Abstract
Background
The association of early changes in the immune infiltrate during neoadjuvant chemotherapy (NACT) with pathological complete response (pCR) in triple-negative breast cancer (TNBC) remains unexplored.
Methods
Multiplexed immunohistochemistry was performed in matched tumor biopsies obtained at baseline and after 3 weeks of NACT from 66 patients from the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer - Triple Negative Breast Cancer (WSG-ADAPT-TN) trial. Association between CD4, CD8, CD73, T cells, PD1-positive CD4 and CD8 cells, and PDL1 levels in stroma and/or tumor at baseline, week 3 and 3-week change with pCR was evaluated with univariable logistic regression.
Results
Compared with no change in immune cell composition and functional markers, transition from ‘cold’ to ‘hot’ (below-median and above-median marker level at baseline, respectively) suggested higher pCR rates for PD1-positive CD4 (tumor: OR=1.55, 95% CI 0.45 to 5.42; stroma: OR=2.65, 95% CI 0.65 to 10.71) and PD1-positive CD8 infiltrates (tumor: OR=1.77, 95% CI 0.60 to 5.20; stroma: OR=1.25, 95% CI 0.41 to 3.84; tumor+stroma: OR=1.62, 95% CI 0.51 to 5.12). No pCR was observed after ‘hot-to-cold’ transition in PD1-positive CD8 cells. pCR rates appeared lower after hot-to-cold transitions in T cells (tumor: OR=0.26, 95% CI 0.03 to 2.34; stroma: OR=0.35, 95% CI 0.04 to 3.25; tumor+stroma: OR=0.00, 95% CI 0.00 to 1.04) and PD1-positive CD4 cells (tumor: OR=0.60, 95% CI 0.11 to 3.35; stroma: OR=0.22, 95% CI 0.03 to 1.92; tumor+stroma: OR=0.32, 95% CI 0.04 to 2.94). Higher pCR rates collated with ‘altered’ distribution (levels below-median and above-median in tumor and stroma, respectively) of T cell (OR=3.50, 95% CI 0.84 to 14.56) and PD1-positive CD4 cells (OR=4.50, 95% CI 1.01 to 20.14).
Conclusion
Our exploratory findings indicate that comprehensive analysis of early immune infiltrate dynamics complements currently investigated predictive markers for pCR and may have a potential to improve guidance for individualized de-escalation/escalation strategies in TNBC.
Keywords: biomarkers, tumor, breast neoplasms, immune evation, programmed cell death 1 receptor, tumor microenvironment
Background
Triple-negative early breast cancer (TNBC) is a heterogeneous disease associated with poor prognosis. However, pathological complete response (pCR) to neoadjuvant chemotherapy (NACT) is relatively frequent and associated with good long-term outcomes.1–3 Consequently, NACT allows individualized therapy approaches for further escalation or de-escalation according to response at surgery (pCR status). However, consensus on the optimal NACT offering the best benefit/risk profile is currently lacking, partially due to lack of a direct comparison between several NACT agents and the biological heterogeneity observed in TNBC.4 Several studies in TNBC demonstrated a number of genetic, molecular, and cell signatures associated with response to different types of therapy.5 6 Elevated tumor-infiltrating lymphocytes (TILs) levels were associated with pCR and improved survival in most, but not all trials.7–12 A recent analysis using multiplexed ion beam imaging revealed distinct patterns of tumor-immune landscape in TNBC characterized by lack of immune infiltrates (frequently termed as ‘cold’), immune cells in contact (‘hot’ or ‘mixed’) or spatially separated from tumor cells (termed ‘altered’, ‘immune excluded’ or ‘compartmentalized’13–15). Interestingly, altered tumor organization translated to longer survival than that associated with hot tumors.15 Capitalizing on the advances in deciphering the molecular interactions in tumor-immune microenvironment, the clinical research on immune checkpoint inhibitors is increasingly gaining traction in TNBC. For instance, high PDL1 expression was positively associated with pCR in response to NACT alone or combined with pembrolizumab, atezolizumab or durvalumab.9 16–19 Yet, PDL1 status does not seem to be predictive of response to immunotherapy in early TNBC,18 20 and available data offer limited insight into marker dynamics during NACT. Marker levels in residual tumors (non-pCR) and their value for prediction of long-term outcomes may not reliably reflect the situation in tumors achieving pCR. In this context, identification of reliable prognostic or predictive markers would allow development of patient-tailored therapeutic approaches that optimize outcome without overtreating patients.
In the present study, we employed multiplexed immune cell detection to explore the value of the inflammatory tumor microenvironment and its early dynamic changes during NACT for prediction of pCR. This research was conducted within the framework of the preplanned exploratory objectives of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer - Triple Negative Breast Cancer (WSG-ADAPT-TN) subtrial in order to identify markers predicting pCR and thus enabling selection of candidates for future therapy de-escalation.21
Methods
Patients
WSG-ADAPT-TN (NCT01815242) is a prospective, multicenter, controlled, non-blinded, randomized, investigator-initiated trial in women ≥18 years of age with histologically confirmed unilateral, primary invasive cT1c-cT4c or cN+ TNBC (centrally confirmed hormone receptor positivity in <1% of tumor nuclei and HER2 score of 0–1+ or 2+ with negative fluorescence in situ hybridization test). Histopathology subtype was assessed by local pathologists at the time of diagnosis. pCR (ypT0/is, ypN0), assessed after 12 weeks of NACT, was a primary endpoint of the study. Surgery was performed within 3 weeks after NACT. In case investigators opted for further NACT, prior histological confirmation of non-pCR by core needle biopsy was obligatory. Recommended poststudy therapy (neoadjuvant and/or adjuvant) followed national guidelines. Core biopsies used for the present analysis were performed at the time of diagnosis and after 3 weeks of NACT (online supplemental figure 1).
jitc-2020-002198supp001.pdf (209.5KB, pdf)
Molecular subtypes of TNBC
The PAM50 assay was used to classify TNBC into five intrinsic molecular subtypes (luminal A, luminal B, HER2-enriched, basal-like and normal-like) as described elsewhere.22 The expression signatures of 50 genes included in PAM50 were compared with each of the five intrinsic molecular subtype centroids using Spearman’s rho correlation levels as a distance measure (the subtype was assigned to the nearest centroid).
Assessment of Ki-67 levels
Ki-67 was assessed by central pathology using rabbit monoclonal anti-Ki-67 antibody 30–9 (Ventana Medical Systems, Tucson, Arizona, USA). Early Ki-67 response in the 3-week biopsy was defined as >30% decrease in Ki-67 versus baseline or <500 invasive tumor cells in the 3-week biopsy as previously described.21
Analysis of TILs levels
Stromal TILs were evaluated by a two-independent observer approach following the guidelines of the International TILs Working Group 201423 as previously described.12 Briefly, the evaluation was based on H&E staining on digital whole slide image (WSI), and consensus was obtained by joint re-evaluation. In single cases, assessment of a third evaluator or additional information to visualize tumor composition (eg, CK5/14 and E-cadherin immunohistochemistry (IHC) staining) was considered. In case of heterogeneous spatial distribution, results were averaged. Brisk focal infiltrates (‘hot spots’), obviously perivascular infiltrates, and tertiary lymphoid structures were excluded from the analysis. For statistical analysis, TILs levels, recorded as percentage, were expressed as a continuous variable.
Multiplexed IHC
Multiplexed IHC was performed on 3 µm-thick sections cut from the formalin-fixed paraffin-embedded tissue blocks from surplus material after central pathology review, which was available for 83 out of 385 study cases. These additional sections were stored at 4°C in the dark to minimize antigen aging over time until further processing. Inclusion criteria for the multiplex study were (1) availability of paired biopsies before and on treatment, (2) sufficient size of the biopsies on visual inspection, (3) representation of cases covering the entire study recruitment time, and (4) sufficient remaining representative tissue for quantitative analysis on microscopic inspection and comparison with the initial H&E staining, resulting in a series of 66 fully evaluable cases.
Assay optimization was performed following the manufacturer’s instructions (OPAL Multiplex IHC Assay Development Guide; Akoya Bioscience, Menlo Park, California, USA). Slides were deparaffinized; initial antigen retrieval was performed by microwave cooking in Tris-buffered saline (TBS) at pH 9; and blocking of unspecific protein binding was performed using Protein Block Serum-free solution (Agilent/Dako, Hamburg, Germany). Subsequent antigen retrieval and deactivation of the preceding staining step were performed according to the previous optimization by microwave cooking either in TBS at pH 9 or citrate buffer at pH 6. Consecutive IHC staining using the OPAL 7-plex fluorescence system was performed using the following primary antibodies: anti-CD4 (clone SP35, Zytomed Systems), anti-CD8 (clone C8/144B, Agilent/Dako), anti-PD1 (clone NAT105, Cell Marque), anti-CK7 (clone OV-TL12/30, Agilent/Dako), anti-CD73 (clone EPR6114, Abcam), anti-PDL1 (clone QR1; Quartett, Berlin, Germany), and a laboratory-developed assay24 25 robustly detecting PDL1 that is not linked to a specific therapy and was validated in several QuIP ring studies (https://www.pdl1portal.eu/). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole. The following fluorophores in combination with the tyramide signal amplification system were used for detection of bound antibodies: Opal 520, Opal 540, Opal 570, Opal 620, Opal 650, or Opal 690. Fluoromount-G mounting medium (Thermo Fisher Scientific, Braunschweig, Germany) was applied to cover slides before imaging.
Multispectral image analysis and quantitative evaluation
WSI scanning was done at ×20 magnification using the Vectra Polaris instrument (Akoya Bioscience, online supplemental figure 2). Three-channel fluorescent WSIs were used to select regions of interest (ROIs) for subsequent targeted scanning of image stacks at ×40 magnification across the visible spectrum (420–720 nm) for multispectral imaging. The full available tumor area including the adjacent stromal area was included, excluding from analysis only ROIs containing obvious artifacts, pre-existing mammary gland tissue, and stromal areas with brisk perivascular inflammatory infiltrates outside of the tumor areas. Spectral libraries were generated using single-stained scans of tonsil tissue for each reagent and image deconvolution was performed with the inForm Advanced Image Analysis software (inForm V.2.4.8, Akoya). Training for the machine learning-based segmentation, tissue classification and cell phenotyping algorithms was performed using the inForm software on 20 images covering the full spectrum of expected variability regarding staining intensities and cell densities. Batch analysis and data export into text format comprised 2188 images representing all evaluable tissue areas in the data set. Visual quality control of all analyzed ROIs was performed by reviewing all composite images in the context of the quantitative output using Image Miner software V.2.7.0 (Definiens, Munich, Germany) (FF), and in-depth control of quantitative output in context of the corresponding H&E-stained sections was performed in 10% of randomly selected cases (FF and MC).
Statistical analysis
Clinical characteristics between patients included in this immune response assessment study and all other patients included in the WSG-ADAPT-TN trial were compared by independent two-sample t-tests, Wilcoxon rank-sum tests, χ2 tests or Fisher’s exact tests. Cellular marker levels at baseline and after week 3 were summarized by median values and 25th and 75th percentiles. Changes between the two time points were evaluated with the Wilcoxon signed-rank test.
Correlations between cellular marker levels and TILs levels (evaluated as a continuous variable) were calculated using Pearson’s correlation coefficient (r). Associations between pCR and patient characteristics, as well as baseline cellular marker levels in stroma and tumor, were evaluated with univariable logistic regression. ORs were calculated per IQR, for example, ORs compared two patients with marker values at the 75th and 25th percentiles. Associations between pCR and changes in cellular marker levels between baseline and week 3, dichotomized as decrease versus increase, were evaluated with logistic regression adjusted for baseline marker levels. A trend test was based on the significance of the continuous marker level. Separate analyses were performed for measurements in stroma and tumor. Dynamics in T cells, PDL1 and PD1-positive CD4 and CD8 cells during 3 weeks of therapy were evaluated by grouping tumors into cold and hot (below-median and above-median marker levels at baseline, respectively), according to a recently proposed nomenclature.14 However, these definitions are currently emerging and might change, depending on tumor type and therapy. For analyses of combined stroma and tumor measurements, tumors were categorized into cold, hot and altered (figure 1). Tumors were considered cold when marker levels in stroma and tumor were below the respective median value, hot when marker levels in tumor were above the median value, irrespective of levels in stroma, or altered when marker levels were below-median in tumor and above-median in stroma. We used univariable logistic regression to evaluate the association between pCR and transitions between the different states under therapy, that is, ‘cold-to-hot’ and ‘hot-to-cold’, from or to altered, and ‘no change’. For all test results, two-sided p values were reported and interpreted as significant at 5%. P values were not corrected for multiple testing because of the exploratory nature of this study. To aid interpretation, ORs for pCR below 0.5 or above 2.0 were considered potentially clinically meaningful. Statistical analyses were performed using STATA SE V.6 software.
Figure 1.
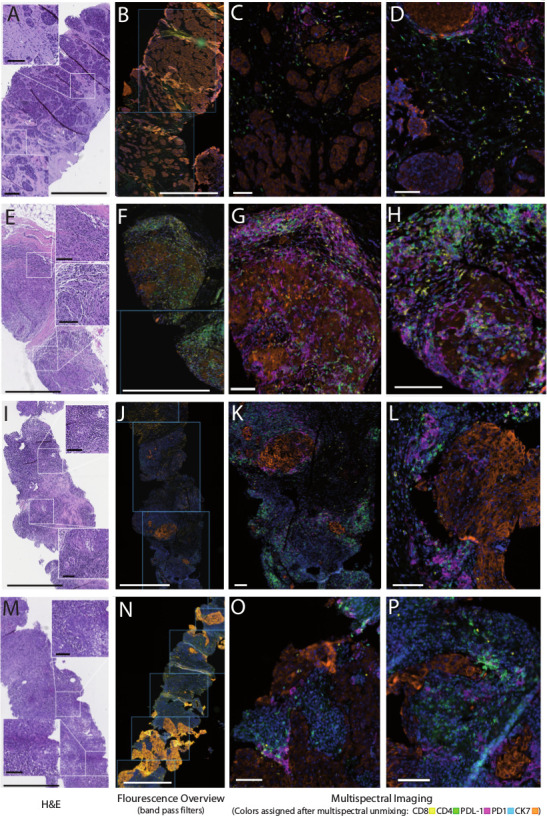
Representative examples for histopathological patterns of the TNBC inflammatory microenvironment and the immunological response. (A–D) Baseline biopsy and (E–H) corresponding paired biopsy under treatment from a patient with largely ‘cold’ tumor at diagnosis and brisk immunological response (cold to ‘hot’ transition). (I–L) Baseline biopsy and (M–P) corresponding paired biopsy under treatment from a patient with largely constant inflammatory pattern before and on treatment with heavily infiltrated stromal compartment but only a few lymphocytes in direct contact with tumor cells, referred to as ‘immune excluded’, ‘compartmentalized’ or ‘altered’ pattern. The left parts of each image set (A, E, I and M) depict H&E staining. (B, F, J, and N) Whole slide image fluorescence scans used to select regions of interest and right parts of each image set (C/D, G/H, K/L, and O/P) show representative examples of multiplexed immunohistochemistry displaying the five markers in the panel in pseudocolors: CK7 (orange), PDL1 (magenta), PD1 (cyan), CD4 (green) and CD8 (yellow) with blue nuclear stain (4′,6-diamidino-2-phenylindole). bars indicate 800 µm in the H&E and overview images and 100 µm in the inserts and the multiplex images. TNBC, triple-negative breast cancer.
Results
Patient characteristics
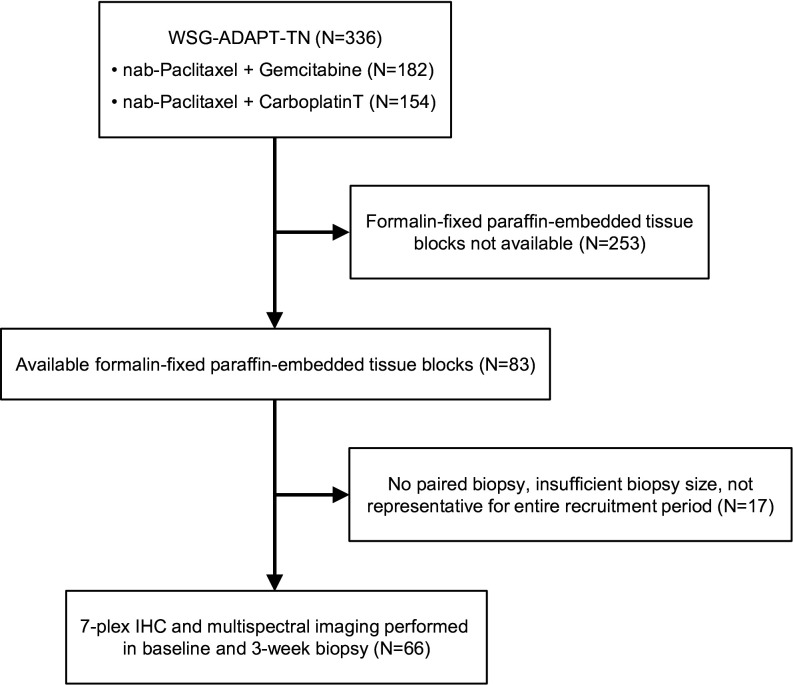
Between June 2013 and February 2015, 336 patients were randomized at 45 centers in the WSG-ADAPT-TN substudy (figure 2). pCR occurred in 36% of patients, that is, 28.4% of patients on nab-paclitaxel+gemcitabine and 44.5% of patients on nab-paclitaxel+carboplatin (p=0.00421). The 7-plex IHC and multispectral imaging were performed in a subset of sequential biopsies from 66 patients obtained at baseline and after 3 weeks of NACT. The median age of this group was 49 years, and 54.5% (n=36/66) were premenopausal (table 1). Most tumors were of basal type according to PAM50 (86.4%, n=57). Most patients had cT2 (65.2%, n=43) and cN0 (77.3%, n=51) cancer. Forty (60.6%) and 26 (39.4%) patients received nab-paclitaxel+gemcitabine or nab-paclitaxel+carboplatin, respectively. pCR was achieved in 36.4% (n=24) of tumors, and early response rate (Ki-67 decrease or low cellularity) was 43.1% (n=28). Characteristics of the 66 patients with immune response assessment were not statistically different from the characteristics of the remaining 270 patients with the exception of a lower rate of early response (43.1%, n=28/65 vs 58.9%, n=116/197, p=0.026; table 1).
Figure 2.
Consolidated Standards of Reporting Trials diagram. IHC, immunohistochemistry; WSG-ADAPT-TN, the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer - Triple Negative Breast Cancer trial.
Table 1.
Patient characteristics for subjects with immune response assessed by 7-plex immunohistochemistry and multispectral imaging and the remaining subjects in the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer - Triple Negative Breast Cancer substudy
| Parameter | Total (N=336) | Immune response assessed (n=66) | Immune response not assessed (n=270) | P value |
| Age (years), median (IQR) | 50 (43–61) | 49 (42–58) | 51 (43–61) | 0.396 |
| Age group (years), n (%) | ||||
| <40 | 59 | 12 (18.2) | 47 (17.4) | |
| 40–50 | 94 | 21 (31.8) | 73 (27.0) | |
| 50–60 | 90 | 18 (27.3) | 72 (26.7) | |
| >60 | 92 | 14 (21.2) | 78 (28.9) | |
| Missing | 1 | 1 (1.5) | 0 (0) | |
| Menopausal status, n (%) | ||||
| Premenopausal | 163 | 36 (54.5) | 127 (47.0) | 0.187 |
| Postmenopausal | 157 | 25 (37.9) | 132 (48.9) | |
| Missing | 16 | 5 (7.6) | 11 (4.1) | |
| Histological subtype, n (%) | ||||
| No special type, ductal | 304 | 60 (90.9) | 244 (90.4) | 0.999 |
| Invasive lobular | 7 | 1 (1.5) | 6 (2.2) | |
| Medullary | 9 | 2 (3.0) | 7 (2.6) | |
| Other | 12 | 2 (3.0) | 10 (3.7) | |
| Missing | 4 | 1 (1.5) | 3 (1.1) | |
| PAM50 subtype, n (%) | ||||
| Basal | 250 | 57 (86.4) | 193 (71.5) | 0.487 |
| HER2 | 19 | 2 (3.0) | 17 (6.3) | |
| LumA | 5 | 0 (0) | 5 (1.9) | |
| Normal | 26 | 6 (9.1) | 20 (7.4) | |
| Missing | 36 | 1 (1.5) | 35 (13.0) | |
| Clinical tumor stage, n (%) | ||||
| 1 | 125 | 20 (30.3) | 105 (38.9) | 0.353 |
| 2 | 188 | 43 (65.2) | 145 (53.7) | |
| 3 | 18 | 2 (3.0) | 16 (5.9) | |
| 4 | 5 | 1 (1.5) | 4 (1.5) | |
| Clinical nodal status, n (%) | ||||
| 0 | 248 | 51 (77.3) | 197 (73.0) | 0.854 |
| 1 | 77 | 13 (19.7) | 64 (23.7) | |
| 2–3 | 11 | 2 (3.0) | 9 (3.3) | |
| Treatment, n (%) | ||||
| nab-paclitaxel+gemcitabine | 182 | 40 (60.6) | 142 (52.6) | 0.242 |
| nab-paclitaxel+carboplatin | 154 | 26 (39.4) | 128 (47.4) | |
| pCR, n (%) | ||||
| No | 206 | 42 (63.6) | 164 (60.7) | 0.992 |
| Yes | 118 | 24 (36.4) | 94 (34.8) | |
| Missing | 12 | 0 (0) | 12 (4.4) | |
| Ki-67, median (IQR) | 72.5 (50–80) | 75 (60–90) | 70 (50–80) | 0.152 |
| Missing | 12 | 0 | 12 | |
| Early response, n (%) | ||||
| No | 118 | 37 (56.9) | 81 (41.1) | 0.026* |
| Yes | 144 | 28 (43.1) | 116 (58.9) | |
| Missing | 74 | 1 (1.5) | 73 (27.0) |
*Among patients with evaluable pCR.
pCR, pathological complete response.
Marker expression levels
Compared with baseline levels, the absolute number of CD4, CD8 and total T cells significantly increased after 3 weeks of NACT in stroma (median change: 29.1, 20.1 and 49.8 cells/megapixel, respectively) and in tumor (median change: 10.3, 21.6 and 39.0 cells/megapixel, respectively; table 2). NACT also induced significant accumulation of PD1-positive CD8 cells in stroma (median change: 3.5 cells/megapixel) and in tumor (median change: 9.5 cells/megapixel). Furthermore, we observed a significant increase of CD73 cells in stroma (median change: 6.4 cells/megapixel) and PDL1-positive cells in tumor (median change: 32.3 cells/megapixel).
Table 2.
Change in marker levels between baseline and week 3
| Marker | Baseline, median (IQR) | Week 3, median (IQR) | Change, median (IQR) | P value for change |
| Stroma (cells/megapixel) | ||||
| CD4 | 63.9 (29.4–119.9) | 102.2 (50.8–199.2) | 29.1 (−8.0 to 112.1) | <0.001 |
| CD8 | 27.8 (17.6–42.0) | 52.1 (32.7–80.3) | 20.1 (0.0–42.1) | <0.001 |
| CD4/CD8 | 2.1 (1.1–3.8) | 2.3 (1.1–3.1) | 0.0 (−1.3 to 0.8) | 0.423 |
| T cell | 102.3 (50.0–167.8) | 168.4 (86.2–270.9) | 49.8 (5.3–153.9) | <0.001 |
| CD73 | 39.9 (25.8–73.3) | 51.2 (30.7–100.1) | 6.4 (−9.7 to 35.3) | 0.015 |
| PD1 in CD4 | 5.8 (3.1–15.0) | 6.1 (2.5–12.2) | −1.0 (−4.5 to 3.3) | 0.265 |
| PD1 in CD8 | 25.5 (11.5–43.4) | 31.9 (20.5–50.9) | 3.5 (−7.4 to 19.2) | 0.019 |
| PDL1 | 54.0 (15.8–146.4) | 68.1 (37.5–149.9) | 6.9 (−25.5 to 78.0) | 0.279 |
| Tumor (cells/megapixel) | ||||
| CD4 | 13.4 (3.9–29.3) | 24.9 (11.8–72.8) | 10.3 (−4.3 to 48.9) | 0.003 |
| CD8 | 19.0 (6.5–29.4) | 35.2 (17.1–74.9) | 21.6 (−1.4 to 43.8) | <0.001 |
| CD4/CD8 | 0.8 (0.3–2.2) | 0.8 (0.3–2.0) | 0.0 (−0.8 to 0.4) | 0.492 |
| T cell | 34.7 (13.6–88.5) | 85.8 (27.9–143.5) | 39.0 (−0.8 to 103.5) | <0.001 |
| CD73 | 33.1 (18.4–80.0) | 43.8 (18.4–74.5) | −1.3 (−25.7 to 22.9) | 0.982 |
| PD1 in CD4 | 11.0 (4.9–22.5) | 12.7 (4.2–33.3) | 1.8 (−5.6 to 10.9) | 0.144 |
| PD1 in CD8 | 15.6 (5.2–38.0) | 29.7 (14.3–44.7) | 9.5 (−5.1 to 26.5) | 0.001 |
| PDL1 | 35.0 (14.1–117.1) | 72.0 (22.5–194.8) | 32.3 (−6.6 to 112.7) | 0.003 |
Correlation between TILs and immune markers
Correlation between the levels of TILs and immune markers either at baseline or their changes during the 3 weeks of NACT was low to moderate (table 3). The highest correlation coefficients (Pearson’s r) were noted for change in T-cell levels in stroma (0.52) and tumor (0.40), baseline PDL1 levels in stroma (0.47) and tumor (0.45), and for change in CD4 cell levels in stroma (0.46).
Table 3.
Pearson’s correlation coefficients between TILs and cellular markers
| Correlation between TILs and | Stroma | Tumor | ||
| Baseline* | Change† | Baseline* | Change† | |
| CD4 | 0.257 | 0.464 | 0.087 | 0.324 |
| CD8 | 0.264 | 0.39 | 0.226 | 0.374 |
| CD4/CD8 | −0.06 | 0.003 | −0.096 | 0.024 |
| T cell | 0.314 | 0.522 | 0.204 | 0.404 |
| CD73 | 0.09 | 0.277 | −0.194 | −0.126 |
| PD1 in CD4 | −0.068 | 0.035 | 0.07 | 0.161 |
| PD1 in CD8 | 0.066 | 0.193 | 0.177 | 0.291 |
| PDL1 | 0.47 | 0.27 | 0.447 | 0.124 |
*Correlation between TILs and immune marker levels measured at baseline.
†Correlation between change in TIL levels and change in immune marker levels (changes between baseline and week 3 assessments).
TIL, tumor-infiltrating lymphocyte.
Association between cellular markers at baseline and pCR
Baseline levels for any of the cellular markers in tumor or stroma were not associated with pCR (online supplemental table 1). Increases in the baseline CD4 to CD8 ratio in stroma of an IQR appeared associated with about a twofold reduced chance of pCR, although the association was not statistically significant.
Association between early dynamic changes in the immune markers and pCR
A decrease in the PD1-positive CD4 count in tumor was significantly associated with lower pCR rates compared with an increase (OR=0.25, 95% CI 0.08 to 0.80, p-trend=0.023; table 4). The pattern in the stroma was similar, though not statistically significant. Lower pCR rates after a marker decrease (compared with an increase) were also observed for CD8, T cells, PD1 in CD8, and PDL1, although trends were not statistically significant.
Table 4.
Association between pCR and changes in cellular marker levels between baseline and week 3
| Marker | Stroma | Tumor | ||||
| pCR, n | Non- pCR, n | OR* (95% CI) | pCR, n | Non- pCR, n | OR* (95% CI) | |
| CD4 | ||||||
| Increase | 17 | 30 | 1.00 | 17 | 28 | 1.00 |
| Decrease | 7 | 12 | 1.24 (0.39 to 3.96) | 7 | 14 | 1.27 (0.37 to 4.41) |
| P-trend | 0.260 | 0.249 | ||||
| CD8 | ||||||
| Increase | 22 | 28 | 1.00 | 21 | 28 | 1.00 |
| Decrease | 2 | 14 | 0.10 (0.01 to 0.66) | 3 | 14 | 0.28 (0.07 to 1.16) |
| P-trend | 0.522 | 0.198 | ||||
| CD4/CD8 | ||||||
| Increase | 15 | 18 | 1.00 | 12 | 20 | 1.00 |
| Decrease | 9 | 24 | 0.69 (0.21 to 2.30) | 12 | 22 | 0.79 (0.26 to 2.34) |
| P-trend | 0.711 | 0.205 | ||||
| T cell | ||||||
| Increase | 20 | 31 | 1.00 | 21 | 28 | 1.00 |
| Decrease | 4 | 11 | 0.60 (0.16 to 2.19) | 3 | 14 | 0.31 (0.07 to 1.41) |
| P-trend | 0.331 | 0.184 | ||||
| CD73 | ||||||
| Increase | 13 | 28 | 1.00 | 11 | 21 | 1.00 |
| Decrease | 11 | 14 | 1.64 (0.58 to 4.62) | 13 | 21 | 1.32 (0.44 to 3.94) |
| P-trend | 0.458 | 0.930 | ||||
| PD1 in CD4 | ||||||
| Increase | 13 | 13 | 1.00 | 18 | 19 | 1.00 |
| Decrease | 11 | 29 | 0.36 (0.12 to 1.07) | 6 | 23 | 0.25 (0.08 to 0.80) |
| P-trend | 0.213 | 0.023 | ||||
| PD1 in CD8 | ||||||
| Increase | 16 | 21 | 1.00 | 19 | 25 | 1.00 |
| Decrease | 8 | 21 | 0.53 (0.17 to 1.61) | 5 | 17 | 0.45 (0.12 to 1.63) |
| P-trend | 0.098 | 0.196 | ||||
| PDL1 | ||||||
| Increase | 15 | 22 | 1.00 | 18 | 27 | 1.00 |
| Decrease | 9 | 20 | 0.50 (0.16 to 1.59) | 6 | 15 | 0.53 (0.16 to 1.81) |
| p-trend | 0.781 | 0.652 | ||||
*OR adjusted for baseline marker value.
pCR, pathological complete response.
For T cells and PDL1 separately in stroma or tumor and both compartments combined, transitions from cold at baseline to hot at week 3 were associated with similar pCR rates as no change (figure 3). Increased ORs appeared to suggest higher pCR rates after cold-to-hot transitions in PD1-positive CD4 (in tumor and stroma separately) and PD1-positive CD8 cells (in tumor and in tumor and stroma combined). Based on PD1-positive CD8 cells, hot-to-cold transitions separately in stroma or tumor and combined were associated with significantly reduced pCR rates compared with no change. However, these results were based on the absence of pCRs in patients with tumors changing from hot to cold. Lower pCR rates in tumors after hot-to-cold transitions compared with no change were also observed for T cells and PD1-positive CD4 cells, but numbers were too small to reach statistical significance. In a combined analysis of changes in tumor and stroma compartments, TNBC with altered T-cell and PD1-positive CD4 cell levels had higher odds of pCR than those with no change (figure 3).
Figure 3.
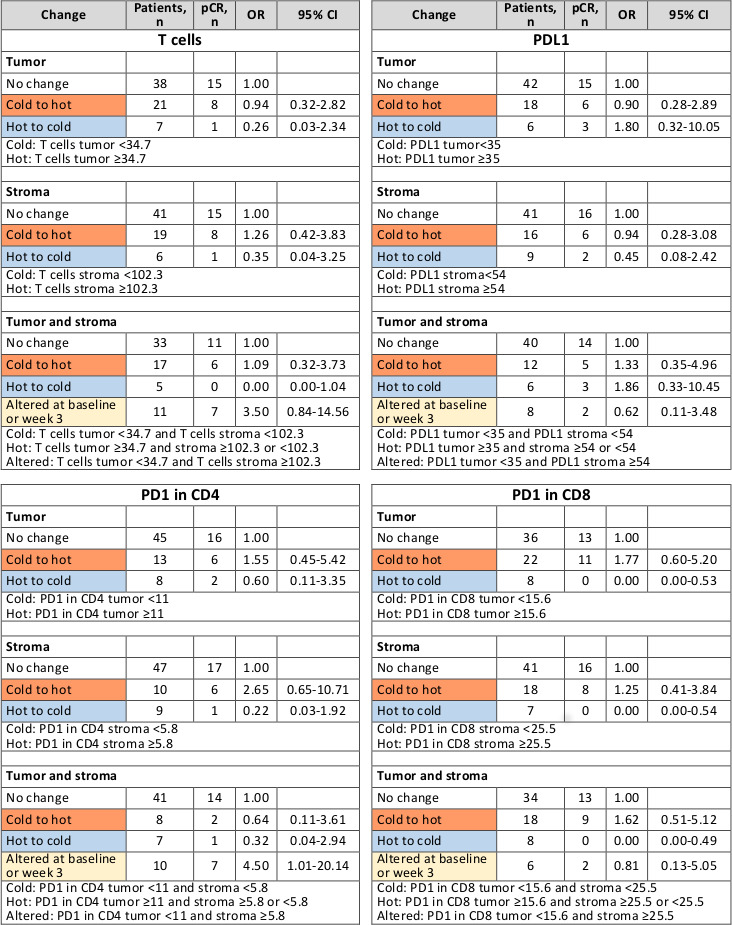
Association between pCR and changes in marker levels in tumor and/or stroma for T cells, PDL1, PD1 in CD4 and PD1 in CD8 based on univariable logistic regression. pCR, pathological complete response.
Discussion
We addressed the role of immune cell infiltrates in TNBC with particular emphasis on early dynamic changes in immune markers that remain underexplored to date and on the spatial distribution of immune cells in tumor subcompartments. This study complements the investigation of early response to NACT and its association with pCR, which was one of the major exploratory objectives of the WSG-ADAPT-TN substudy, in addition to assessment of clinical response/resistance after one cycle of NACT. These objectives are clinically highly relevant given their potential to guide individualized de-escalation/escalation strategies.
We present a unique cohort as we evaluated dynamic changes in immune markers induced early during NACT (sequential assessments at baseline and after only one cycle of combination chemotherapy) and their association with pCR as opposite to prior approaches relying exclusively on post-treatment samples. We observed increases in the levels of CD4, CD8, PD1-positive CD8 and T cells and in both tumor and stroma, CD73 in stroma and PDL1 in tumor compartments. Thus far, investigations of immune marker levels in post-NACT residual tumor specimens demonstrated increased levels of stromal and intratumoral TILs, either referring to total TILs23 or specifically to CD4+ or CD8+ lymphocytes7 26 27. Expression of stromal PD1 was shown to increase after NACT, whereas PDL1 on TILs and tumor cells did not change.26 In the NeoTRIP study, one cycle of nab-paclitaxel+carboplatin±atezolizumab increased TIL levels.28 Interestingly, nab-paclitaxel+carboplatin reduced PDL1 expression on immune cells; however, addition of atezolizumab increased PDL1 expression on immune and tumor cells.28 Previously, baseline TIL levels were found to correlate with PDL1 expression,17 which is in line with our results. Lee and colleagues reported that PDL1 expression decreased in the residual tumor or was maintained at low levels in tumor bed or stroma in 35.3% and 13.3% of patients, respectively.27 Furthermore, in the residual tumor tissue, the number of stromal CD4+/CD8+/FOXP3+ TILs increased or remained stable in 70% of patients. In our study, 3 weeks of NACT induced the same effect on stromal T-cell infiltration in a similar proportion of patients (71.2%), whereas more patients had corresponding changes in PDL1 expression in tumor (72.7%) and stroma (75.8%). Potentially, this variability in findings could result from differences in the population of investigated lymphocytes and the definitions used for low/high PDL1 expression. Alternatively, the timing of the biopsy could play a role as it was suggested that the levels of TILs and PDL1 decrease during the natural history of TNBC.29 This could therefore explain the more pronounced changes after one NACT cycle observed in our study compared with the changes observed in residual tumors after two to six NACT cycles.27
Baseline immune cell infiltrates were not predictive of pCR in our exploratory substudy cohort. Lack of predictive power may be due to a relatively low number of samples available for multiplexed IHC and the accuracy of this approach involving the exact phenotyping (restricted to CD4+ and CD8+ lymphocytes) as opposed to the H&E-based approaches including all mononuclear cells (including natural killer cells, plasma cells, and others in addition to CD4+ and CD8+ lymphocytes). Additionally, a potential trend favoring slightly higher inclusion of cases with unfavorable outcome could be associated with more vital tumor and less signs of regression in the on-treatment biopsies in patients with delayed pathological response. Available evidence on association of high TILs levels, immune checkpoint and T-cell differentiation markers prior to NACT is conflicting with studies indicating either a high predictive value,9 26 28 30–32 no predictive value33 or even a negative prognostic/predictive role.34 Collectively, available data suggest that improved scoring approaches, concise immune cell phenotyping and increased numbers of patients in concert with harmonization of treatments are needed for consensus between different studies.35 36
Thus far, only a few studies investigated the association between patient outcomes and NACT-induced changes in immune markers (and mostly in postsurgery specimens). Low stromal PDL1 expression after NACT was associated with shorter disease-free survival,27 while increased CD8+ TILs correlated with improved breast cancer-specific survival.32 However, since these findings were obtained in residual tumor tissue, changes in immune markers may not reflect the dynamics occurring in tumors particularly sensitive to NACT, that is, in those who will achieve pCR. Therefore, results from surgical specimens could be biased towards ‘no response’ and therapy resistance or may have more necrosis/regression in responders. Furthermore, biopsies and surgical specimens are differently processed thus introducing tissue fixation-related variability. Our strategy reduces these risks as 3-week biopsies capture the entire spectrum of responses to NACT and they are processed identically as pretherapy biopsies. This approach revealed that hot-to-cold change in PDL1 expression (stroma) and in T-cell number (either stroma or tumor) was associated with low pCR rate. Conversely, tumors with increases in stromal CD4+ and intratumoral CD8+ cells more frequently achieved pCR. Additionally, altered T-cell status (cold in tumor, hot in stroma) at baseline or at week 3 was predictive of pCR. In a recent analysis of the WSG-ADAPT-TN data, TIL levels in non-paired biopsies increased during 3 weeks of NACT and higher TIL numbers at either time point corresponded to higher pCR rates.37 It is well established that CD8 T cells directly promote the apoptosis of cancer cells, whereas the role of CD4 TILs is twofold. CD4 helper T cells initiate and maintain antitumor functions of CD8 T cells, while CD4 regulatory T cells may suppress immune response. Therefore, association of increased CD4 and CD8 number with pCR indicates an ongoing active adaptive immune response in tumors responding well to NACT. We also found that increased number of PD1-positive CD8 and CD4 cells and altered distribution of PD1-positive CD4 cells were associated with pCR. Classically, PD1 expression marks an exhausted population of CD8+ TILs, and PD1 interaction with PDL1 on tumor cells leads to inhibition of proliferation, cytokine release and cytotoxic functions of TILs. However, we observed that pronounced increase in PD1 and decrease in PDL1 during NACT were associated with pCR. Although this finding appears to challenge the current paradigm on the role of PD1–PDL1 interaction in immune escape mechanisms, a decrease in PDL1 early during NACT could also indicate an active elimination of tumor cells. Furthermore, induced PD1 expression on immune cells appears to prevent lymphovascular invasion.38 Considering the favorable outcome in tumors with changes in CD8+ TILs and PDL1 in our study, early assessment of tumor response to NACT could potentially select patients for therapy de-escalation. Given that high pCR rates were obtained already after 12 weeks of NACT in the WSG-ADAPT-TN study,21 patients with CD8+ TILs increase accompanied by PDL1 decrease could benefit from this shorter therapy. Conversely, high PDL1 levels could be used to identify candidates for therapy escalation with immunotherapy. Addition of pembrolizumab to NACT was recently shown to increase pCR rate in the KEYNOTE-522 and I-SPY two trials.20 39 This observation appears to support the hypothesis that PDL1 blockade may sensitize cancer cells to chemotherapy.40 Recently, atezolizumab+chemotherapy improved pCR rates over chemotherapy in the IMpassion031 study41 but not in the NeoTRIP study.42 However, in both studies, PDL1-positive disease was associated with higher odds for pCR than PDL1-negative disease. Yet, PDL1 status was not predictive for atezolizumab response. Interestingly, in unresectable, locally advanced or metastatic TNBC, PDL1 status is predictive of response to atezolizumab with first-line atezolizumab plus nab-paclitaxel being approved only for PDL1-positive TNBC based on the prolonged overall survival in the Impassion130 study.43
In conclusion, our multiplexed IHC approach provided important insights into the immune infiltrate dynamics during tumor response to NACT. These results might help to find immune markers to identify tumors most likely to respond to chemotherapy, thus potentially allowing for individualized de-escalation/escalation strategies in TNBC. Nevertheless, both TILs and multiplex immune markers need to be further investigated in prospective trials to confirm their value for prediction of tumor response to NACT and to investigate their function in response to immunotherapy.
Acknowledgments
The authors thank Ms Nicole Krönke for excellent technical assistance for multiplexed immunohistochemistry staining and Ms Madlen Garke for expertise in the evaluation of tumor-infiltrating lymphocyte levels. Medical writing and editorial support were provided by Lukasz Wujak, Lukasz Wujak MedComms, Warsaw, Poland, and were funded by the West German Study Group, Moenchengladbach, Germany.
Footnotes
Contributors: MG, FF, OG, UN, and NH conceptualized the study. MG, OG, SK, E-MG, HF, MB, MW, JH, CU, BA, CS, CK-L, RK, RW, UN, and NH conducted the investigation. FF, VV, MC, and HHK performed the imaging analysis. MH and KJ performed formal analysis. MG, FF, MH, KJ, OG, and NH wrote the original draft. All authors reviewed the manuscript.
Funding: This project was partially funded by the German Ministry of Education and Research, DLR project management (e:Med, grant numbers #01ZX1710A and #01ZX1608A to FF (institution)). The WSG-ADAPT-TN trial was financially supported by Celgene and Teva (institution).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data used for this analysis are available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The ADAPT trial was approved by ethics committee of the University of Cologne, Germany. All patients provided written informed consent.
References
- 1. Houssami N, Macaskill P, von Minckwitz G, et al. Meta-Analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012;48:3342–54. 10.1016/j.ejca.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 2. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 3. von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 4. Omarini C, Guaitoli G, Pipitone S, et al. Neoadjuvant treatments in triple-negative breast cancer patients: where we are now and where we are going. Cancer Manag Res 2018;10:91–103. 10.2147/CMAR.S146658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–67. 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688–98. 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014;25:611-618. 10.1093/annonc/mdt556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loi S, Dushyanthen S, Beavis PA, et al. Ras/Mapk activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 2016;22:1499–509. 10.1158/1078-0432.CCR-15-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denkert C, von Minckwitz G, Brase JC, et al. Tumor-Infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983–91. 10.1200/JCO.2014.58.1967 [DOI] [PubMed] [Google Scholar]
- 10. Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 2019;30:1941–9. 10.1093/annonc/mdz395 [DOI] [PubMed] [Google Scholar]
- 11. Mao Y, Qu Q, Zhang Y, et al. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One 2014;9:e115103. 10.1371/journal.pone.0115103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolberg-Liedtke C, Gluz O, Heinisch F, et al. Association of TILs with clinical parameters, Recurrence Score® results, and prognosis in patients with early HER2-negative breast cancer (BC)-a translational analysis of the prospective WSG PlanB trial. Breast Cancer Res 2020;22:47. 10.1186/s13058-020-01283-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 14. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197–218. 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- 15. Keren L, Bosse M, Marquez D, et al. A structured Tumor-Immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 2018;174:1373–87. 10.1016/j.cell.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 17. Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1B open-label, multicohort KEYNOTE-173 study. Ann Oncol 2020;31:569–81. 10.1016/j.annonc.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 18. Harbeck N, Zhang H, Barrios CH, et al. LBA11 IMpassion031: results from a phase III study of neoadjuvant (neoadj) atezolizumab + chemotherapy in early triple-negative breast cancer (TNBC). Ann Oncol 2020;31:S1144. 10.1016/j.annonc.2020.08.2239 [DOI] [Google Scholar]
- 19. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279–88. 10.1093/annonc/mdz158 [DOI] [PubMed] [Google Scholar]
- 20. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 21. Gluz O, Nitz U, Liedtke C. Comparison of Neoadjuvant Nab-Paclitaxel+Carboplatin vs Nab-Paclitaxel+Gemcitabine in Triple-Negative Breast Cancer: Randomized WSG-ADAPT-TN Trial Results | JNCI: Journal of the National Cancer Institute | Oxford Academic. J Natl Cancer Inst. Published online 2018. https://academic.oup.com/jnci/article/110/6/628/4698155 (Accessed July 15, 2020). [DOI] [PubMed] [Google Scholar]
- 22. Gluz O, Kolberg-Liedtke C, Prat A, et al. Efficacy of deescalated chemotherapy according to PAM50 subtypes, immune and proliferation genes in triple-negative early breast cancer: primary translational analysis of the WSG-ADAPT-TN trial. Int J Cancer 2020;146:262–71. 10.1002/ijc.32488 [DOI] [PubMed] [Google Scholar]
- 23. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs Working group 2014. Ann Oncol 2015;26:259–71. 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheel AH, Baenfer G, Baretton G, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology 2018;72:449–59. 10.1111/his.13375 [DOI] [PubMed] [Google Scholar]
- 25. Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol 2017;35:3867–76. 10.1200/JCO.2017.74.7642 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Dong T, Xuan Q, et al. Lymphocyte-Activation gene-3 expression and prognostic value in neoadjuvant-treated triple-negative breast cancer. J Breast Cancer 2018;21:124–33. 10.4048/jbc.2018.21.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J, Kim D-M, Lee A. Prognostic role and clinical association of tumor-infiltrating lymphocyte, programmed death ligand-1 expression with neutrophil-lymphocyte ratio in locally advanced triple-negative breast cancer. Cancer Res Treat 2019;51:649–63. 10.4143/crt.2018.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bianchini G, Huang C-S, Egle D, et al. LBA13 tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol 2020;31:S1145–6. 10.1016/j.annonc.2020.08.2241 [DOI] [Google Scholar]
- 29. Kim I, Sanchez K, McArthur HL, et al. Immunotherapy in triple-negative breast cancer: present and future. Curr Breast Cancer Rep 2019;11:259–71. 10.1007/s12609-019-00345-z [DOI] [Google Scholar]
- 30. Cerbelli B, Botticelli A, Pisano A, et al. Cd73 expression and pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Virchows Arch 2020;476:569–76. 10.1007/s00428-019-02722-6 [DOI] [PubMed] [Google Scholar]
- 31. West NR, Milne K, Truong PT, et al. Tumor-Infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 2011;13:R126. 10.1186/bcr3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and Foxp3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 2015;17:124. 10.1186/s13058-015-0632-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ono M, Tsuda H, Shimizu C, et al. Tumor-Infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 2012;132:793–805. 10.1007/s10549-011-1554-7 [DOI] [PubMed] [Google Scholar]
- 34. Asano Y, Kashiwagi S, Goto W, et al. Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression. J Transl Med 2018;16:87. 10.1186/s12967-018-1458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amgad M, Stovgaard ES, Balslev E, et al. Report on computational assessment of tumor infiltrating lymphocytes from the International Immuno-Oncology biomarker Working group. NPJ Breast Cancer 2020;6:16. 10.1038/s41523-020-0154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez-Ericsson PI, Stovgaard ES, Sua LF, et al. The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J Pathol 2020;250:667–84. 10.1002/path.5406 [DOI] [PubMed] [Google Scholar]
- 37. Liedtke C, Feuerhake F, Garke M, et al. Impact of tumor-infiltrating lymphocytes on response to neoadjuvant chemotherapy in triple-negative early breast cancer: translational subproject of the WSG-ADAPT Tn trial. JCO 2018;36:12102 10.1200/JCO.2018.36.15_suppl.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeong J, Lim JCT, Lee B, et al. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer 2019;7:34. 10.1186/s40425-019-0499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 2020;6:676–84. 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw 2020;18:479–89. 10.6004/jnccn.2020.7554 [DOI] [PubMed] [Google Scholar]
- 41. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090–100. 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 42. Gianni L, Huang C-S, Egle D. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study SABCS 2019. https://www.abstractsonline.com/pp8/#!/7946/presentation/1911 [DOI] [PubMed] [Google Scholar]
- 43. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-002198supp001.pdf (209.5KB, pdf)
Data Availability Statement
Data used for this analysis are available upon reasonable request to the corresponding author.