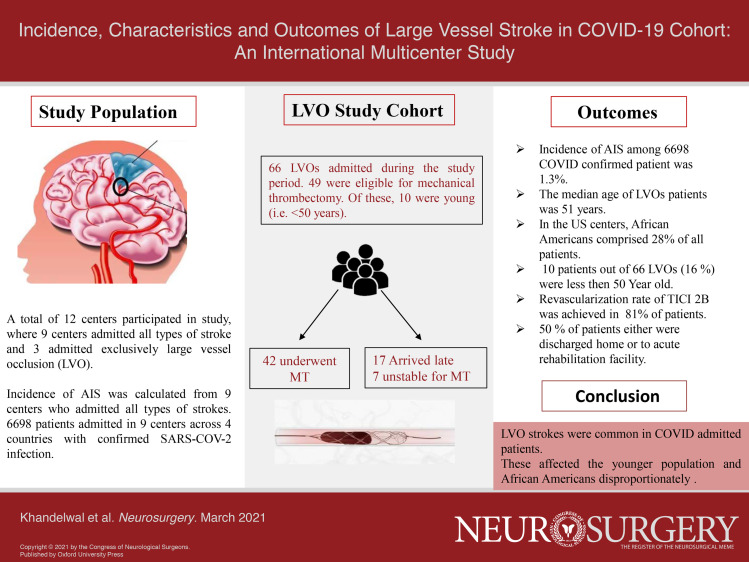
Out of 6698 COVID-19 patients admitted to 9 stroke centers, the incidence of stroke was found to be 1.3% (interquartile range [IQR] 0.75%-1.7%). The median age of LVOs patients was 51 yr (IQR 50-75 yr), and in the US centers, African Americans comprised 28% of patients. Out of 66 LVOs, 10 patients (16%) were less than 50 yr of age. Among the LVOs eligible for MT, the average time from symptom onset to presentation was 558 min (IQR 82-695 min). A total of 21 (50%) patients were either discharged to home or discharged to acute rehabilitation facilities.
CONCLUSION
LVO was predominant in patients with AIS and COVID-19 across 2 continents, occurring at a significantly younger age and affecting African Americans disproportionately in the USA.
Keywords: COVID-19, Acute ischemic stroke, Large vessel occlusion, Epidemiology
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ACE
angiotensin converting enzyme
- AIS
acute ischemic stroke
- CMV
cytomegalovirus
- CRP
C-reactive protein
- CTA
CT angiogram
- DIC
disseminated intravascular coagulopathy
- ER
emergency room
- HERMES
Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials
- IQR
interquartile range
- MRA
MR angiogram
- MT
mechanical thrombectomy
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
- sICH
symptomatic intracerebral hemorrhage
- tPA
tissue plasminogen activator
- VZV
varicella zoster virus
A novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected more than 8 million people across the globe. The incidence of acute ischemic stroke (AIS) in coronavirus disease 2019 (COVID-19) patients is just beginning to be elucidated, but whether the clinical profile of AIS in such patients is different from the pre-COVID-19 era is yet to be established.1-5 Recent studies have shown that patients with COVID-19 appear to have a heightened risk of AIS compared with patients with influenza.6 Given that SARS-CoV-2 affects multiple systems, which potentially increases the risk of embolic stroke, including coagulation, endothelium, cardiac rhythm, and hypoxia,7,8 it is important to understand if acute LVO stroke (LVOs), the most disabling stroke subtype, may be occurring at an increased rate in COVID-19 patients. In addition, it is important to determine whether the clinical characteristics of LVOs in COVID-19 patients and outcomes of treatment with mechanical thrombectomy (MT), with or without IV tissue plasminogen activator (tPA), differ from those in non-COVID-19 patients. While stroke is not typically the primary presentation of COVID-19, recent evidence has been emerging regarding acute stroke in COVID-19 patients. Most alarmingly, stroke is being increasingly reported in young adults with COVID-19.9,10
METHODS
Patient Selection
We conducted a cross-sectional, international multicenter retrospective study of laboratory-confirmed COVID-19 patients with acute LVOs consecutively admitted between March 1 and May 1 in 12 thrombectomy capable stroke centers located in the USA, United Kingdom, Spain, and Italy. The ethical review boards of participating institutions approved the study. Given the retrospective nature of the study, patient consent was waived. Patients with AIS were identified as patients presenting with focal neurological symptoms. Diagnosis of AIS required confirmation by brain computed tomography (CT) or magnetic resonance imaging (MRI). LVO was defined as an occlusion of internal carotid artery, M1 segment of middle cerebral artery, or basilar artery confirmed by CT angiogram (CTA) or MR angiogram (MRA) as per the individual institutional protocol.
Data Collection and Analysis
We collected data on the total number of hospitalized COVID-19 and AIS patients, with detailed data on LVO/AIS patients in 9 of 12 stroke centers to define the incidence of AIS and proportion LVOs. The other 3 centers were exclusive MT referral centers and admitted all stroke subtypes, and hence were excluded for AIS incidence and LVOs proportion calculation, as they would not necessarily reflect the true burden of AIS in COVID-19 patients. The total number of AIS patients included those presenting to the emergency room (ER) and/or patients developing stroke during hospitalization. We did not include any outpatient AIS at these centers during this period. For characterizing detailed demographic and clinical features of LVOs patients eligible for MT as well as their response to MT, all 12 centers were included.
RESULTS
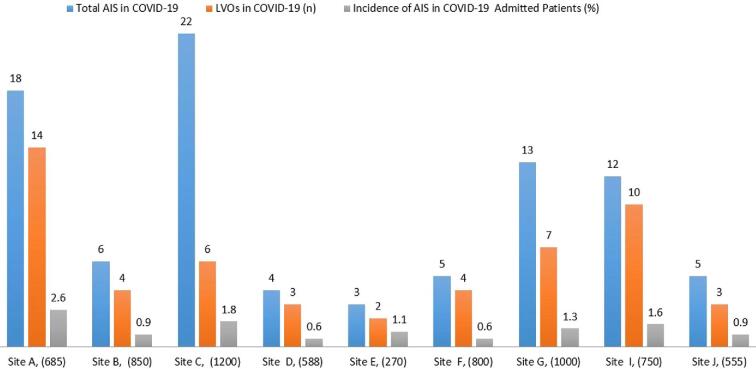
Out of a total of 6698 patients admitted with COVID-19 positive at 9 stroke centers during the study period, the incidence of stroke was 88/6698 (1.3%, IQR 0.75%-1.7%). Of these, 53/88 (60%) were LVOs (Figure 1). To define the clinical characteristics of COVID-19 positive LVOs, acute LVOs from all 12 centers were analyzed and a total of 66 patients were identified. In this cohort, the median age of LVOs patients was 51 yr (IQR 50-75 yr). Out of 66, a total of 42 (63%) of these patients underwent MT (Table 1). Out of 66 patients, 24 patients (36%) either arrived late or were too unstable for MT. The median age in this subgroup of patients undergoing MT was 54 yr (IQR 52-76 yr). Eight of these (8/42, 19%, IQR 32-46 yr) patients who underwent MT were under 50 yr of age (Table 2). One (1/8, 12%) patient in this young MT cohort died during hospitalization. Among the 8 US centers, African Americans (AAs) constituted 28% of all LVOs patients. The rate of internal carotid artery-middle cerebral artery (ie, ICA-MCA tandem) occlusion was 21%. The median time from symptom onset to presentation to an ER was 558 min (IQR, 83-695 min).
FIGURE 1.
X-axis shows the name of the hospital and total number of COVID admissions during the study period. Y-axis shows the number of AIS (blue bar), LVOs (red bar), and incidence of AIS in COVID-19 (%) (green bar) admitted patients. (1) UH, NJ, USA, stands for University Hospital, New Jersey Medical School, Rutgers, New Jersey, USA; (2) RWJ, NJ, USA stands for Robert Wood Johnson Hospital & Medical School, Rutgers, New Jersey, USA; (3) WMC, NY, US stands for Westchester Medical Center at NY Medical College, Valhalla, New York, USA; (4) BK/NYU, NY, USA stands for Brookdale Hospital Center, NYU School of Medicine, New York City, USA; (5) NH, NY, USA Eastern Region, Northwell Health, Long Island, New York, USA; (6) SJH, DMC stands for Michigan, Saint Joseph Health, Detroit Medical Center, Michigan, USA; (7) RCH, CA stands for University of California Riverside, Riverside Community Hospital, California, USA; (8) CU stands for Careggi University, Florence, Italy, and GOMN stands for Grande Ospedale Metropolitano Niguarda, Milan. Italy; (9) U of V stands for Hospital Clínico Universitario de Valladolid, Spain; and (10) RSUH, UK stands for Royal Stoke University Hospital, University Hospital of North Midlands, Stoke-on-Trent, United Kingdom. LVOs is large vessel occlusion stroke; AIS is acute ischemic stroke.
TABLE 1.
Large Vessel Occlusion in COVID-19 Confirmed Patients: Demographics, Comorbidities, Hospitalization Events, and Outcomes
| Characteristics | Young strokea (18-49 yr) | Nonyoung stroke (>50 yr) | Overall group |
|---|---|---|---|
| Large vessel occlusion (LVO) in COVID-19 (n) | 10 | 56 | 66 |
| Median age (yr) (range) | 36 (27-49) | 73 (51-87) | 51 (27-87) |
| MT performed (%) | 8 (80) | 34(60) | 42 (63) |
| Characteristics of LVO undergoing MTb | |||
| Median NIHSS (IQR) | 11 (15-22) | 18 (14-24) | 18 (12-24) |
| Sex | |||
| Male, n (%) | 5 (62) | 16(47) | 21 (50) |
| Female, n (%) | 3 (37) | 18(53) | 21(50) |
| Race/ethnicity, n (%) | |||
| White | 2 (25) | 22 (64) | 24 (57) |
| African American | 2 (25) | 9(26) | 11(26) |
| Hispanic/Latino | 1 (12.5) | 4 (11) | 5 (11) |
| Asian/Pacific Islander/others | 1 (12) | 1 (3) | 2 (5) |
| Stroke sign and symptoms | |||
| Median NIHSS (IQR) | 11(15-22) | 18 (14-24) | 13 (12-24) |
| LVO, n (%) | |||
| Right MCA | 1 (12) | 9 (26) | 11 (26) |
| Left MCA | 6 (75) | 13 (38) | 19 (45) |
| Distal ICA + right or left MCA occlusion (tandem) | 1 (12) | 8 (23) | 9 (21) |
| BA | 0 | 4 (11) | 4 (9) |
| Median time from last known well to ER (min) | 408.5 | 345 | 558.5 |
| IQR | 80-747 | 82-555 | 82-695 |
| Laboratory studies (median, IQR) | |||
| D-dimer, ng/mL | 1683 (202-4332) | 912 (223-3260) | 997 (223-3286) |
| White-cell count x 103/mm3. | 8.71 (8-11.725) | 7.4 (3.5-9.77 | 8 (5.2- 10) |
| Ferritin, ng/mL | 1064 (10-1100) | 368 (0-654) | 511 (12-720) |
| C-reactive protein, mg/L | 59 (22-107) | 39 (5.95-41.5) | 48 (6.15-56.25) |
| Absolute neutrophil count, % | 7.85 (5.05-11.1) | 4.3 (4-8.6) | 9.5 (5.15- 10) |
| Absolute lymphocyte count, %. | 5.75 (2.1-17) | 1.5(1-8.5) | 5.4 (1.3-10) |
| COVID-19 symptoms, n(%) | |||
| Fever | 2 (25) | 11 (32) | 12 (28) |
| Cough | 5/8 (62) | 18/(52) | 24 (57) |
| SOB | 2/8 (25) | 15/34(44) | 16(38) |
| Chest X-ray infiltrate | |||
| Unilateral | 3/8 (37.5) | 9/34 (26) | 12/42 (28) |
| Bilateral | 4/8(50) | 28/34(82) | 32/42 (76) |
| Intubation on presentationc | 2/8 (25) | 5(14) | 7/42 (16) |
| Angiographic results | |||
| TICI 0-2A | 1/8(12) | 7/34 (20) | 8/42 (19) |
| TICI 2B/3 | 7/8 (87) | 27/34 (80) | 34/42 (81) |
| >PH1 | 2/8 (25) | 4/32 (11) | 6/40 (14) |
| sICH | 0 | 0 | 0 |
| Preexisting conditions/comorbidities, n (%) | |||
| Hypertension | 1 (12) | 28 (82) | 29 (69) |
| Hyperlipidemia | 1 (12) | 18 (52) | 19 (45) |
| Diabetes mellitus | 4 (50) | 9 (26) | 13 (30) |
| Afib | 0 (0) | 10 (34) | 10 (23) |
| Smoking (active) | 0 | 8 (23) | 8 (19) |
| Known, hypercoagulable | 0 | 0 | 0 |
| Treatment | |||
| IV-rtPA | 3/8 (37) | 16/34 (47) | 19/42 (45) |
| Anticoagulation | 3/8 (37) | 3/34 (8) | 6/42 (14) |
| Antithrombotic agent | 5/8 (75) | 25/34 (73) | 31/40 (73) |
| Statins | 7/8 (87.5) | 24/32 (70) | 31/40 (73) |
| Azithromycin | 3/8 (37.5) | 10/34 (29) | 13/42 (30) |
| Hydroxychloroquine | 1/8 (12.5) | 14/34 (41) | 15/40 (35) |
| Disposition, n (%) | |||
| Home | 3/8 (37) | 5/34 (23) | 8/42 (19) |
| Acute rehabilitation | 3/8 (37) | 10/34 (35) | 13/42 (30) |
| Skilled nursing facility | 1/8 (12) | 4/34 (11) | 5/42 (11) |
| Currently still inpatient | 0 | 6/34 (17) | 6/42 (14). |
| Death in hospital, n, (%) | 1/8 (12) | 9/32 (28) | 10/42 (23) |
IQR, interquartile range; VTE, venous thromboembolism; IV-rtPA, intravenous recombinant tissue plasminogen activator; ICA, internal carotid artery; BA, basilar artery; MCA, middle cerebral artery. Reference range: C-reactive protein (CRP), 0-5 mg/L; D-dimer, 90-500 ng/mL; ferritin, 20-400, ng/mL; absolute neutrophil % x 10 * 3/ul, absolute lymphocyte % 10 * 3/ul; NIHSS, National Institutes of Health Stroke Scale ranging from 0 to 42, with a higher number indicating more severe stroke; TICI, thrombolysis in cerebral infarction score, ranging from TICI 0 (no vessel recanalization), TICI 2A (<50% vessel recanalization), TICI 2B (>50% vessel recanalization), TICI 2C (>90% vessel recanalization) to TICI 3 (complete vessel recanalization); PH, parenchymal hemorrhage with mass effect: PH1 < 30% of the infarcted area with mild space-occupying effect and PH2 > 30% of the infarcted area with significant space-occupying effect; sICH, symptomatic intracerebral hemorrhage; DM, diabetes mellitus; HTN, hypertension.
aYoung was defined as age less than 50 yr.
bThe clinical, laboratory, angiographic, and neurological outcomes were calculated in COVID-19 patients who underwent mechanical thrombectomy.
cNot including intubations required for general anesthesia in the operating room.
TABLE 2.
Clinical Characteristics of 8 Young Patients Presenting With Large Vessel Occlusion Strokes Who Underwent MT
| Variablea | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Age | 27 | 33 | 37 | 38 | 39 | 40 | 49 | 49 |
| Sex | Male | Female | Male | Male | Female | Male | Male | Female |
| Race | AS | AA | H | C | H | AA | H | C |
| NIHSSb | 18 | 17 | 22 | 6 | 12 | 26 | 19 | 25 |
| Region, country | NYC, USA | NJ, USA | NJ, USA | NYC, USA | NYC, USA | NYC, USA | NYC, USA | Spain |
| Stroke risk factors | None | None | DM | None | None | HTN, DM | DM. | None |
| Location of LVO | Left distal ICA to MCA | Left MCA | Left MCA | Right MCA | Left MCA | Left MCA | Left MCA | Left MCA |
| Time to presentation (h) | 12 | 2.2 | 4 | 1 | 12 | 15 | 1 | 2.5 |
| TICI score | 2A | 2B | 2C | 2B | 3 | 2C | 2B | 3 |
| COVID-19 symptoms | Cough | Cough, fever, chills | Cough | Cough, fever, SOB | None | Cough | SOB | None |
| Chest X-ray | Infiltrate B/L lungs | Unremarkable | Infiltrate in both lungs | Infiltrate in both lungs | Unremarkable | Right lung infiltration | Unremarkable | Right lung infiltration |
| Fibrinogen, mg/dL | 970 | 339 | 368 | 623 | Not sent | 542 | 170 | 403 |
| D-dimer, ng/mL | 5446 | 4964 | 2312 | 2439 | Not Sent | 769 | 601 | 1054 |
| Ferritin, ng/mL | 592 | 970 | 703 | 1533 | Not sent | 207 | 130 | 100 |
| CRP, mg/L | 22 | 107 | 83 | Not sent | 56 | 19 | 9.3 | |
| White cell count, per mm3 | 17.4 | 8 | 8 | 5.7 | 9.42 | 26 | 8 | 12.3 |
| Treatment | IVrtPA, DAPT, converted to lovenox on day13 | Aspirin 81 mg | Aspirin 81 mg | IV-rtPA, aspirin 81 mg | Xarelto | Full dose lovenox | Aspirin 81 mg | Aspirin 100 mg |
| Outcome | Acute rehab | Death | Acute rehab | Home | Home | Acute rehab | Home | Home |
aReference ranges are as follows: C-reactive protein (CRP) 0-5 mg/L, D-dimer 90-500 ng/mL, ferritin 20-400 ng/mL.
bScores on the National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42, with higher numbers indicating more severe stroke.
MCA, middle cerebral artery; ICA, internal cerebral artery; LVO, large vessel occlusion; TICI score, thrombolysis in cerebral infarction score, ranging from TICI 0 (no vessel recanalization), TICI 2A (<50% vessel recanalization), TICI 2B (>50% vessel recanalization), TICI 2C (>90% vessel recanalization) to TICI-3 (complete vessel recanalization); IV-rtPA, intravenous tissue recombinant plasminogen activator; DM, diabetes mellitus; HTN, hypertension; SOB, shortness of breath; AA, African American; C, Caucasian; H, Hispanic; A, Asian; M, male; F, female; NYC, New York City; NJ is New Jersey.
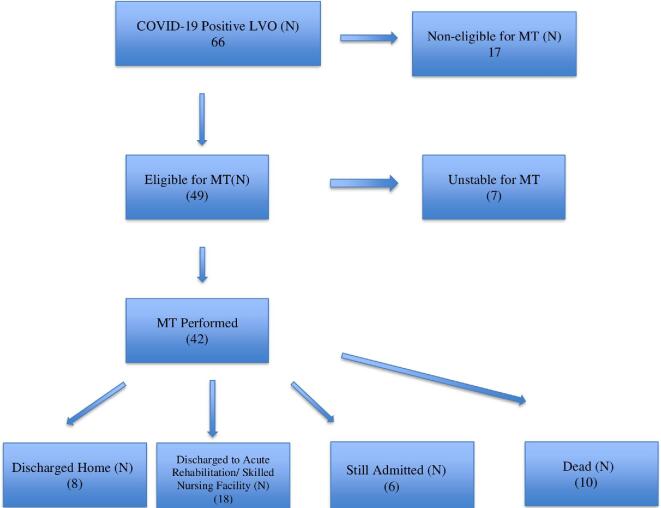
The majority of patients had elevated serological markers of inflammation with a mean C-reactive protein (CRP) and D-dimer of 48 and 997, respectively. With regard to outcomes of reperfusion therapy in this LVO cohort, the thrombolysis in cerebral infarction (TICI) > 2b reperfusion rate was 81% (34/42) and parenchymal hematoma type 1 (PH1) rate was 14% (5/42) with no symptomatic intracerebral hemorrhage (sICH). Out of 42 patients, 21 (50%) patients who underwent MT were discharged home or to an acute rehabilitation center, while the remainder were discharged to a skilled nursing facility or remain hospitalized over the study period (Figure 2). The majority of patients in the COVID-19 LVO cohort (53/66, 81%) presented to the ER. A total of 13 (13/66, 19%) patients had LVOs during hospitalization. The incidence of LVOs in AA and young patients in non-COVID-19 cohort was 6% and 14%, respectively (data collected from 9 out of 12 centers).
FIGURE 2.
Breakdown of disposition of strokes due to large vessel occlusion (LVO) in COVID-19 admitted patients. Noneligible denotes nonreceipt of EVT by accepted LVO trial criteria. Unstable denotes medically unfit to undergo the procedure.
DISCUSSION
Over the past several months, the SARS-CoV-2 pandemic has rapidly encompassed the globe, inflicting millions with COVID-19. Stroke can be one of the presenting features or complications of SARS-CoV-2. We report a demographic and clinical profile of AIS in COVID-19 patients, which is distinct from AIS pre-COVID-19.
Our findings of an incidence of AIS of 1.3% (IQR .75-1.7) is similar to AIS incidence in a recent study of 2 centers in New York. This is lower than the initial report from Mao et al and closer to the report by Yaghi et al.1,2,6 Furthermore, our study shows a substantially higher rate of LVOs in COVID-19, including tandem occlusions, as compared to other stroke subtypes. The reported incidence of LVO in AIS is estimated to be 20% to 38% of total strokes outside the context of COVID-19.11 In our cohort, LVO incidence was much higher at 60% (53 of 88 patients) at 9 centers. These LVOs occur in a much younger age group than would otherwise be expected, and in the USA, they also seem to affect African Americans disproportionately as compared to historical controls.4,12-14 We believe that the pathophysiology of COVID-19 likely leads to this higher incidence of LVO in AIS. Another possibility is that those with non-LVO strokes are not presenting to hospitals for evaluation.15 A few recent series have noted a decline in both stroke volume and patient reluctance to attend the ER. We noted a similar worrisome trend in our overall cohort with 17 out of 66 LVO patients arriving with near-complete infarcts, which were too late for possible MT.13,15,16
Proposed Mechanism of LVO in COVID-19 Patients
Our results are consistent with an increased risk of AIS in those without traditional risk factors as an emerging hallmark of COVID-19.6 Early speculation surrounding vascular occlusion in COVID-19 also focused on disseminated intravascular coagulopathy (DIC), given that this phenomenon befalls the critically ill.17 Markers of inflammation, including CRP, demonstrated consistent elevation in our cohort. However, the laboratory findings in these patients do not always support a formal diagnosis of DIC, and one would have expected a similar incidence in prior historical coronavirus epidemics.
It is increasingly understood that SARS-CoV-2 trespass of the blood-brain barrier occurs at the level of the lung alveolar capillary network or alternatively via the ganglia responsible for taste and smell. In both instances, this is at least partly facilitated by affinity for endothelial angiotensin converting enzyme (ACE) II receptors. Hematogenous spread subsequently invites thrombosis by complex interplay of endothelial damage, antiphospholipid antibody formation, reactive oxygen species generation, and downstream phenomena.18 As an ACE2-seeking virus, we suggest that capillary endothelial damage likely predisposes to recurrent stroke.19 Cerebral involvement of influenza virus, varicella zoster virus (VZV), cytomegalovirus (CMV), and human immunodeficiency virus (HIV) has been demonstrated to be a risk factor for stroke.6,20 It remains unclear if SARS-CoV-2 leads to increased risk of stroke by weakening the intima of arteries (thereby increasing the risk for dissection), by causing a generalized hypercoagulable state, or by a combination of both factors. Nonetheless, AIS in those without traditional risk factors is an emerging hallmark of COVID-19, unprecedented in the modern era of previous viral pandemics, and a distinguishing characteristic compared with other coronavirus infections.6
Stroke in the Young
Despite many different definitions, AIS in those younger than 50 yr of age is considered “young” per the majority of large series.12 Approximately 10% to 15% of all ischemic strokes occur in young adults under 50 yr old. Of those, LVOs constitute approximately one-third of overall ischemic strokes. Oxley et al recently reported cases of LVOs in COVID-19 patients less than 50 yr of age.9 We noted a similar trend with 10 (16%) cases being under 50 yr of age. Out of these 10 younger LVOs cases, 8 underwent MT. Interestingly, in our international cohort, the majority of cases in the young age group (8/10) were from the NYC metropolitan area. The mean age of this young cohort of LVO was 35 yr (range 32-46). All of the cases had elevated markers of inflammation.
There is also an over-representation of AA in the USA in our MT cohort (28%), suggesting a disproportionately higher race-ethnic-related incidence consistent with the overall higher rates of COVID-19 seen among African Americans.14,21 Our findings are reassuring with regard to treatment outcomes with reperfusion rates, and intracranial hemorrhage rates similar to historical MT data.13 Similar revascularization rates have been reported in HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials), a large meta-analysis of pre-COVID MT trials for LVO. Compared with this era, MT appears to be similarly efficacious, but the propensity for LVO in COVID-19 to affect younger patients and patients with concurrent acute disease (eg, respiratory disease) complicates comparison.13 As the majority of COVID-19 LVOs patients who underwent MT were not primarily sick with COVID-19 syndrome but rather came to the ER for their stroke symptoms, their mortality rate was lower than the rates reported in patients primarily hospitalized for COVID-19.22,23 It remains to be seen that the long-term outcomes on these patients will contribute to our understanding of this process.
Limitations
This study has several limitations. There are no long-term outcome data for AIS patients beyond the study period. We did not collect the total number of stroke admissions and compared them to previous years or to the non-COVID-19 cohort as our focus was to define the incidence of AIS and characteristic of LVO strokes in admitted COVID-19 patients. We included patients who had LVOs but were not candidates for MT; however, there is certainly a possibility that some patients who developed LVOs in hospital were not screened for AIS if they were too unwell and had limited neurological examinations while on ventilators. As mentioned previously, there is a possibility that patients with minor strokes and less-disabling strokes are less likely to seek medical attention during this pandemic, which may have skewed the data in favor of LVO strokes. We do not have long-term follow-up data; hence, disposition was used in lieu of 90-d modified Rankin Scale (mRS). In order to bring these data in an expedited fashion to the medical community, comparison was made to historical data rather than the pre-COVID-19 data at each center.
CONCLUSION
This international multicenter study reports a multicentric observational experience of a substantially higher proportion of the LVOs subtype occurring at a younger-than-usual median age among all strokes in COVID-19 patients. These data have implications for urgent research into thromboprophylaxis to prevent large arterial emboli in patients with COVID-19, including those who may not have traditional vascular risk factors.
Funding
This study did not receive any funding or financial support.
Disclosures
Dr. Dileep Yavagal discloses relationships with; Modest; Medtronic, Inc, Cerenovus, Rapid Medical, Neuralanalytics, Vascular Dynamics, Inneuroco, Inc, Poseydon Medical, LLC, Deck Therapeutics.
Acknowledgments
The authors wish to thank Dr Ralph Sacco (University of Miami Hospital, Miami, Florida, USA) for guidance, and Dr Ivo Bach and Dr Pratit Patel (University Hospital, Newark, New Jersey, USA), Dr Ketevan Berekashvili (Brookdale, New York, USA), and Dr Mercedes de Lera (Universitario de Valladolid, Spain) for census data collection.
Notes
This article was posted to the SSRN on June 8, 2020 under the title “Incidence, Characteristics and Outcomes of Large Vessel Stroke in COVID-19 Cohort: A Multicentric International Study.”
Contributor Information
Priyank Khandelwal, Department of Neurological Surgery, University Hospital Newark, New Jersey Medical School, Rutgers, New Jersey, USA.
Fawaz Al-Mufti, Department of Neurology, Radiology, and Neurosurgery, Westchester Medical Center at NY Medical College, Valhalla, New York, USA.
Ambooj Tiwari, Department of Neurology, Brookdale and Jamaica Hospital Center, NYU School of Medicine, Brooklyn, New York, USA.
Amit Singla, Department of Neurological Surgery, University Hospital Newark, New Jersey Medical School, Rutgers, New Jersey, USA.
Adam A Dmytriw, Neuroradiology and Neurointervention Service, Brigham & Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Mariangela Piano, Department of Neuroradiology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
Luca Quilici, Department of Neuroradiology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
Guglielmo Pero, Department of Neuroradiology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
Leonardo Renieri, Department of Radiology, Neurovascular Unit, Careggi University Hospital, Florence, Italy.
Nicola Limbucci, Department of Radiology, Neurovascular Unit, Careggi University Hospital, Florence, Italy.
Mario Martínez-Galdámez, Department of Interventional Neuroradiology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
Miguel Schüller-Arteaga, Department of Interventional Neuroradiology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
Jorge Galván, Department of Interventional Neuroradiology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
Juan Francisco Arenillas-Lara, Department of Neurology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
Zafar Hashim, Department of Radiology, University Hospital of North Midlands, Stoke-on-Trent, United Kingdom.
Sanjeev Nayak, Department of Radiology, University Hospital of North Midlands, Stoke-on-Trent, United Kingdom.
Keith Desousa, Department of Neurology, Eastern Region, Northwell Health, Long Island, New York, New York, USA.
Hai Sun, Department of Neurological Surgery, Rutgers Robert Wood Johnson Medical School, New Jersey Medical School, New Brunswick, New Jersey, USA.
Pankaj K Agarwalla, Department of Neurological Surgery, University Hospital Newark, New Jersey Medical School, Rutgers, New Jersey, USA.
Anil Nanda, Department of Neurological Surgery, University Hospital Newark, New Jersey Medical School, Rutgers, New Jersey, USA.
J Sudipta Roychowdhury, Department of Neurology & Radiology, Robert Wood Johnson University Hospital, Rutgers, New Jersey, USA.
Emad Nourollahzadeh, Department of Neurology & Radiology, Robert Wood Johnson University Hospital, Rutgers, New Jersey, USA.
Tannavi Prakash, Department of Neurological Surgery, University Hospital Newark, New Jersey Medical School, Rutgers, New Jersey, USA.
Chirag D Gandhi, Department of Neurosurgery, Westchester Medical Center at NY Medical College, Valhalla, New York, USA.
Andrew R Xavier, Department of Neurology, Saint Joseph Health, Detroit Medical Center, Detroit, Michigan, USA.
J Diego Lozano, Department of Radiology, University of California Riverside, Riverside, California, USA.
Gaurav Gupta, Department of Neurological Surgery, Rutgers Robert Wood Johnson Medical School, New Jersey Medical School, New Brunswick, New Jersey, USA.
Dileep R Yavagal, Department of Neurology, Miller School of Medicine, Miami, Florida, USA.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1.Mao L, Jin H, Wang Met al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaghi S, Ishida K, Torres Jet al. SARS-CoV-2 and stroke in a new york healthcare system. Stroke. 2020;51(7):2002-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahammedi A, Saba L, Vagal Aet al. Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology. 2020;297(2):201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari A, Berekashvili K, Vulkanov Vet al. Etiologic subtypes of ischemic stroke in SARS-CoV-2 patients in a cohort of New York City hospitals. Front Neurol. 2020;11:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntaios G, Michel P, Georgiopoulos Get al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51(9):e254-e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkler AE, Parikh NS, Mir Set al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess DC, Eldahshan W, Rutkowski E.. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleasure SJ, Green AJ, Josephson SA.. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection. JAMA Neurol. 2020;77(6):679-680. [DOI] [PubMed] [Google Scholar]
- 9.Oxley TJ, Mocco J, Majidi Set al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avula A, Nalleballe K, Narula Net al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra K, Gornbein J, Saver JL.. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekker MS, Boot EM, Singhal ABet al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17(9):790-801. [DOI] [PubMed] [Google Scholar]
- 13.Goyal M, Menon BK, van Zwam WHet al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 14.Dmytriw AA, Phan K, Schirmer Cet al. Ischaemic stroke associated with COVID-19 and racial outcome disparity in North America. J Neurol Neurosurg Psychiatry. 2020;91(12):1362-1364. [DOI] [PubMed] [Google Scholar]
- 15.Siegler JE, Heslin ME, Thau Let al. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29(8):104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kansagra AP, Goyal MS, Hamilton Set al. Collateral effect of COVID-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollias A, Kyriakoulis KG, Dimakakos Eet al. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Li X, Chen Met al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Xiao M, Zhang Set al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagel MA, Mahalingam R, Cohrs RJet al. Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets. 2010;10(2):105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. [DOI] [PubMed] [Google Scholar]
- 22.Berenguer J, Ryan P, Rodríguez-Baño Jet al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escalard S, Maïer B, Redjem Het al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020;51(8):2540-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]