Abstract
Objective:
Prior research demonstrated that the δ-opioid receptor (OPRD1) rs678849 variant influences opioid use in African Americans treated with methadone. We examined whether this variant moderated cocaine and opioid use in our clinical cohort of methadone and disulfiram treated recipients.
Methods:
Cocaine and opioid co-dependent patients were stabilized for two weeks on methadone and subsequently randomized into groups treated with either methadone+placebo (n = 37) or methadone+disulfiram (250 mg/day, n = 33) for 12 weeks.
Results:
A drop in cocaine positive urines was found in the OPRD1 CC genotype group compared to T-allele carrier patients treated with methadone+disulfiram (P < 0.0001), but not in the methadone+placebo group. No difference in opioid positive urines were found among each genotype group in either treatment group.
Conclusion:
These findings suggested that rs678849 genotype may predict treatment response of disulfiram for cocaine use in patients with co-occurring opioid and cocaine dependence.
Keywords: Disulfiram, methadone, polymorphism, cocaine, opioid, ORPD1
Introduction
Opioid dependence (OD, as defined in DSM-IV) is a chronic disease that accounted for 28% of all drug abuse treatment admissions in 2015 (SAMHSA-TEDS, 2018) and imposed an annual economic burden of 56 billion dollars in the United States in 2007 (Birnbaum et al., 2011). Cocaine dependence (CD) co-occurs with opioid dependence in 30–50% of methadone-maintained populations (Grella et al., 1997). This comorbidity worsens most outcomes, resulting in decreased treatment completion, increased relapse, and poorer mental health outcomes (Villano et al., 2002,Bovasso and Cacciola, 2003,Tzilos et al., 2009,King et al., 2014,White et al., 2014,Oviedo-Joekes et al., 2015). As such, there is a critical need for clinical trials to examine the effectiveness treatment of OD/CD comorbidity. Although there are no FDA approved drugs for OD/CD nor CD alone (Kenna et al., 2007), some drugs have demonstrated efficacy for both disorders. Methadone is primarily used for treatment of OD, but reduces co-occurring cocaine use when given in doses > 60 mg (Faggiano et al., 2003,Peles et al., 2006). Disulfiram, when given with methadone in opioid users, reduces cocaine, but not opioid positive urines in opioid users (Petrakis et al., 2000,Kosten et al., 2013). Many suspect that disulfiram prevents relapse through its inhibition of dopamine β-hydroxylase, which decreases conversion of dopamine to norepinephrine, thus lowering norepinephrine tone and reducing stress-related reinstatement of cocaine use (Gaval-Cruz and Weinshenker, 2009).
Methadone treatment for OD acts through the opioid receptor family, which includes the μ-opioid receptor (MOR), ĸ-opioid receptor (KOR), and δ-opioid receptor (DOR). Methadone acts as a full agonist at MOR at typical treatment doses, resulting in a release of dopamine which produces rewarding effects similar to endogenous and illicit opioids (Kreek et al., 2005). Methadone binds to KOR and DOR at much lower affinities, and likely does not illicit the typical dysphoria from KOR activation or analgesia from DOR activation (Kristensen et al., 1995,Chavkin, 2011).
Despite not being activated by methadone, DOR function may play an important role in the treatment of co-occurring CD/OD, as evidenced by animal studies that demonstrate DOR’s role in response to cocaine and tolerance to morphine (Unterwald et al., 1993,Zhu et al., 1999,Nitsche et al., 2002). Methadone may affect cocaine use through receptor heterodimers that DOR forms with MOR. The summation of opioid signaling by heterodimers of DOR and MOR may be an understudied part of opioid signaling, as these heteromers activate cell signaling pathways differently than either receptor alone in vitro (Kabli et al., 2014).
Approximately 25–35% of methadone-maintained patients have opioid-positive urine drug screens during treatment, even with adherence to methadone and supplemental psychosocial treatments (Zhang et al., 2013,Nielsen et al., 2016). These outcomes may be explained by genetic background, as heritability of illness is 70% in CD and 50% in OD (Khokhar et al., 2010). Variants in opioid receptor genes have been associated with CD, OD, and overall risk of substance use disorders (Yuferov et al., 2010,Levran et al., 2012,Schwantes-An et al., 2016).
The single nucleotide polymorphism (SNP) rs678849 is located in the first intron of OPRD1. The OPRD1 variant rs678849 may be important for examining the effect of genotype on cocaine and opioid abuse. The CC genotype of rs678849 has been found to be associated with response to buprenorphine and methadone treatment of OD in African Americans (AA), but not in EAs (Crist et al., 2013a). Those participants carrying the CC genotype responding better to buprenorphine but worse to morphine than those carrying a T allele. In European American (EA) females, two other OPRD1 variants, rs581111 and rs529520, were found to be associated with treatment response to treatment of OD with methadone or buprenorphine (Clarke et al., 2014). Crist et al., also found that during a 14-week treatment trial with topiramate OPRD1 rs678849 T-allele carriers in cocaine- and alcohol-dependent AAs used less cocaine than did those with the CC genotype (Crist et al., 2016). These results have yet to be replicated in a multi-ethnic cohort and have not specifically tested disulfiram as a pharmacotherapy, but suggest that T-allele carriers respond better to cocaine pharmacotherapy.
The aims of this study were two-fold. First, since the OPRD1 rs678849 variant is associated with treatment response to methadone pharmacotherapy for OD, we hypothesized that this variant also might be associated with disulfiram treatment in CD participants. We expect that T-allele carriers treated with methadone+disulfiram will have fewer cocaine positive urines than those patients having the CC genotype. Secondly, this study aimed to replicate and confirm the association of the OPRD1 rs678849 variant with response to medication-assisted treatment of OD/CD patients from a multi-ethnic cohort. We hypothesized that methadone-maintained patients with a CC genotype will have fewer opioid positive urines than T-allele carriers.
Material and methods
Participants
From 2005–2006 and 2006–2008, OD/CD patients (n = 93) stabilized on methadone maintenance at Yale University (n = 40) and Baylor College of Medicine (n = 53), respectively, were evaluated during a two-week screening period. Patient screening included physical examination and psychiatric interview with the MINI (English Version 5.0.0, July 1, 2006) (Sheehan et al., 1998) and the ASI (McLellan et al., 1992) to confirm DSM-IV-TR OD and CD diagnoses. DSM-IV-TR was used in our analyses as this was the classification in place during the patients’ enrollment. This study used DSM-IV-TR for OD and CD diagnosis, as this was the standard for diagnosis during the participants enrollment (APA, 2000). DSM-5 was introduced in 2013 and modified the classifications of substance use disorders (SUDs) (APA, 2013). Previously in DSM-IV, SUDs were classified as substance use or substance dependence, with the latter classification having a higher degree of severity. DSM-5 combined these two classification to create the category SUD. In addition, the criterion of legal problems was deleted from DSM-V and replaced with cravings or a strong desire or urge to use a substance. SUD also is classified on the basis of severity, i.e., mild, moderate, or severe in DSM-V.
Patients were retained in the study if they had at least one urine sample positive for cocaine, and continued treatment and follow-up. Women of childbearing age were retained provided they agreed to use adequate contraception and agreed to monthly pregnancy tests. Patients were excluded for current diagnosis of other drug and alcohol dependence (other than tobacco), acute or unstable medical illness, history of severe mental illness (such as psychosis, schizophrenia, or bipolar disorder), current suicidality, a positive urine pregnancy test for women, or inability to read and understand the consent form. This consent, as well as specific consent for genetic studies, was signed by all screened patients, and approved by Institutional Review boards of both institutions, and the Research and Development Committee of the Michael E. DeBakey Veterans Affairs Medical Center. Ninety-three patients were screened and 74 were included in the study. After applying exclusion criteria, the 74 patients were of African American (n = 9), Hispanic (n = 8), and Caucasian (n = 57) ethnicity as determined by self-report. Participants had a mean age of 39 years old (SD = 10.4) and were mostly white males (64%) (Table 1). Participants had abused cocaine for a mean of 12 years (SD = 8.1) and heroin for a mean of 9 years (SD = 9.2). All 74 patients were treated with methadone. Patients were randomly assigned into the methadone+placebo group or methadone+disulfiram treatment group. The final sample consisted of 70 participants with genotype data for OPRD1 rs678849. Among the genotyped participants, there were 25 with the CC, 28 with the CT, and 17 with the TT genotype.
Table 1.
Demographic and clinical characteristics by treatment and OPRD1 genotype.
| Characteristic | Methadone+placebo |
Methadone+disulfiram |
||
|---|---|---|---|---|
| CC | CT/TT | CC | CT/TT | |
| N | 17 | 20 | 8 | 25 |
| % Male | 65 | 65 | 75 | 60 |
| % Caucasian | 59 | 85 | 63 | 84 |
| % African American | 29 | 10 | 13 | 4 |
| % Hispanic | 12 | 5 | 25 | 12 |
| Age, Years (SD) | 39 (12) | 40 (10) | 40 (11) | 38 (10) |
| ASI Employed | 5 (3) | 3 (3) | 4 (3) | 3 (3) |
| Alcohol Last 30 Days | 3 (4) | 3 (5) | 1 (2) | 2 (3) |
| Alcohol, Years | 4 (7) | 5 (7) | 3 (5) | 3 (6) |
| Intoxicated Last 30 Days | 0 (0) | 0 (1) | 0 (0) | 0 (2) |
| Intoxicated, Years | 0 (0) | 3 (6) | 3 (5) | 3 (6) |
| Heroin Last 30 Days | 23 (13) | 11 (13) | 19 (15) | 10 (14) |
| Heroin, Years | 10 (12) | 10 (7) | 12 (10) | 9 (8) |
| Methadone, Last 30 Days | 8 (12) | 19 (14) | 14 (15) | 13 (15) |
| Methadone, Years | 2 (7) | 2 (4) | 2 (4) | 3 (7) |
| Cocaine Last 30 Days | 18 (10) | 19 (8) | 16 (12) | 17 (10) |
| Cocaine, Years | 15 (10) | 14 (6) | 10 (7) | 9 (7) |
| Marijuana Last 30 Days | 3 (7) | 2 (7) | 1 (1) | 1 (2) |
| Marijuana, Years | 6 (9) | 5 (9) | 7 (7) | 7 (8) |
| Illegal drug, Last 30 Days | 4 (9) | 5 (11) | 7 (13) | 4 (10) |
Study design
Study participants were stabilized on methadone maintenance at 60 mg per day for two weeks. Methadone dose started at 25 mg per day and increased 5 mg per day until patients achieved a 60 mg per day maintenance dose. Patients then were assigned randomly to methadone+placebo or methadone+disulfiram treatment groups and treated for ten weeks. The disulfiram (250 mg daily) was dissolved into the patient’s daily methadone dose to assure medication compliance. Individual manualized cognitive behavioral therapy (Carroll, 1997) was provided weekly to all participants. Supervised urine samples were obtained thrice weekly and tested for illicit opioids and cocaine metabolites as described previously (Nielsen et al., 2012).
Genotyping & statistical analyses
DNA purification, genotyping, and statistical analyses were performed as previously described (Nielsen et al., 2012,Kosten et al., 2013). Briefly, we used the Gentra Puregene Buccal Cell Kit (Qiagen, Valencia, CA), following the manufacturer’s recommendations, to isolate DNA using from pelleted buccal cells that were obtained by the centrifugation of 10 ml Scope® mouthwash that was used to rinse the subject’s mouth for 60 seconds.
Genotypes were determined using a 5′-fluorogenic exonuclease assays (TaqMan®, Applied Biosystems, Foster City, CA). The OPRD1 rs678849 variant was genotyped using the TaqMan® primer-probe sets (Applied Biosystems) assay ID C_11264519_1. PCR amplifications were performed using Platinum® quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA) on a GeneAmp® PCR system 9700 (Applied Biosystems).
A repeated-measures analysis of covariance (ANCOVA) was used to analyze the number of cocaine- or opioid-positive urines over the total number of samples (six) for each two-week period. All analyses were corrected for any possible confounding effects, by including the proportion of each subject from the founder populations and sex as covariates in the model. Data from the methadone+disulfiram treatment group was compared to the methadone+placebo group over time to determine if the effect of either treatment (methadone+placebo or methadone+disulfiram) is moderated by the rs678849 OPRD1 using R version 2.9.1 (R_Development_Core_Team, 2009). Genotypes CT and TT were grouped as in the study by Crist et al. (Crist et al., 2013b). We compared condition (methadone+placebo or methadone+disulfiram), OPRD1 genotype (0 = CC genotype, 1 = CT and TT genotypes), time (each two-week period), and interactions between condition and time, and between condition and genotype. We compared condition (disulfiram or placebo), OPRD1 genotype (0=CT/TT genotype, 1=CC genotype), time (each 2-week period), and interactions between condition and time and between condition and OPRD1. We analyzed all individuals who had complete data (n = 55) and unbalanced repeated measures ANCOVA for all genotyped individuals (n = 70). The two analyses yielded similar results; therefore, all analyses were conducted with all genotyped individuals (n =70). ANCOVA and χ2 tests also was used to analyze demographic and MINI/ASI data.
Population structure was determined by genotyping ten ancestry informative markers (AIMs) and comparing our cohort against CEPH-HGDP samples (1,035 subjects of 51 populations) as previously described (Nielsen et al., 2012,Kosten et al., 2013). Lao et al have shown this approach obtains 94.6% of the maximum informativity value (Lao et al., 2006). Sex was confirmed by genotyping SRY (Kosten et al., 2013). Sex, recruitment site, and population structure (three of the four population structure values) were run as covariates in each statistical model. Corrections for multiple testing were performed to evaluate experiment-wise significance by applying the Bonferroni correction for the four analyses conducted (methadone+placebo versus methadone+disulfiram group and the CC genotype group versus T-allele carriers for both cocaine and opioid use. This study was designed as an evaluation of the role of OPRD1 rs678849 under these four conditions with experiment-wise significant being P < 0.05/4 = 0.013). Effect size was calculated as a partial eta-squared statistic using condition or variant variance over residual variance. The three general cut-offs for effect size are the following: a large effect is 0.14, a medium effect is 0.06, and a small effect is 0.01 (Cohen, 1988). Hardy-Weinberg equilibrium was calculated for the OPRD1 rs678849 variant and resulted in non-significant p-values indicating that the genotypes were observed in the expected ratios (P = 0.113).
Results
Cocaine Treatment Outcomes
The overall cohort showed a disulfiram treatment effect (F = 16.1; df = 1,364; P < 0.0001, with an effect size of 0.04) similar to results we had reported previously (Kosten et al., 2013). Positive cocaine urine rates decreased from 79% during the baseline two weeks to 62% for the methadone+disulfiram group and to 75% for the methadone+placebo group during the last two weeks of treatment.
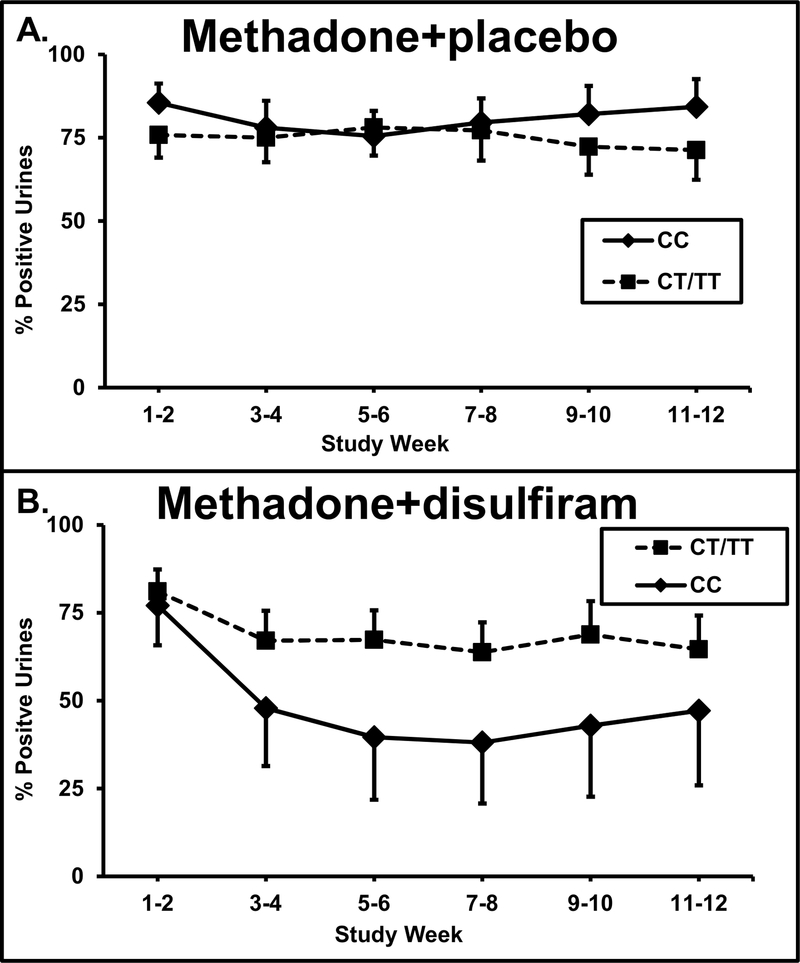
The 70 patients that were genotyped for the OPRD1 alleles were divided into two groups based on their OPRD1 rs678849 genotypes. Patients with one or two T alleles (CT and TT genotypes; T-allele carriers) were compared to those with a CC genotype. As shown in Figure 1, disulfiram reduced cocaine positive urines more than any other group when used in CC genotype patients treated with methadone. There was a treatment by genotype interaction (F = 8.89; df = 1,364; experiment-wise P = 0.0001, with an effect size of 0.05) on cocaine-positive urines.
Fig. 1.
Percentage of cocaine positive urine toxicology screens for two-week time blocks across the 12-week trial for the methadone+placebo treatment group. A. Percentage of cocaine positive urine toxicology screens in the methadone group with the CC genotype (diamond symbols, solid line, n = 17) and those with CT/TT genotypes (square symbols, dashed lines, n = 20) are displayed Standard error bars are shown at each time point. B. Percentage of cocaine positive urine toxicology screens when treated with methadone+disulfiram (dashed line) or methadone+placebo (solid line) in those with the CT/TT genotype (square symbols) and those with CC genotypes (diamond symbols) are shown. Standard error bars are shown at each time point.
Patients with the CC genotype had better outcomes with methadone+disulfiram treatment than with methadone+placebo treatment (F = 27.7; df = 1,127; point-wise P <0.0001, experimental-wise P <.001, with an effect size of 0.22). In these CC participants, the cocaine positive urines in the methadone+disulfiram group dropped from 77% at week 1–2 to 47% at week 11–12 for a 39% drop, while the cocaine positive urines in the methadone+placebo group showed little change of 86% to 84% over the same period (10%). Those patients with the CT/TT genotype showed no difference in cocaine positive urines between methadone+disulfiram and methadone+placebo treatments. For the CT/TT patients, the cocaine positive urines in the methadone+disulfiram group dropped from 81% to 65% (20% drop), while the methadone+placebo group showed little change from 76% to 71% (10% drop).
Opioid treatment outcomes
No association was found with experiment-wise significance for differences in treatment outcome in opioid-positive urines. Neither genotype nor disulfiram/placebo treatment groups showed main effects on opioid-positive urines for the combined cohort.
Discussion
The first aim of this study was to replicate the findings of Clark’s, who found a decrease in opioid-positive urines in AA participants carrying in OPRD1 rs678849 GG participants (Crist et al., 2013a). Our results did not support Clark’s finding, either methadone alone, or with methadone plus disulfiram.
Our second aim was to examine the role of OPRD1 rs678849 in moderation of disulfiram treatment response for CD in a multi-ethnic population. In the CC, but not in the T-allele carrier participants, the methadone patients when treated with disulfiram decreased the number of cocaine-positive urines while in those treated with placebo had minimal decrease in cocaine-positive urines. In contrast, the T-allele participants did better than CC carriers in a clinical trial on CD with topiramate (Crist et al., 2016). This lack of a precise replication may have resulted from multiple factors. First, not only did the cocaine pharmacotherapies differ, but also the comorbid substance dependencies differed, alcohol with topiramate and opioid with disulfiram. Second, the underlying mechanisms for both medications are not direct effects on DOR and are quite different between the enzyme inhibition of disulfiram, and the glutamate and gamma amino butyrate action of topiramate. Thus, indirect pharmacogenetic effects on the DOR and cocaine use might be the opposite for these two medications in different comorbid patient populations.
We previously examined eight other pharmacogenetic associations in this clinical trial and found a genotype dependent effect on treatment response for polymorphisms in the following genes: α1A-adrenoceptor (ADRA1A), dopamine β-hydroxylase (DBH), ankyrin repeat and kinase domain-containing 1 (ANKK1), dopamine receptor D2 (DRD2), dopamine transporter (SLC6A3), 5,10-methylene tetrahydrofolate reductase (MTHFR), serotonin-transporter-linked polymorphic region (SLC6A4, 5-HTTLPR), and tryptophan hydroxylase 2 (TPH2) (Nielsen et al., 2012,Spellicy et al., 2012,Kosten et al., 2013,Shorter et al., 2013,Spellicy et al., 2013,Kampangkaew et al., 2019). These multiple associations highlight the pleiotropic way methadone+disulfiram can improve cocaine treatment outcomes in OD/CD co-dependent patients.
The OPRD1 rs678849 locus is associated with a function that might affect disulfiram and methadone treatment of CD/OD. Crist et al., reported that the T-allele of rs678849 in intron 1 of OPRD1 is located in a binding site for USF2 (Crist et al., 2013a), a transcription factor that may regulate how much DOR is transcribed from the OPRD1. The C-allele at this site abolishes USF2 binding and may reduce DOR levels. Reduced DOR signaling enhances chronic methadone’s effects of internalizing and degrading MOR (Zhu et al., 1999,Nitsche et al., 2002,He et al., 2011).
Genetic variations in the OPRD1 gene appear to be centrally involved in the vulnerability to develop and maintain addictions, and are involved in the efficacy of their treatment. The OPRD1 variants rs2236861, rs2236857 and rs3766951 have been found to be associated with vulnerability to develop opioid addiction. The OPRD1 intron 1 variant rs508448 (Gao et al., 2017) and a haplotype of the OPRD1 intron 1 variants rs2236857 and rs581111 were found to be associated with risk for heroin dependence (Nelson et al., 2014) while the OPRD1 exon 1 variant rs1042114 was associated with OD (Zhang et al., 2008). Recently, two OPRD1 variants, rs204047 and rs797397, were found to be associated with plasma R, S-methadone concentrations(Fang et al., 2020). The OPRD1 variant rs204047 (Fang et al., 2020) and rs529520 (Luo et al., 2017)was found to be related to methadone dosage among participants in methadone maintenance treatment (MMT).
In additional studies, the OPRD1 variant rs204047 was associated with diastolic blood pressure and heart rate in MMT patients (Fang et al., 2020). Furthermore, OPRD1 variants rs9322447 and rs609148 had a role in personality traits such as Openness and the variant rs2234918 with Agreeableness(Luo et al., 2008). A role of the OPRD1 variant rs569356 was observed in anorexia nervosa (Brown et al., 2007). Hence, our finding an association with rs678849, although it has a small effect size, is in line with these other associations of OPRD1 variants.
This study has several limitations. These results may be less generalizable to cocaine abusers that are not opioid dependent. Additionally, the effect size of the most significant findings is small, but consistent with most pharmacogenetic studies. Overall, efforts to replicate pharmacogenetics studies often are hampered by small effect sizes, small samples size, and the constraints of randomized clinical trials. These limitations lead to replication rates as low as 3.4%, which is similar to genome-wide association study (GWAS) replication rates (Aslibekyan et al., 2013). Many academic pharmacogenetic studies on new pharmacotherapies involve moderately sized groups. These human pharmacotherapy studies require substantial energy in terms of cost and time to perform. However, promising candidate pharmacotherapies often will be followed up with larger studies for confirmation and for developing FDA approved pharmacotherapies of cocaine addiction by matching genetically identified patients with appropriate medications. Given that medication assisted treatment for co-occurring substance use disorders has not been successful, further research using genetic backgrounds to guide medication-assisted treatment may allow pharmacotherapy to be personalized, thereby improving outcomes.
Acknowledgements
Supported by: NIH/NIDA 5 P50 DA018197-05 (TK), NIH/NIDA RO1 DA15477, for DN through MD Anderson’s Cancer Center Support Grant DA026120 NIH/NIDA DA026120, and the Toomim Family Fund. This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX. We thank Steve Herrera for his contribution to the figures. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Clinical Trial: Pharmacogenetics of Disulfiram for Cocaine, clinicaltrials.gov/ct2/show/NCT00149630, NIDA-18197-2, NCT00149630
Conflicts of interest
There are no conflicts of interest.
CONFLICTS OF INTEREST The authors declare no conflicts of interest
References
- APA (2000). Diagnostic and Statistic Manual of Mental Disorders (DSM-IV-TR). 4th ed, text rev. edn. Washington, DC: American Psychiatric Association. [Google Scholar]
- APA (2013). Diagnostic and Statistic Manual of Mental Disorders (DSM-5). 5th edn. Washington, DC: American Psychiatric Association. [Google Scholar]
- Aslibekyan S, Claas SA, Arnett DK (2013). To replicate or not to replicate: the case of pharmacogenetic studies: Establishing validity of pharmacogenomic findings: from replication to triangulation. Circ Cardiovasc Genet 6(4):409–412; discussion 412. doi: 10.1161/CIRCGENETICS.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL (2011). Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain medicine 12(4):657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Bovasso G, Cacciola J (2003). The long-term outcomes of drug use by methadone maintenance patients. J Behav Health Serv Res 30(3):290–303. [DOI] [PubMed] [Google Scholar]
- Brown KM, Bujac SR, Mann ET, Campbell DA, Stubbins MJ, Blundell JE (2007). Further evidence of association of OPRD1 & HTR1D polymorphisms with susceptibility to anorexia nervosa. Biol Psychiatry 61(3):367–373. doi: 10.1016/j.biopsych.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Carroll KM (1997). Manual-guided psychosocial treatment. A new virtual requirement for pharmacotherapy trials? Arch Gen Psychiatry 54(10):923–928. [DOI] [PubMed] [Google Scholar]
- Chavkin C (2011). The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology 36(1):369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Crist RC, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ et al. (2014). Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J 14(3):303–308. doi: 10.1038/tpj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Crist RC, Doyle GA, Kampman KM, Berrettini WH (2016). A delta-opioid receptor genetic variant is associated with abstinence prior to and during cocaine dependence treatment. Drug Alcohol Depend 166:268–271. doi: 10.1016/j.drugalcdep.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ et al. (2013a). An Intronic Variant in OPRD1 Predicts Treatment Outcome for Opioid Dependence in African-Americans. Neuropsychopharmacology 38(10):2003–2010. doi: 10.1038/npp.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Ambrose-Lanci LM, Vaswani M, Clarke TK, Zeng A, Yuan C et al. (2013b). Case-control association analysis of polymorphisms in the delta-opioid receptor, OPRD1, with cocaine and opioid addicted populations. Drug Alcohol Depend 127(1–3):122–128. doi: 10.1016/j.drugalcdep.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P (2003). Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev (3):CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Fang CP, Wang SC, Tsou HH, Chung RH, Hsu YT, Liu SC et al. (2020). Genetic polymorphisms in the opioid receptor delta 1 (OPRD1) gene are associated with methadone dose in methadone maintenance treatment for heroin dependence. J Hum Genet 65(4):381–386. doi: 10.1038/s10038-019-0718-x. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang Y, Lang M, Yuan L, Reece AS, Wang W (2017). Contribution of Genetic Polymorphisms and Haplotypes in DRD2, BDNF, and Opioid Receptors to Heroin Dependence and Endophenotypes Among the Han Chinese. OMICS 21(7):404–412. doi: 10.1089/omi.2017.0057. [DOI] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D (2009). Mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv 9(4):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE (1997). Patterns and predictors of cocaine and crack use by clients in standard and enhanced methadone maintenance treatment. Am J Drug Alcohol Abuse 23(1):15–42. [DOI] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB et al. (2011). Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron 69(1):120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Kabli N, Fan T, O’Dowd BF, George SR (2014). Mu-delta opioid receptor heteromer-specific signaling in the striatum and hippocampus. Biochemical and Biophysical Research Communications 450(1):906–911. doi: 10.1016/j.bbrc.2014.06.099. [DOI] [PubMed] [Google Scholar]
- Kampangkaew JP, Spellicy CJ, Nielsen EM, Harding MJ, Ye A, Hamon SC et al. (2019). Pharmacogenetic role of dopamine transporter (SLC6A3) variation on response to disulfiram treatment for cocaine addiction. Am J Addict 28(4):311–317. doi: 10.1111/ajad.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM (2007). Pharmacotherapy of dual substance abuse and dependence. CNS Drugs 21(3):213–237. doi: 2133 [pii]. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Ferguson CS, Zhu AZ, Tyndale RF (2010). Pharmacogenetics of drug dependence: role of gene variations in susceptibility and treatment. Annu Rev Pharmacol Toxicol 50:39–61. doi: 10.1146/annurev.pharmtox.010909.105826. [DOI] [PubMed] [Google Scholar]
- King VL, Brooner RK, Peirce J, Kolodner K, Kidorf M (2014). Challenges and outcomes of parallel care for patients with co-occurring psychiatric disorder in methadone maintenance treatment. J Dual Diagn 10(2):60–67. doi: 10.1080/15504263.2014.906132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J et al. (2013). Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry 73(3):219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA (2005). Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev 57(1):1–26. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Christensen CB, Christrup LL (1995). The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci 56(2):PL45–50. [DOI] [PubMed] [Google Scholar]
- Lao O, van Duijn K, Kersbergen P, de Knijff P, Kayser M (2006). Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. American Journal of Human Genetics 78(4):680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Yuferov V, Kreek MJ (2012). The genetics of the opioid system and specific drug addictions. Hum Genet 131(6):823–842. doi: 10.1007/s00439-012-1172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Li X, Qin S, Luo Z, Luo X, Hu P et al. (2017). Impact of SNP-SNP interaction among ABCB1, ARRB2, DRD1 and OPRD1 on methadone dosage requirement in Han Chinese patients. Pharmacogenomics 18(18):1659–1670. doi: 10.2217/pgs-2017-0072. [DOI] [PubMed] [Google Scholar]
- Luo X, Zuo L, Kranzler H, Zhang H, Wang S, Gelernter J (2008). Multiple OPR genes influence personality traits in substance dependent and healthy subjects in two American populations. Am J Med Genet B Neuropsychiatr Genet 147B(7):1028–1039. doi: 10.1002/ajmg.b.30701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G et al. (1992). The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 9(3):199–213. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL et al. (2014). Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict Biol 19(1):111–121. doi: 10.1111/j.1369-1600.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Harding MJ, Hamon SC, Huang W, Kosten TR (2012). Modifying role of serotonergic 5-HTTLPR & TPH2 variants on disulfiram treatment of cocaine addiction: a preliminary study. Genes, Brain and Behavior 11:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N (2016). Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev (5):CD011117. doi: 10.1002/14651858.CD011117.pub2. [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE (2002). Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci 22(24):10906–10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Sordo L, Guh D, Marsh DC, Lock K, Brissette S et al. (2015). Predictors of non-use of illicit heroin in opioid injection maintenance treatment of long-term heroin dependence. Addict Behav 41:81–86. doi: 10.1016/j.addbeh.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Peles E, Kreek MJ, Kellogg S, Adelson M (2006). High methadone dose significantly reduces cocaine use in methadone maintenance treatment (MMT) patients. J Addict Dis 25(1):43–50. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T et al. (2000). Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95(2):219–228. [DOI] [PubMed] [Google Scholar]
- R_Development_Core_Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- SAMHSA-TEDS. Discharges from Substance Use Treatment Services: Tables. Data received through March 16, 2018. 2018. [Google Scholar]
- Schwantes-An TH, Zhang J, Chen LS, Hartz SM, Culverhouse RC, Chen X et al. (2016). Association of the OPRM1 Variant rs1799971 (A118G) with Non-Specific Liability to Substance Dependence in a Collaborative de novo Meta-Analysis of European-Ancestry Cohorts. Behav Genet 46(2):151–169. doi: 10.1007/s10519-015-9737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shorter D, Nielsen DA, Huang W, Harding MJ, Hamon SC, Kosten TR (2013). Pharmacogenetic randomized trial for cocaine abuse: disulfiram and alpha1A-adrenoceptor gene variation. Eur Neuropsychopharmacol 23(11):1401–1407. doi: 10.1016/j.euroneuro.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellicy CJ, Kosten TR, Hamon SC, Harding MJ, Nielsen DA (2012). The MTHFR C677T Variant is Associated with Responsiveness to Disulfiram Treatment for Cocaine Dependency. Front Psychiatry 3:109. doi: 10.3389/fpsyt.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellicy CJ, Kosten TR, Hamon SC, Harding MJ, Nielsen DA (2013). ANKK1 and DRD2 pharmacogenetics of disulfiram treatment for cocaine abuse. Pharmacogenet Genomics 23(7):333–340. doi: 10.1097/FPC.0b013e328361c39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos GK, Rhodes GL, Ledgerwood DM, Greenwald MK (2009). Predicting cocaine group treatment outcome in cocaine-abusing methadone patients. Exp Clin Psychopharmacol 17(5):320–325. doi: 10.1037/a0016835. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Cox BM, Kreek MJ, Cote TE, Izenwasser S (1993). Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse 15(1):33–38. [DOI] [PubMed] [Google Scholar]
- Villano CL, Rosenblum A, Magura S, Fong C (2002). Improving treatment engagement and outcomes for cocaine-using methadone patients. Am J Drug Alcohol Abuse 28(2):213–230. [DOI] [PubMed] [Google Scholar]
- White WL, Campbell MD, Spencer RD, Hoffman HA, Crissman B, DuPont RL (2014). Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. J Psychoactive Drugs 46(2):114–122. doi: 10.1080/02791072.2014.901587. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ (2010). Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci 1187:184–207. doi: NYAS5275 [pii] 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kranzler HR, Yang BZ, Luo X, Gelernter J (2008). The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry 13(5):531–543. doi: 10.1038/sj.mp.4002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chow EP, Zhuang X, Liang Y, Wang Y, Tang C et al. (2013). Methadone maintenance treatment participant retention and behavioural effectiveness in China: a systematic review and meta-analysis. PLoS One 8(7):e68906. doi: 10.1371/journal.pone.0068906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP et al. (1999). Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 24(1):243–252. [DOI] [PubMed] [Google Scholar]