Abstract
Background
Clinical trials of immunotherapy have excluded patients with pre-existing autoimmune disease. While the safety and efficacy of single agent ipilimumab and anti-PD1 antibodies in patients with autoimmune disease has been examined in retrospective studies, no data are available for combination therapy which has significantly higher toxicity risk. We sought to establish the safety and efficacy of combination immunotherapy for patients with advanced melanoma and pre-existing autoimmune diseases.
Methods
We performed a retrospective study of patients with advanced melanoma and pre-existing autoimmune disease who received combination ipilimumab and anti-PD1 at 10 international centers from March 2015 to February 2020. Data regarding the autoimmune disease, treatment, toxicity and outcomes were examined in patients.
Results
Of the 55 patients who received ipilimumab and anti-PD1, the median age was 63 years (range 23–83). Forty-six were treated with ipilimumab and nivolumab and nine with ipilimumab and pembrolizumab.
Eighteen patients (33%) had a flare of their autoimmune disease including 4 of 7 with rheumatoid arthritis, 3 of 6 with psoriasis, 5 of 10 with inflammatory bowel disease, 3 of 19 with thyroiditis, 1 of 1 with Sjogren’s syndrome, 1 of 1 with polymyalgia and 1 of 1 with Behcet’s syndrome and psoriasis. Eight (44%) patients ceased combination therapy due to flare. Thirty-seven patients (67%) had an unrelated immune-related adverse event (irAE), and 20 (36%) ceased combination immunotherapy due to irAEs. There were no treatment-related deaths. Patients on immunosuppression (OR 4.59; p=0.03) had a higher risk of flare.
The overall response rate was 55%, with 77% of responses ongoing. Median progression free survival and overall survival were 10 and 24 months, respectively. Patients on baseline immunosuppression had an overall survival of 11 months (95% CI 3.42 to 18.58) compared with 31 months without (95% CI 20.89 to 41.11, p=0.005).
Conclusions
In patients with pre-existing autoimmune disease, not on immunosuppression and advanced melanoma, combination ipilimumab and anti-PD1 has similar efficacy compared with previously reported trials. There is a risk of flare of pre-existing autoimmune disorders, particularly in patients with inflammatory bowel disease and rheumatologic conditions, and patients on baseline immunosuppression.
Keywords: autoimmunity, programmed cell death 1 receptor, CTLA-4 antigen, melanoma, immunotherapy
Introduction
Combination immunotherapy with ipilimumab, an anti-CTLA4 inhibitor antibody, and anti-PD1 antibodies such as pembrolizumab and nivolumab, have demonstrated efficacy across multiple cancers and are approved first line treatment for BRAF-wild type and mutated melanoma,1 renal cell carcinoma,2 non-small lung cancer,3 mesothelioma,4 hepatocellular carcinoma5 and Microsatellite Instability-High (MSI-H) colorectal carcinoma.6
CTLA4 and PD1 are fundamental in immune regulation. Immune checkpoint inhibitors targeting these can cause interruption of this homeostasis and lead to immune-related adverse events (irAEs).7 Clinical trials testing ipilimumab and anti-PD1 alone or in combination have excluded patients with pre-existing autoimmune diseases due to concerns regarding severe irAEs or exacerbation of autoimmune disorders. However, previous retrospective studies suggest the use of single-agent ipilimumab8 and single-agent anti-PD19–12 is safe in patients with pre-existing autoimmune disease.
Two other retrospective studies assessing irAEs in patients with inflammatory bowel disease (IBD)13 and pre-existing autoimmune diseases14 included a small number of patients who received combination immunotherapy, 10 patients and 3 patients, respectively. However, these were not powered to assess the safety and efficacy as compared with monotherapy.
The safety and efficacy of combination therapy, which is known to have a higher risk of toxicity, has not been assessed in patients with pre-existing autoimmune diseases. As the indications for combination immunotherapy broaden and the use extends to the treatment of other malignancies, the question of safety and efficacy in this population is significant, perhaps more so given the rate of malignancies is higher in patients with a pre-existing autoimmune condition.15
We conducted an international, multicenter, retrospective cohort study to assess the safety and efficacy of combination immune checkpoint inhibitors in patients with pre-existing autoimmune disease.
Methods
Patients
Following approval of institutional review boards, data were extracted from the medical records of patients at 10 international participating centers.
Patients who had received at least one dose of combination ipilimumab and anti-PD1 between 2015 and February 2020 with a concomitant diagnosis of an autoimmune disorder were included. Qualifying autoimmune disorders included but were not limited to the following: rheumatologic (rheumatoid arthritis (RA), systemic lupus erythematosus, psoriatic arthritis, vasculitis, polymyalgia rheumatica, scleroderma, Sjogren’s syndrome), gastrointestinal (Crohn’s disease, ulcerative colitis, celiac disease), neurologic (Guillain-Barre syndrome (GBS), transverse myelitis, multiple sclerosis, myasthenia gravis, chronic inflammatory demyelinating polyneuropathy), endocrine (Graves’ disease, Hashimoto’s thyroiditis, type 1 diabetes mellitus), dermatologic (psoriasis, eczema, erythema nodosum) and other (sarcoidosis, asthma, idiopathic thrombocytopenic purpura). Autoimmune disorders were diagnosed based on each center’s standard of diagnosis, for most conditions, a history and serological testing confirmed the diagnosis. For patients with IBD and dermatologic conditions, all had a biopsy confirming the diagnosis.
Study design
Baseline patient demographics were collected including age, gender, Eastern Cooperative Group Performance Status (ECOG) and prognostic factors including eighth edition of the American Joint Committee on Cancer pathologic stage, presence of brain and liver metastases and serum lactate dehydrogenase level. Severity of the baseline autoimmune disorder was assessed by clinical activity as deemed by the treating clinician, use of baseline immunosuppression and dose, and recent flare of autoimmune disease.
The safety of combination immunotherapy was assessed by worsening of the autoimmune disorder or flare requiring systemic or immunosuppressive therapy or interruption to immunotherapy as well as the incidence and management of conventional irAE. Flare of autoimmune disease was as diagnosed by the patient’s treating oncologist, and where necessary in conjunction with autoimmune disease experts. Severity of irAE and flare was defined by Common Terminology Criteria for Adverse Events criteria.16 The efficacy of combination immunotherapy in this population was measured either by Response Evaluation Criteria in Solid Tumours V.1.117 or by clinical assessment of fluorodeoxyglucose-positron emission tomography (FDG-PET)imaging and assessment of objective response rate, duration of response, progression-free survival (PFS) and overall survival (OS).
Statistical analysis
Categorical and continuous variables are summarized using percentages and medians. No formal hypothesis testing was performed. OS and PFS were estimated using the Kaplan-Meier method and compared using the log-rank test; all patients were censored at last available follow-up. PFS was defined as time of treatment start to disease progression (as determined by the treating clinician); OS was defined as treatment start to death for any reason. Univariate and multivariable Cox regression analyses served to determine predictive variables. All analyses were performed by IBM SPSS Statistics and R statistics. Data analysis was performed on June 21, 2020.
Results
Patient characteristics
Fifty-five eligible patients were identified. All patients were followed for more than 3 months with a median follow-up time of 14 months. The median age of patients was 63 years (range 23–83), 69% had M1c or d disease.
Pre-existing autoimmune diseases included Crohn’s disease (N=3), ulcerative colitis (N=7), Graves’ disease (N=8), Hashimoto’s thyroiditis (N=11), RA (N=7), psoriasis (N=6), multiple sclerosis (N=2) and others (table 1). Three patients (5%) had more than one autoimmune disease. At commencement of combination immunotherapy, 10 patients (18%) had active symptoms of their autoimmune condition. Thirteen patients (24%) were receiving immunosuppressive therapy (five on corticosteroids, five on steroid-sparing agents and three on both).
Table 1.
Baseline patient characteristics
| Demographics | No (%) | Detail |
| Age, median (range), years | 63 (23–83) | |
| Gender | ||
| Male | 26 (47) | |
| Female | 29 (53) | |
| ECOG ≤1 | 53 (96) | |
| AJCC stage | ||
| III/M1a/M1b | 17 (31) | |
| M1c | 21 (38) | |
| M1d | 17 (31) | |
| LDH | ||
| Normal | 40 (73) | |
| Elevated | 15 (27) | |
| Mutation status | ||
| BRAF/NRAS wild type |
29 (53) | |
| BRAF | 17 (31) | |
| NRAS | 9 (16) | |
|
Autoimmune disorder (AD)* |
||
| Rheumatologic | 11 (20) | Rheumatoid arthritis—7, Sjogren’s syndrome—1, Behcet’s syndrome—1*, polymyalgia—1, uveitis—1* |
| Gastrointestinal | 14 (25) | Ulcerative colitis—7, Crohn’s disease—3, Celiac disease—4 |
| Endocrine | 21 (38) | Graves’ disease—8, Hashimoto’s thyroiditis—11*, type I diabetes—2* |
| Dermatologic | 7 (13) | Psoriasis—6, alopecia areata—1* |
| Neurologic | 2 (4) | Multiple sclerosis—2 |
| Hematologic | 1 (2) | ITP |
| Other | 2 (4) | Sarcoidosis—2 |
| Activity of AD | ||
| Clinically active | 10 (18) | |
| Not clinically active | 45 (82) | |
| Immunosuppression | 13 (24) | |
| Corticosteroids | 5 (9) | |
| SSA | 5 (9) | Sulfasalazine—1, mesalazine—2, plaquenil—1, methotrexate and plaquenil—1 |
| Corticosteroids+SSA | 3 (5) | Sulfasalazine, methotrexate, plaquenil |
| No immunosuppression | 42 (76) |
*Three of 55 patients had two concurrent autoimmune diseases.
AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Group Performance Status; ITP, immune thrombocytopenic purpura; LDH, lactate dehydrogenase; SSA, steroid-sparing agent.
Patients received ipilimumab in combination with either nivolumab (N=46) or pembrolizumab (n=9). Forty patients (73%) received an ipilimumab dose of 3 mg/kg and nivolumab 1 mg/kg 3 weekly for four doses followed by maintenance nivolumab 3 mg/kg. Fifteen (27%) had an alternate regimen with a lower dose of ipilimumab of 1 mg/kg; six with nivolumab 3 mg/kg every 3 weeks for four doses followed by maintenance nivolumab, and nine with pembrolizumab 2 mg/kg every 3 weeks for four doses followed by maintenance pembrolizumab. Anti-PD1 monotherapy was given previously to nine patients (16%), resulting in no flares of autoimmune disease and three (33.3%) patients had experienced previous grade 1 or 2 irAEs.
Flare of pre-existing autoimmune disease
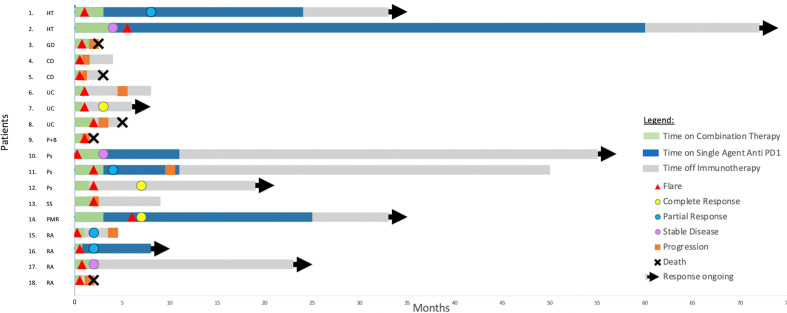
Eighteen patients (33%) experienced a flare of their autoimmune disease (figure 1, table 2), most often in rheumatic and gastrointestinal disorders. This included 4 of 7 with RA, 3 of 6 with psoriasis, 3 of 7 patients with ulcerative colitis, (including one of two who had undergone previous subtotal colectomy), 2 of 3 with Crohn’s disease, 1 of 8 with Graves’ disease, 2 of 11 with Hashimoto’s thyroiditis, 1 of 1 with Sjogren’s syndrome, 1 of 1 with polymyalgia rheumatica, and 1 of 1 with concurrent Bechet’s syndrome and psoriasis. Flare of autoimmune conditions was based on clinical history and clinician diagnosis. For the patients with RA and Sjogren’s syndrome, this was confirmed with serological testing. For patients with a flare of IBD, four of the five patients who experienced a flare underwent a biopsy for histopathological confirmation. The median time to flare was 19 days (range 4–167). The median time to flare for rheumatologic conditions was 16 days and for IBD was 28 days. Sixteen of the 18 (89%) of the flares occurred during combination therapy, and 2 of 18 (11%) occurred on single-agent PD1 maintenance treatment.
Figure 1.
Patients with an autoimmune flare: treatment, response, flare and progression timelines: CD, Crohn’s disease; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; P+B, psoriasis+Behcet’s syndrome; PMR, polymyalgia rheumatica; Ps, psoriasis; RA, rheumatoid arthritis; SS, Sjogren’s syndrome; UC, ulcerative colitis.
Table 2.
Rates of flare of autoimmune disease (AD)
| No (%) | Details | |
| Flare of AD | ||
| No | 37 (67) | |
| Yes | 18 (33) | |
| Time to flare (range) days | 19 (4–167) | |
| Grade of flare of AD | ||
| G1, 2 | 11 (61) | |
| G3 | 5 (28) | Ulcerative colitis—1, Crohn’s disease—1, RA—2, psoriasis—1 |
| G4 | 2 (11) | Ulcerative colitis—2 |
| Flare by AD subtype | ||
| Rheumatologic | 7/11 (64) | RA—4/7, Sjogren’s syndrome—1/1, Behcet’s syndrome and psoriasis —1/1, polymyalgia rheumatica—1/1 |
| Gastrointestinal | 5/14 (56) | Ulcerative colitis—3/7, Crohn’s disease—2/3 |
| Dermatologic | 3/7 (43) | Psoriasis—3/6 |
| Endocrine | 3/21 (11) | Hashimoto’s thyroiditis—2/11, Graves’ disease—1/8 |
| Neurologic | 0/2 (0) | |
| Hematologic | 0/1 (0) | |
| Other | 0/2 (0) | |
| Flare by AD activity at baseline | ||
| Clinically active | 5/10 (50) | |
| Clinically inactive | 13/45 (29) | |
| On immunosuppression | 7/13 (54) | |
| Not on immunosuppression | 11/42 (26) | |
| Immunosuppression for AD flare | ||
| Oral steroids | 4 (22) | |
| Intravenous steroids | 3 (17) | |
| Steroid and SSA* | 6 (33) | Ciclosporin—1 for psoriasis, sulfasalazine—2 for ulcerative colitis and polymyalgia, infliximab—2 for ulcerative colitis and Crohn’s disease, methotrexate—2 for RA, leflunomide—1 for RA |
| No immunosuppression | 5 (28) | |
| IO dosing after flare | ||
| Both drugs ceased | 5 (28) | IBD—2, Behcet’s syndrome—1, RA—1, Sjogren’s syndrome—1 |
| Anti-PD1 alone continued | 3 (17) | |
| Both continued | 7 (39) | |
| Ceased due to PD | 3 (17) |
*Some patients received two SSAs.
IBD, inflammatory bowel disease; IO, immuno-oncology therapy; PD, progressive disease; RA, rheumatoid arthritis; SSA, steroid-sparing agent.
Thirteen of the 18 (72%) patients with a flare were managed with corticosteroids and six (33%) required additional immunosuppressive agents which included ciclosporin, sulfasalazine, infliximab, methotrexate and leflunomide. Seven patients (39%) were hospitalized for management of a flare (three with ulcerative colitis, two with Crohn’s disease and two with RA). Two patients (11%) required admission to intensive care and vasopressor support for severe flares of ulcerative colitis, which had been quiescent prior to treatment and off immunosuppression. One patient suffered diarrhea and shock and the other a duodenal perforation, both recovered and were responsive to intravenous steroids and sulfonamides. Five patients (28%) permanently ceased both drugs due to the flare (two with IBD, one with RA, one with Behcet’s syndrome, one with Graves disease). The median number of doses administered prior to cessation was 1 (range 1–8). Eight of the 18 (44%) patients ceased either both immunotherapy agents or continued on anti-PD1 agent alone due to the flare.
There was a numerical trend to flare of autoimmune disease in patients who had clinically active disease more than those who had clinically inactive disease (50% vs 29%, OR 1.59; 95% CI 0.34 to 7.38, p=0.56). There were also more flares in patients on immunosuppression than in those not on immunosuppression for their autoimmune disorders at immunotherapy commencement (39% vs 26%, OR 4.59; 95% CI 1.16 to 18.04, p=0.03). Notably, in the patients with RA with severe flare (defined as severe pain, joint swelling and limiting self-care), all had quiescent symptoms prior to commencement of therapy. In the four patients with IBD who had a severe flare, none were on immunosuppression and all had been in clinical remission for greater than 24 months prior to commencement of combination immunotherapy.
Immune-related adverse events
Thirty-seven patients (67%) experienced an irAE unrelated to their autoimmune disease (table 3). Twenty-one patients (38%) had grade 3–4 irAEs, these included colitis (N=9), hepatitis (N=5), colitis and hepatitis (N=2), pneumonitis (N=2), thyroiditis (N=1), myasthenia gravis (N=1) and GBS (N=1). Nine patients (17%) experienced both flare of autoimmune disease and an irAE. All patients with a grade 3 or 4 immune-related colitis underwent diagnostic colonoscopy or flexible sigmoidoscopy and histopathological confirmation.
Table 3.
Rates of irAE (unrelated to pre-existing autoimmune disease)
| No (%) | Details | |
| irAE | ||
| No | 18 (33) | |
| Yes | 37 (67) | |
| irAE grade | ||
| G1, 2 | 16(43) | Colitis—5, hepatitis—2, hypophysitis—4, thyroiditis—2, rash, arthritis, type 1 diabetes |
| G3 | 15 (27) | Colitis—7, hepatitis—3, colitis+hepatitis—2*, pneumonitis—2, thyroiditis |
| G4 | 6 (15) | Colitis—2, hepatitis—2, Guillain-Barre syndrome, myasthenia gravis |
| Immunosuppression for irAE | ||
| Oral steroids | 7 (19) | |
| IV steroids | 7 (19) | |
| Steroid and SSA | 9 (24) | Colitis—4, hepatitis—3, colitis+hepatitis—2 |
| IVIg | 1 (3) | Guillain-Barre syndrome |
| Plasmapheresis | 1 (3) | Myasthenia gravis |
| IO dosing after irAE | ||
| Both drugs ceased | 15 (41) | Colitis—6, hepatitis—3, colitis+hepatitis—2, pneumonitis, Guillain-Barre syndrome, myasthenia gravis, type 1 diabetes mellitus |
| Anti-PD1 continued alone | 2 (5) | |
| Anti-CTLA4 continued alone | 3 (8) | |
| Both continued | 17 (46) |
*Two patients experienced both grade 3 colitis and hepatitis.
irAE, immune-related adverse event; IVIg, intravenous immunoglobulin; SSA, steroid-sparing agent.
Twenty-five patients (68%) required immunosuppression for the management of irAE. Seven patients were managed with oral steroids, seven with intravenous steroids, and nine patients were managed with steroids and steroid-sparing agents; one patient with GBS was managed with intravenous immunoglobulin and one patient with myasthenia gravis was treated with plasmapheresis. Both drugs were ceased due to irAE in 15 patients (41%).
A further subanalysis was undertaken to compare the outcomes of patients who had a flare of their autoimmune disorder with patients who had an irAE alone or both an irAE and a flare. Ten patients had no flare or irAE, 27 patients had an irAE alone, 9 patients had a flare of their autoimmune disorder alone, and 9 had both a flare and an irAE (online supplemental table 1). There was a slightly higher use of immunosuppression to manage patients with both a flare and irAE (89%) compared with irAE alone (70%) or flare alone (78%). More patients who experienced both a flare and irAE were alive at the time of analysis (78%) compared with no irAE or flare (60%), irAE alone (70%) and flare alone (67%). Unfortunately the small numbers in this subanalysis limit statistical evaluation.
jitc-2020-002121supp001.pdf (203KB, pdf)
Patient outcomes
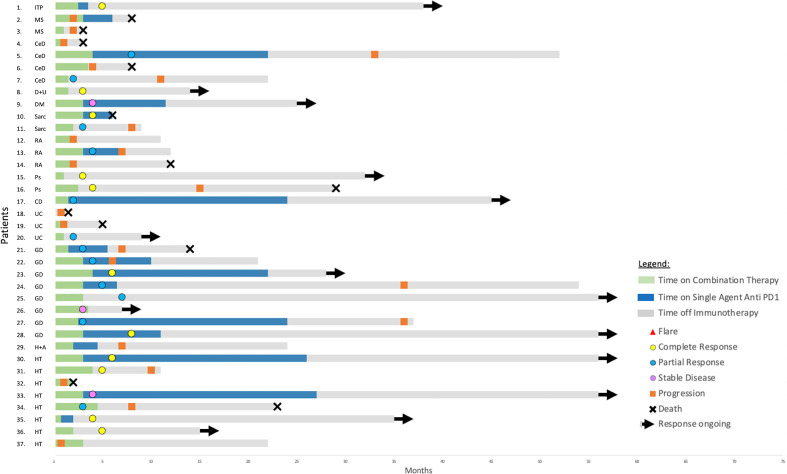
A partial or complete response was observed in 30 patients (55%), 30% partial and 25% complete. Seventy-seven per cent of responses are ongoing, with a median follow-up time of 14 months (figures 1 and 2). The objective response rate was not statistically different in those who had a flare of their autoimmune disease versus those who did not (44% vs 59%, p=0.39) nor in patients who were on baseline immunosuppression versus not (46% vs 57%, p=0.72).
Figure 2.
Patients without an autoimmune flare: treatment, response and progression timelines: CD, Crohn’s disease; CeD, celiac disease; DM, diabetes mellitus; D+U, diabetes and uveitis; GD, Graves’ disease; H+A, Hashimoto’s thyroiditis and alopecia areata; HT, Hashimoto’s thyroiditis; ITP, immune thrombocytopenic purpura; MS, multiple sclerosis; P+B, psoriasis+Behcet’s syndrome; PMR, polymyalgia rheumatica; Ps, psoriasis; RA, rheumatoid arthritis; Sarc, sarcoidosis; SS, Sjogren’s syndrome; UC, ulcerative colitis.
Fourteen patients had a complete response (online supplemental table 2). All of these patients had a performance status of ECOG 0. At commencement of combination immunotherapy, two of the complete responders (14%) had active symptoms of their autoimmune condition and were receiving corticosteroids. Three (21%) of these patients experienced a flare of their autoimmune diseases (one with ulcerative colitis, one with psoriasis, one with polymyalgia). Twelve (86%) patients experienced an irAE with seven of these being grade 3 or 4 (online supplemental table 3). Eleven patients remain in complete response.
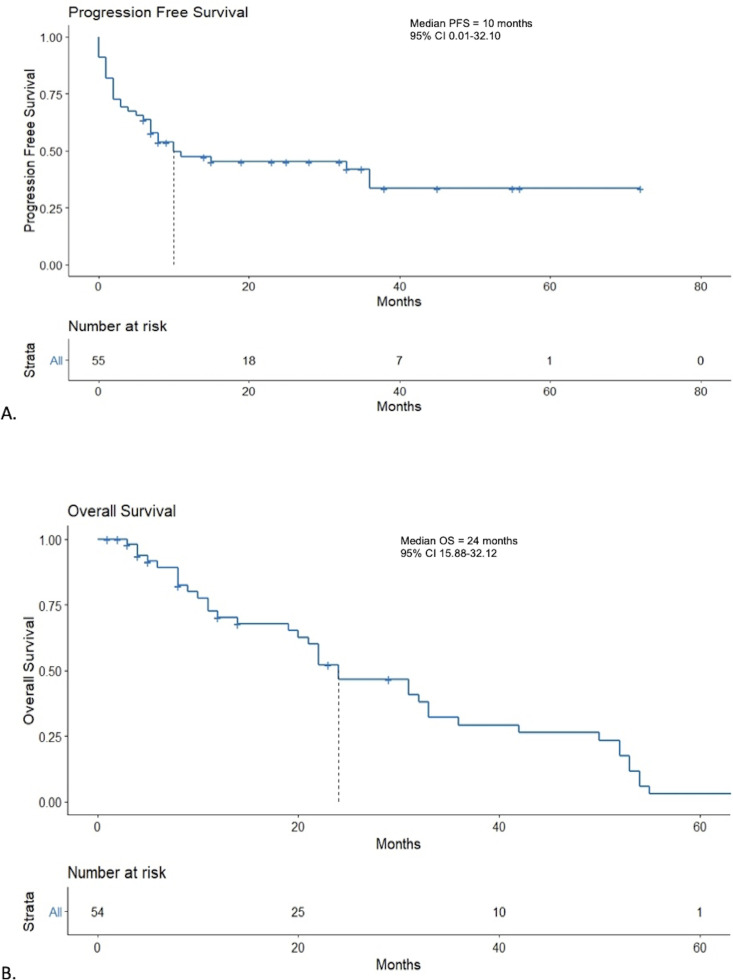
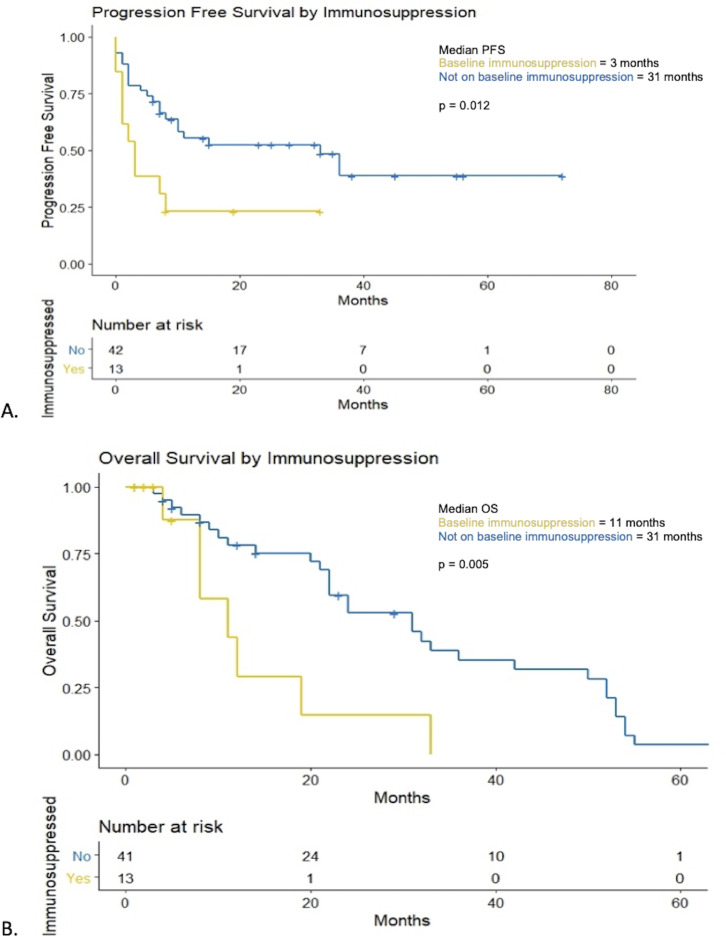
The median PFS was 10 months (95% CI 0.01 to 32.10) (figure 3A). Patients with flare of their autoimmune disease had a median PFS of 5 months compared with 11 months in those without a flare (p=0.73) (figure 4A). At 12 months, PFS rates were 33% vs 22% in those with a flare of their autoimmune disease versus no flare, respectively. Patients on baseline immunosuppression had a median PFS of 3 months (95% CI 0.71 to 5.30) compared with 33 months (95% CI 8.38 to 57.62) for patients not on baseline immunosuppression (p=0.012) (figure 5A). At 12 months, PFS rates were 15% vs 48% in those on immunosuppression versus no immunosuppression, respectively.
Figure 3.
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS). (A) Kaplan-Meier curves for PFS. (B) Kaplan-Meier curves for OS.
Figure 4.
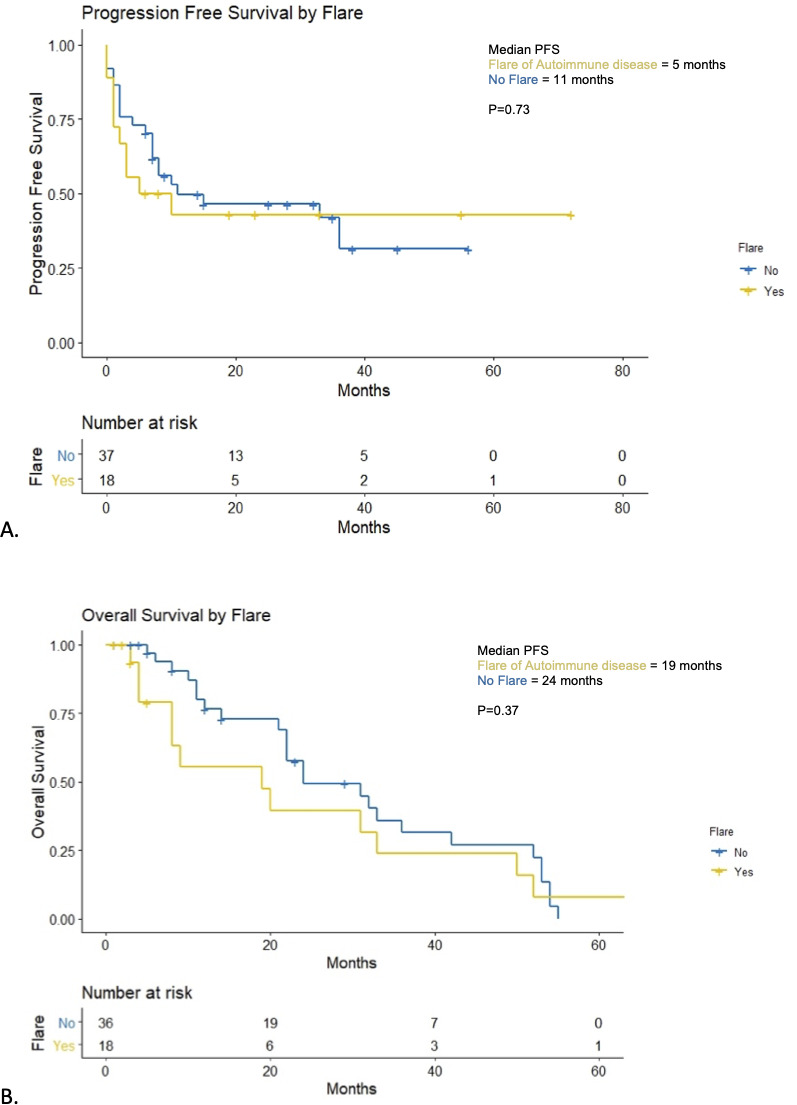
Progression-free survival (PFS) and overall survival (OS) in patients with flare of autoimmune disease. (A) Kaplan-Meier curves for PFS for patients with flare of autoimmune disease versus no flare. (B) Kaplan-Meier curves for OS for patients with flare of autoimmune disease versus no flare.
Figure 5.
Progression-free survival (PFS) and overall survival (OS) in patients on baseline immunosuppression. (A) Kaplan-Meier curves for PFS for patients on baseline immunosuppression versus no immunosuppression at baseline. (B) Kaplan-Meier curves for OS for patients on baseline immunosuppression versus no immunosuppression at baseline.
At the time of analysis, a total of 17 patients (31%) had died. One patient was lost to follow up following progression of disease and was not included in the OS analysis. Median OS was 24 months (95% CI 15.88 to 32.12) (figure 3B). Median OS in patients who had a flare of their autoimmune disease was 19 months vs 24 months in those who did not (p=0.37) (figure 4B). Twelve-month OS rates were 39% in patients who had a flare of autoimmune disease vs 64% in those who did not. Median OS in patients who were on baseline immunosuppression was 11 months (95% CI 3.42 to 18.58) compared with 31 months (95% CI 20.89 to 41.11, p=0.005) (figure 5B). Twelve-month OS rates were 23% in patients on immunosuppression vs 66% in those who were not.
Endocrine autoimmune disorders
Given there were a significant number of patients with an underlying endocrine autoimmune condition, a further subanalysis was performed to assess the difference in outcomes (online supplemental table 4). Endocrine autoimmune disorders were defined as endocrine only disorders and not patients who had an additional autoimmune condition (18 with thyroiditis, 1 with type 1 diabetes mellitus). Patients with an endocrine autoimmune disorder in patients versus patients with non-endocrine autoimmune disorders were less likely to have a flare 16% vs 42% (two-sided Fisher’s exact p=0.05). The rates of irAEs were similar across both groups. The median OS was worse in the patients with a non-endocrine autoimmune disorder at 22 months vs 35 months in patients with an endocrine autoimmune disorder (p=0.046).
Subsequent therapies
Nineteen patients (35%) were given subsequent therapy following combination immunotherapy, some of these had multiple lines of therapy. Alternate anti-PD1 therapy was given in 11 patients (58%), BRAF-MEK inhibitors in 8 patients (42%), 1 patient (5%) was treated with chemotherapy and 5 patients (26%) were enrolled on clinical trials. Of the patients given subsequent therapies, 58% had a partial or complete response.
Discussion
Therapy with ipilimumab in combination with anti-PD1 agents is associated with higher rates of irAEs compared with anti-PD1 therapy alone.1 The safety and efficacy of combination therapy in patients with autoimmune disorders is unknown. To our knowledge, this is the first study to examine this issue. The results of this study suggest that efficacy of combination ipilimumab and anti-PD1 therapy is comparable in patients with autoimmune disorders (not on baseline immunosuppression) with the clinical trial population in patients.1 18–20
Flares of pre-existing autoimmune disorders were common, affecting 33% of patients. These events were, for the most part, managed easily by standard treatment protocols. It is known that ipilimumab is associated with increased rates of colitis21 and anti-PD1 higher rates of arthropathy.22 Therefore, it is not surprising that in our study, the combination of the two demonstrates an increased risk of flare in both rheumatologic and gastrointestinal autoimmune conditions. In particular, IBD flares seemed to be idiosyncratic and often life-threatening.
Patients with a flare of their autoimmune disease had numerically worse survival outcomes than those without. While this was not statistically significant, a potential explanation for this may be the early cessation of therapy in 44% of patients and the addition of immunosuppression in 62% contributed to the worse PFS and OS outcomes compared with other patients without flare of their autoimmune disease.
The rate of irAEs otherwise appeared similar to rates observed in clinical trial population.1 18–20
Patients on immunosuppression at baseline had similar disease characteristics to patients not on immunosuppression and were noted to have worse outcomes with a lower OS and PFS which have been substantiated in prior studies.7 9 Although overall response rates were similar, the inferior survival rates may be in part driven by attenuation of the therapeutic T-cell response by concomitant immunosuppressive treatment. Therefore, in patients with pre-existing autoimmune diseases that are not rheumatologic or IBD, weaning or cessation of immunosuppression prior to immunotherapy should be considered in patients without clinically active disease.
The rates of flare were higher in patients with non-endocrine autoimmune conditions versus those with an endocrine autoimmune condition. This is suggestive that perhaps it is safer for patients with endocrine disorders to be on combination immunotherapy. Additionally, patients with non-endocrine autoimmune disorders had worse PFS and OS outcomes perhaps owing to the higher rates of flare leading to discontinuation and/or higher levels of baseline immunosuppression.
A previous study assessing the safety and activity of single-agent ipilimumab in 30 patients with a range of concomitant autoimmune disorders found that 27% of patients experienced a flare of their autoimmune disorder and 33% experienced grade 3–4 irAEs with a 20% response rate.8 Another retrospective analysis in the same population with anti-PD1 antibodies suggested patients were at risk of mild flare of their autoimmune disorder with 38% of patients experiencing a flare and 10% experiencing grade 3–4 irAEs with a 33% response rate.9 Both studies concluded there was reasonable activity in patients with baseline autoimmunity, but with greater immune toxicities often of a mild nature. Findings in our study demonstrate a higher rate of flare of autoimmune disease and mirror the higher rates of irAEs in patients who receive combination therapy.
A retrospective assessment of patients specifically assessing IBD has shown relative safety with the use of immunotherapy.13 There was a higher rate of any grade gastrointestinal adverse events in patients with pre-existing IBD, 41% vs 11%. In this analysis, use of CTLA4 or combination therapy was associated with a higher risk of gastrointestinal adverse events compared with anti-PD1. However, 4 out of 102 patients in t study of immune checkpoint inhibitors in IBD experienced colonic perforation, 3 having previously received anti-PD1 therapy and 1 receiving combination with ipilimumab and anti-PD1 therapy. Comparatively, the rates of flare of IBD in our study were 56% with two of these resulting in intensive care unit admission. Therefore, it is paramount that patients with IBD who develop a suspected gastrointestinal toxicity or flare are thoroughly investigated to ensure appropriate and timely management.
As experience builds in these populations with the use of immunotherapy, management of flares of autoimmune disease will improve. However, recent recommendations have been published for the management of patients with autoimmune diseases.7 In our study, most patients were managed with corticosteroids, with severe cases of psoriasis, RA and IBD managed with a form of additional immunosuppression to steroids.
There are several limitations of our study. First, the retrospective analysis of patient data adds an inherent selection bias within the cohort reflecting patients who have been deemed suitable for combination ipilimumab and PD-1 therapy by the treating physician. Most of the patients had clinically inactive disease not requiring immunosuppression prior to treatment. Second, the short overall follow-up time of patients may prevent suitable analysis of OS and PFS. Third, we acknowledge patient numbers may have prevented detection of significant differences between groups.
This highlights the importance for this patient population to be considered in further clinical trials so that responses, impacts of immunosuppression and flare management may be evaluated in a prospective manner.7
Conclusions
Combination immunotherapy with ipilimumab and PD1 inhibitors may flare pre-existing autoimmune diseases particularly rheumatologic and gastrointestinal disorders, and those disorders that are clinically active and/or require immunosuppression. Rates of irAE were not increased.
Our results support that combination immunotherapy for patients with pre-existing autoimmune disease is efficacious for patients with advanced melanoma. However, with pre-existing IBD and rheumatologic conditions, the risk of severe flare is significant and these patients should be informed of this risk. Thus, close monitoring and thorough investigation of concerning symptoms are essential in these patients if treated with combination immunotherapy followed by prompt treatment with consultation of irAE management guidelines.
Footnotes
Contributors: Conception and design—LJB and MC. Collection and assembly of data—LJB. Provision of study material or patients—LJB, AW, PB, CA, JP, PO, AH, CL, DBJ, SS, GVL, AM and MC. Data analysis and interpretation—LJB and MC. Manuscript writing—LJB. Co-writing the manuscript— MC. All authors interpreted the data, reviewed the manuscript, gave final approval of the manuscript and are accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CA—received travel support from Amgen, Bristol-Myers Squibb and Roche. DBJ—advisory role for Array Biopharma, Bristol-Myers Squibb, Iovance, Jansen, Merck and Novartis; has received research funding from Bristol-Myers Squibb and Incyte. PO—received research funding from and has advised Neon Therapeutics, Bristol-Meyers Squibb, Merck, CytomX, Pfizer, Novartis, Celldex, Amgen, Array, AstraZeneca/MedImmune, Armo BioSciences and Roche/Genentech. CL—honoraria from Amgen, Bristol-Myers Squibb, Incyte, Merck Sharpe & Dome, Novartis, Pfizer, Pierre Fabre and Roche; has an advisory role for Amgen, Bristol-Myers Squibb, Merck Serono, Merck Sharpe & Dome, Novartis, Roche and Sanofi; has received research funding from Bristol-Myers Squibb and Roche; and received travel support from Bristol-Myers Squibb and Merck Sharpe & Dome. SS—honoraria from Amgen, Bristol-Myers Squibb, Merck and Merck Serono; has an advisory role for Amgen; received research funding from Bristol-Myers Squibb and Genentech; and has received travel support from Genentech. GVL—consultant or advisory role to Aduro Biotech, Amgen, Array Biopharma, Boehringer Ingelheim International, Bristol-Myers Squibb, Highlight Therapeutics, Merck Sharpe & Dohme, Novartis Pharma, QBiotics Group Limited, Regeneron Pharmaceuticals, SkylineDX outside the submitted work. AM—advisory role to Bristol-Meyers Squibb, MSD, Novartis, Roche and Pierre-Fabre outside the submitted work. MC—consultant or advisory role to BMS, MSD, Amgen, Novartis, Pierre Fabre, Roche, Sanofi, Merck and Co, Ideaya, Regeneron, Nektar, Eisai, Oncosec and Q biotics outside the submitted work; received honoraria from Bristol-Meyers Squibb, MSD and Novartis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. Deidentified participant data are available from LJB upon request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Each individual site had ethics approval, either prospective informed consent or retrospective consent, depending on the requirement of the individual institutions.
References
- 1. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 4. Baas P, Scherpereel A, Nowak A, et al. ID:2908 first-line nivolumab + ipilimumab vs chemotherapy in unresectable malignant pleural mesothelioma: CheckMate 743. Journal of Thoracic Oncology 2020;15:e42itle:e42. 10.1016/j.jtho.2020.08.004 32093860 [DOI] [Google Scholar]
- 5. Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 2020:2–9. 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Overman MJ, Lonardi S, Wong KYM, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (PTS) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. JCO 2019;37:635. 10.1200/JCO.2019.37.4_suppl.635 [DOI] [Google Scholar]
- 7. Haanen J, Ernstoff MS, Wang Y, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 2020;31:724–44. 10.1016/j.annonc.2020.03.285 [DOI] [PubMed] [Google Scholar]
- 8. Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–40. 10.1001/jamaoncol.2015.4368 [DOI] [PubMed] [Google Scholar]
- 9. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-Pd-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 10. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–12. 10.1200/JCO.2017.77.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 2018;168:121–30. 10.7326/M17-2073 [DOI] [PubMed] [Google Scholar]
- 12. Kennedy LC, Bhatia S, Thompson JA, et al. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J Natl Compr Canc Netw 2019;17:750–7. 10.6004/jnccn.2019.7310 [DOI] [PubMed] [Google Scholar]
- 13. Abu-Sbeih H, Faleck DM, Ricciuti B, et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J Clin Oncol 2020;38:576–83. 10.1200/JCO.19.01674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol 2019;71:2100–11. 10.1002/art.41068 [DOI] [PubMed] [Google Scholar]
- 15. Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119–36. [PMC free article] [PubMed] [Google Scholar]
- 16. Common terminology criteria for adverse events. Definitions. Published online 2020. [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18. Lebbé C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019;37:867–75. 10.1200/JCO.18.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–81. 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 20. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellaguarda E, Hanauer S. Checkpoint Inhibitor–Induced colitis. Am J Gastroenterol 2020;115:202–10. 10.14309/ajg.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 22. Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 2018;48:553–7. 10.1016/j.semarthrit.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-002121supp001.pdf (203KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. Deidentified participant data are available from LJB upon request.