Abstract
Cancer is one of the leading causes of mortality and morbidity worldwide. Recent improved therapies have resulted in more cancer survivors and living longer. Despite these advances, the majority of patients will develop adverse events from anticancer therapies. Foot alterations including nail toxicities, hand foot syndrome, edema, xerosis, hyperkeratosis, and neuropathy are frequent among cancer patients. These untoward conditions may negatively impact quality of life, and in some cases may result in the interruption or discontinuation of cancer treatments. Appropriate prevention, diagnosis and management of podiatric adverse events are essential in order to maintain foot function and health-related quality of life, both of which are critical for the care of cancer patients and survivors. This article shows results related to complaint and impact on quality of life of the Oncology Foot Care program and reviews publications specific to podiatric adverse events related to cancer treatments.
Keywords: Hand-foot syndrome, chemotherapy, targeted therapy, podiatry, paronychia, hyperkeratosis, neuropathy, hand-foot-skin reaction
INTRODUCTION
Currently, cancer is one of the leading causes of morbidity and death in many high-income countries. Worldwide, an estimated 14.1 million people were diagnosed with cancer in 2012, many of whom died from this disease.1 Despite these daunting statistics, improvements in cancer therapies have resulted in a greater number of people surviving cancer, with an estimated 32.5 million of cancer survivors in 2012. Anticancer therapies may result in adverse events (AE) which may negatively impact the normal functioning of the feet and lower extremities. Symptomatic podiatric adverse events (pAE) are common in patients treated for cancer and have considerable negative impact on their well-being and quality of life (QoL). Several questionnaires are available to assess objectively this impact, such as the HFS-14.2 These events may have a negative effect on the patient’s ability to use footwear, bear weight, ambulate, or perform instrumental or self-care activities of daily life and also may cause treatment discontinuation or modification.3–6 Because of the potential serious impact and rapid development of complications, podiatric and other medical staff must learn about the prevention and management of the multiple pAE frequently observed in oncology patients. This article shows the impact of pAE on QoL in the Oncology Foot Care program,7 a program developed to raise awareness and training to podiatrist in the Netherlands. Furthermore, this article reviews publications specific to pAE related to cancer treatments and include: peripheral neuropathy, hand-foot syndrome, hand-foot-skin reaction, nail toxicity, xerosis, edema and lymphoedema.
Awareness and Education
To date, no podiatric screening and treatment strategies have been developed in order to prevent or mitigate pAE in cancer patients. Additionally, podiatrists and podologists may not be aware of complications that their treatments can cause during anticancer therapies. The understanding of (p)AE related to anticancer therapies by the patient, podiatrist and oncology team is essential to optimally communicate, manage and treat these patients.
In 2012, the Netherlands Medical Foot Academy developed an educational program for podiatrists and podologists (“The Oncology Foot Care”).7 The main goal of the program is to encourage awareness and screening of potential complications due to anticancer therapy, to keep the feet of cancer patients in optimal condition during and after therapy, and to encourage communication with the oncology team. A special information booklet is given to cancer patients to make them aware and seek professional help if any pAE arises. Podiatrists could provide valuable information about the feet condition to cancer patients, which ensure consistent anticancer therapy for a better QoL.
The Oncology Foot Care program7 includes psychological topics such as QoL (i.e. psychological, social and emotional characteristics) and risk factors of distress in cancer patients (i.e. age, life events). Furthermore, patients report the impact of pAE on QoL (i.e. general activity, mood, walking ability and life enjoyment) using an 11-point Numeric Rating Scale before and after treatment, where 0 equal “no complaint, positive outcome” and 10 equal “worst imaginable, negative outcome”.
From April to July 2016, the Oncology Foot Care program7 included a total of 291 patients. The mean age was 65.3 ± 11.7 years and the majority of patients (66.3%) were female. The oncology team referred 10.7% of patients, and 18.9% were referred by others health care professionals. The most common cancer was breast cancer (40.2%), followed by colon cancer (10.3%). Before the cancer diagnosis, 61.5% did not report foot problems. In 67.7% of patients, pAE were attributed to cancer therapies. The mean number of visits was 5.9 ± 3.9. Table 1 shows the impact on complaint and QoL of the received foot therapy. A total of 106 patients scored their complaint before foot therapy ≥8, and after podiatric intervention improved to 4.8 ± 3.0 (P < 0.001). Similar results were scored on the QoL.
Table 1.
Numeric Rating Scale results before and after foot therapy regarding complaint and impact in QoL.
| Before treatment | After treatment | |
|---|---|---|
| Complaint | 5.9 | 3.2* |
| General activity | 5.0 | 3.0* |
| Mood | 4.5 | 2.8* |
| Walking ability | 5.1 | 3.0* |
| Normal work | 4.9 | 3.1* |
| Enjoyment in life | 4.4 | 2.8* |
P value <0.001
Most common podiatric adverse events and foot care in cancer patients and survivors Peripheral Neuropathy
Peripheral Neuropathy (PN) is among the most frequent AE associated with cancer therapies. Usually begins after several months of cancer treatment, and could be a persistent complication. The clinical findings include numbness, diminished or absent temperature sensitivity, and alteration of two-point discrimination, touch, vibration, proprioception and muscle strength, which can lead to imbalance.8 Chemotherapy induced peripheral neuropathy (CIPN) is predominantly a sensory neuropathy, known to be a primary dose-limiting toxicity, with a “stocking-glove” distribution, which could extend up to the knees depending on the amount, frequency and length of chemotherapy cycles.9 CIPN develops particularly with the use of platinum, taxanes, alkaloids, thalidomide, lenalidomide, and bortezomib therapies, used to treat solid tumors and hematologic malignancies.10 Sensory nerves are mainly affected compared to motor neurons, probably due to the lack of protection by the blood-brain barrier and less myelination.8
Patients with CIPN may also experience walking difficulties, foot discomfort, and increased propensity to falls.11 One factors is the acute onset of CIPN without adaptation time. Severe neuropathy is also associated with depression,12 and often a reason for patients’ discontinuation of anticancer therapy, with negative impact on QoL.13
The risk of developing neuropathy may be higher in patients who have other risk factors, such as diabetes mellitus and obesity.9
There is no consensus to assess CIPN and pain, and it is frequently misdiagnosed and undertreated.14 However, there is a unified terminology criteria for grading any AE of anticancer therapy, published by the US Department of Health and Human Services on June 14, 2010 (Table 2). Additionally, multiple specific validated scales, such as EORTC QLQ-CIPN20,15 FACT/GOG-Ntx16 and Neurotoxicity Questionnaire,8 could be used to measure the impact and severity of neurotoxicity in patients with CIPN. Unfortunately, most of these assessments combine negative and positive symptoms in one question. Since neuropathic pain medication only affects the positive symptoms, the pain reducing effect cannot be assessed adequately, therefore we recommend using the Neuropathic Pain Symptom Inventory.17
Table 2.
General grading of Common Terminology Criteria for Adverse Events (CTCAE) v4.03.
| Grade | General Characteristics |
|---|---|
| 1-Mild | Minor; no specific medical intervention indicated; asymptomatic laboratory finding only; radiographic finding only; marginal clinical relevance; mild symptoms and intervention not indicated; non-prescription intervention indicated |
| 2-Moderate | Intervention indicated; minimal, local, noninvasive intervention indicated; limiting instrumental Activities of Daily Living (ADL). Though many of the skin manifestations have little clinical significance, they cause significant psychosocial impact to the patients. AEs that could cause significant psychosocial impact to the patient are graded as 2. |
| 3-Severe | Medically significant but not life-threatening; impatient or prolongation of hospitalization indicated; important medical events that do not result in hospitalization but may jeopardize the participant or may require intervention either to prevent hospitalization or to prevent the event from becoming life-threatening or potentially resulting in death; limiting basic self care ADL; disabling resulting in persistent or significant disability or incapacity.* |
| 4-Life Threatening | Life-threatening consequences; urgent intervention indicated; urgent operative intervention indicated; participant is at risk of death at time of event if immediate intervention is not undertaken. |
| 5 | Death |
Source: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed on 12.30.16
Foot care and preventive approach for PN is showed in Figure 1. In a previous randomized clinical trial, duloxetine18 and venlafaxine19 showed superiority over placebo to control pain associated with CIPN. Another relatively-positive result was seen in a randomized clinical trial with combination of ketamine 1.5%, amitriptyline 3% and baclofen 0.75% gel.9,20
Figure 1.

Prevention and management strategies for podiatric adverse events.
CIPN affects negatively proprioceptive feedback, thus disrupting normal locomotion and increased variability in one’s gait.21 One study showed a significantly better balance after 36 weeks of exercise, improved time to regain balance, and QoL compared to the control group.22 Other poorly studied interventions such as sensorimotor training, whole-body vibration or Tai Chi may also improve balance.23 No literature was found regarding the effect of shoes on balance in CIPN. Loss of sensitivity is one of the aspects that leads to inadequate footwear (too big or too small) in the majority of elderly, contributing to an increased foot injury due to shearing forces and skin irritation.24 Patients with peripheral neuropathy preferred light weighted shoes, such as sandals, with a molded foot bed and tight fitting.25
Hand-foot syndrome
Hand-foot syndrome (HFS) is a common complication of cytotoxic agents (Table 3). Extravasation with accumulation of the drug in the stratum corneum has been hypothesized as a potential mechanism of toxicity.26 The clinical findings include as first symptoms swelling, numbness, a feeling of tightness/stiffness or pain in the palms and soles. This is followed 2–4 days later by bright erythema and edema, which is symmetrical and well-defined.
Table 3.
Anticancer therapies and associated podiatric adverse events.
| Cytotoxic chemotherapy | Targeted therapy | Radiation therapy | Surgical procedures | Stem cell transplants | |
|---|---|---|---|---|---|
| Paronychia | ± | + | − | − | ± |
| Neuropathy | + | ± | ± | − | − |
| Hand foot syndrome | + | − | − | − | − |
| Hand foot skin reaction | − | + | − | − | − |
| Xerosis/fissures | ± | + | − | − | ± |
| Edema/lymphedema | + | + | ± | ± | − |
| Skin and soft tissue infections | ± | ± | + | + | ± |
Onycholysis could be associated with severe reactions. Without appropriate interventions the lesions can blister, desquamate, form crusts, ulcerate, or even progress to epidermal necrosis (Figure 2). Healing occurs without scarring unless there has been skin ulceration or necrosis.26 With each subsequent cycle of chemotherapy, the reaction will appear more quickly, be more severe and will take longer to heal. Common culprits include 5-flourouracil, its prodrug capecitabine, doxorubicin, liposomal doxorubicin, docetaxel, and cytarabine. The incidence of HFS varies depending on the offending agent, ranging from 15–45%.27
Figure 2.

Hand foot syndrome from cytotoxic chemotherapy (i.e. doxorubicin, fluorouracil, capecitabine)
Hand-foot-skin reaction
The multikinase inhibitors (MKIs), such as sorafenib, sunitinib, axitinib, pazopanib, regorafenib, may cause hand-foot-skin reaction (HFSR) in 20%–60% of the treated patients.6,28–34 It will occur in areas of friction or pressure in the palms and soles, within the first few weeks, as painful blisters, followed by hyperkeratotic areas, similar to calluses (Figure 3). This leads to increased pressure points and painful areas on the feet that may limit mobility and weight bearing. Hyperkeratotic-HFSR is a painful complication most frequently seen over sites of pressure or friction during the early weeks of MKIs-therapy.5 Higher risk is found in Asians, women and highly active people.
Figure 3.
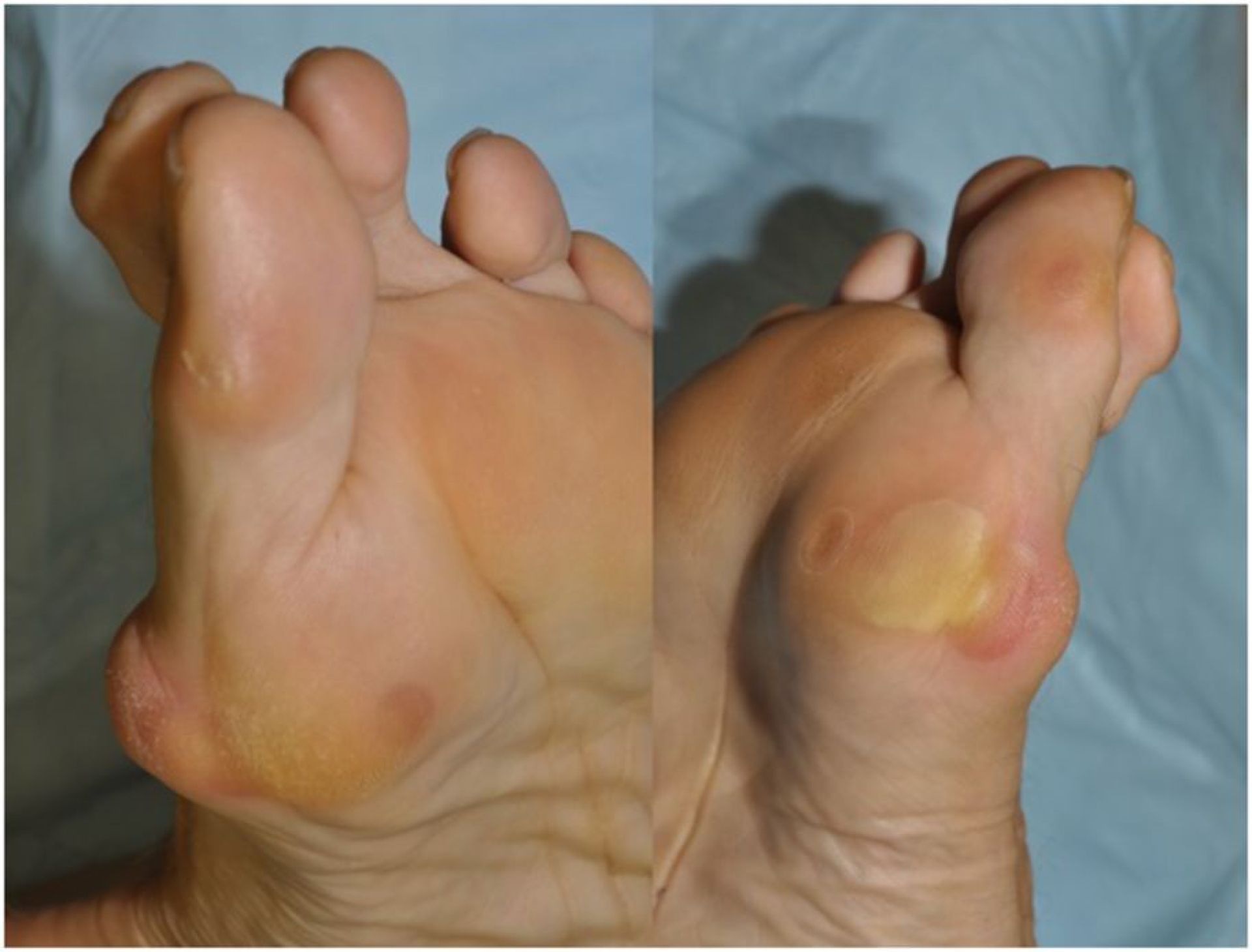
Hand foot skin reaction from targeted therapy (i.e. multikinase inhibitors such as sorafenib, regorafenib, sunitinib).
Podiatric management of HFS and HFSR
HFS and HFSR could be a therapeutic challenge, especially when patients are active and when a long standing is needed for the patient’s daily life. There are few reports in podiatric literature that discuss preventive therapies for these conditions. Routine podiatric care is highly recommended. Many of the symptoms experienced in the early and late stages of HFS and HFSR are commonly managed by podiatrists, such as dystrophic toenails/onycholysis, corns and calluses, skin fissures, blisters, neuropathy, and ulcerations. All of the above can be severely painful and debilitating, however, can be treated conservatively with minimal discomfort.
Patient education regarding this condition before starting chemotherapy is important. A preventive approach is based on the use of moisturizer before starting chemotherapy. Avoiding mechanical trauma (e.g. in footwear) such as friction, heat, pressure, irritants and adhesives may help to limit the reaction.35 Additionally, hands and feet cooling during chemotherapy administration have had variable success in preventing the reaction in patients receiving chemotherapies that may cause HFS, such as paclitaxel, docetaxel, or doxorubicin.26 An early podiatric evaluation could prevent the progression of HFS and HFSR. Maintenance good footwear and hygiene play an important role in reducing or minimizing infections that may impact QoL and may limit the use of anticancer therapy.29 A prophylaxis with urea 10% cream in association with supportive care in patients treated with sorafenib reduced HFSR rates, extended the time to first occurrence of HFSR, and improved patient QoL compared with best supportive care.36 Application of topical non occlusive polymers in patients with HFSR decreased the SRRC score (scaling, roughness, redness, cracks) and improved QoL compared to baseline.37
Nail toxicity and infections
Around 35% of patients undergoing anticancer therapies may suffer any nail AE.38,39 Patients receiving epidermal growth factors receptors inhibitors and taxanes are at high risk for developing nail changes, which typically appear after 2 months of treatment.40 Paronychia, onycholysis, granulation tissue formation and subungual abscesses with potential secondary infection are often painful and may impact QoL.39
Cosmetic nail changes are usually asymptomatic and do not require medical intervention. These nail changes are noted when anticancer drugs affect the nail matrix, but usually are reversible after discontinuation of the anticancer treatment.41 However, nail toxicity that affects the nail fold and nail bed may become symptomatic (Figure 4). If untreated, it will progress to paronychia or nail detachment and possible secondary infection, requiring invasive intervention, even dose modification.38,39 Prophylactic actions include modifications in shoe gear (proper supportive and contoured shoe). Additionally, cold therapy using frozen socks significantly reduced the incidence of docetaxel-induced foot nail toxicity.42
Figure 4.

Onycholysis, brittle nails, and paronychia from taxane chemotherapy (i.e. paclitaxel, docetaxel)
The most common causative infective organism of acute paronychia is Staphylococcus aureus. The combination of amoxicillin with clavulanic acid is suggested as first-line treatment for acute bacterial paronychia, together with appropriate surgical drainage if severe.43 In cases of chronic paronychia, the most common organism seen is Candida albicans.44 Topical antifungals such as ciclopirox 8%, amorolfine, efinaconazol may be used. A case study showed that topical 1% povidone-iodine/ dimethylsulfoxide has been very effective in alleviating the signs and symptoms of severe paronychia associated with chemotherapy.45
A study of 127 patients with paronychia, showed that corticosteroid ointment and phenol chemical matricectomy significantly improved paronychia severity.46 For recalcitrant cases of chronic paronychia, an alternative therapy with intralesional triamcinolone can be used in the affected nailfold with positive results.47 If conservative therapy fails, a surgical approach may be necessary. One of the most widely used is a wedge resection of the affected nail fold. Regarding permanent nail procedures, it is important to keep in mind that most of the nail changes in cancer patient will usually resolve after the end of their treatment.
Xerosis
Since anticancer agents affect all rapidly proliferating cells, epidermal keratinocytes will have a lower rate of turnover, resulting in decreased proliferation and altered differentiation, which results in increased transepidermal water loss and dryness.39 It could present as painful fissures in the lateral aspects of the soles and heels (Figure 5).48,49 Treatment includes frequent hydration with ointments or creams containing keratolytics (salicylic acid 6%, urea 10–40%), if possible under occlusion (socks or plastic dressings).48 For faster healing, liquid bandages (5-hydroqxiquinoline) could be use on fissures.
Figure 5.

Paronychia and heel fissures with targeted therapies (i.e. erlotinib, cetuximab, panitumumab, afatinib).
Edema and Lymphoedema
Increased extracellular fluid accumulation in the lower extremities and feet can result as a consequence of radiation or surgical interventions in the pelvis, groin, or leg, in addition to chemotherapeutic agents.50 The end result appears to be the same, namely increased diameter of the leg and foot, with concomitant increased weight, skin changes, infections, and possible ulcerations (Figure 6 and 7). Treatment and prevention of leg and foot edema is critical in order to prevent infections and loss of mobility. This is usually achieved through compression garments or manual lymphatic drainage performed by a lymphoedema therapist.50
Figure 6.

Foot edema from systemic therapies and skin and soft tissue infection on dorsal foot (i.e. chemotherapy, targeted therapy).
Figure 7.

Leg edema due to chemotherapy.
Conclusions
Despite the fact that most of the pAEs observed in patients receiving anticancer therapies are not life-threatening, their manifestation may result in dose interruptions or discontinuation, and decreased QoL. To date, there is no podiatric focus on cancer patients and survivors. Moreover, there is a need of clinical trials to develop guidelines for foot care and screening to prevent or reduce complications due to cancer treatments, similar as the current standardized screening guidelines for patients with conditions such as diabetes and arthritis.
A proper education is imperative to provide cancer patients and survivors with the most effective actions against pAE and to positively impact on their QoL. In addition, an accurate communication with the oncology team is needed to properly adjust the cancer treatment, and to give an optimal foot treatment at the right moment. The ultimate goal of managing these patients is avoiding treatment modifications or interruptions to attain a maximum benefit from the anticancer agents and the most optimal QoL.
Funding sources:
MEL was supported in part by the NIH/NCI Cancer Center Support Grant P20 CA008748 and the RJR Oncodermatology Fund at Memorial Sloan Kettering Cancer Center. Funding/Sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication
Footnotes
Conflict of interest statement:
MEL has a consultant advisory role with Amgen, AstraZeneca, Boehringer Ingelheim, EMD Serono, Foamix, Galderma, Genentech, Helsinn, Merck, Novartis, Novocure.
Mischa Nagel is CEO of Supplement and De Medische Voet in Amsterdam, The Netherlands
References
- 1.de Souza JA, Hunt B, Asirwa FC, et al. : Global Health Equity: Cancer Care Outcome Disparities in High-, Middle-, and Low-Income Countries. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34: 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V, Dalenc F, Chevreau C, et al. : HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. The oncologist 16: 1469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KK, Gorcey L, McLellan BN: Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 71: 787, 2014. [DOI] [PubMed] [Google Scholar]
- 4.McLellan B, Ciardiello F, Lacouture ME, et al. : Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald JB, Macdonald B, Golitz LE, et al. : Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol 72: 203, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Gomez P, Lacouture ME: Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. The oncologist 16: 1508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel MPM, Kopsky DJ, Lacouture ME, et al. : Foot care in Oncology: the cancer patient From Ankle to Toe. The Asco Post 22: 2015. [Google Scholar]
- 8.Hausheer FH, Schilsky RL, Bain S, et al. : Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Seminars in oncology 33: 15, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Schneider BP, Hershman DL, Loprinzi C: Symptoms: Chemotherapy-Induced Peripheral Neuropathy. Advances in experimental medicine and biology 862: 77, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Smith EM, Bridges CM, Kanzawa G, et al. : Cancer treatment-related neuropathic pain syndromes--epidemiology and treatment: an update. Current pain and headache reports 18: 459, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Cavaletti G, Alberti P, Marmiroli P: Chemotherapy-induced peripheral neurotoxicity in cancer survivors: an underdiagnosed clinical entity? American Society of Clinical Oncology educational book / ASCO. American Society of Clinical Oncology. Meeting: e553, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Tofthagen C, Donovan KA, Morgan MA, et al. : Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 21: 3307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ: Chemotherapy induced peripheral neuropathic pain. Korean J Anesthesiol 67: 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zedan AH, Vilholm OJ: Chemotherapy-induced polyneuropathy: major agents and assessment by questionnaires. Basic & clinical pharmacology & toxicology 115: 193, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie Smith EM, Barton DL, Qin R, et al. : Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 22: 2787, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun EA, Welshman EE, Chang CH, et al. : Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 13: 741, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Bouhassira D, Attal N, Fermanian J, et al. : Development and validation of the Neuropathic Pain Symptom Inventory. Pain 108: 248, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Smith EM, Pang H, Cirrincione C, et al. : Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama 309: 1359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand JP, Deplanque G, Montheil V, et al. : Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 23: 200, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Barton DL, Wos EJ, Qin R, et al. : A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19: 833, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall TF. Effects of chemotherapy-induced-peripheral-neuropathy on spatiotemporal gait parameters and fall risk in cancer patients after the completion of chemotherapy drug treatment: Seton Hall University Dissertations and Theses 2016.
- 22.Streckmann F, Kneis S, Leifert JA, et al. : Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 25: 493, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Streckmann F, Zopf EM, Lehmann HC, et al. : Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports medicine (Auckland, N.Z.) 44: 1289, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Lopez Lopez D, Losa Iglesias ME, Becerro de Bengoa Vallejo R, et al. : Optimal choice of footwear in the elderly population. Geriatric nursing (New York, N.Y.) 36: 458, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Paton JS, Roberts A, Bruce GK, et al. : Does footwear affect balance?: the views and experiences of people with diabetes and neuropathy who have fallen. Journal of the American Podiatric Medical Association 103: 508, 2013. [DOI] [PubMed] [Google Scholar]
- 26.von Moos R, Thuerlimann BJ, Aapro M, et al. : Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. European journal of cancer (Oxford, England : 1990) 44: 781, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Heidary N, Naik H, Burgin S: Chemotherapeutic agents and the skin: An update. Journal of the American Academy of Dermatology 58: 545, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Anderson R, Jatoi A, Robert C, et al. : Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). The oncologist 14: 291, 2009. [DOI] [PubMed] [Google Scholar]
- 29.McLellan B, Ciardiello F, Lacouture ME, et al. : Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 26: 2017, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer A, Wu S, Ho AL, et al. : The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs 31: 787, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Dranitsaris G, Vincent MD, Yu J, et al. : Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 23: 2103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balagula Y, Wu S, Su X, et al. : The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs 30: 1773, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Lacouture ME, Wu S, Robert C, et al. : Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. The oncologist 13: 1001, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Lacouture ME, Reilly LM, Gerami P, et al. : Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 19: 1955, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Lorusso D, Di Stefano A, Carone V, et al. : Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 18: 1159, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Ren Z, Zhu K, Kang H, et al. : Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol 33: 894, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Ionescu MA, Fabbrocini G, Cristaudo A, et al. : Topical nonocclusive polymers in hand-foot skin reaction. Journal of the American Academy of Dermatology 72: AB65, 2013. [Google Scholar]
- 38.Capriotti K, Capriotti JA, Lessin S, et al. : The risk of nail changes with taxane chemotherapy: a systematic review of the literature and meta-analysis. Br J Dermatol: 2015. [DOI] [PubMed] [Google Scholar]
- 39.Robert C, Sibaud V, Mateus C, et al. : Nail toxicities induced by systemic anticancer treatments. Lancet Oncol 16: 71133, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Garden BC, Wu S, Lacouture ME: The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol 67: 400, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Capriotti K, Capriotti JA, Lessin S, et al. The risk of nail changes with taxane chemotherapy: a systematic review of the literature and meta-analysis: Br J Dermatol. 2015. September;173(3):842–5. doi: 10.1111/bjd.13743. Epub 2015 Jul 29. [DOI] [PubMed] [Google Scholar]
- 42.Scotte F, Banu E, Medioni J, et al. : Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer 112: 1625, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Lomax A, Thornton J, Singh D: Toenail paronychia. Foot Ankle Surg 22: 219, 2016. [DOI] [PubMed] [Google Scholar]
- 44.Frain-Bell W: Chronic paronychia. Short review of 590 cases. Trans St John’s Hosp Dermatol Soc 38: 29, 1957. [Google Scholar]
- 45.Capriotti K, Capriotti JA: Chemotherapy-associated paronychia treated with a dilute povidone-iodine/dimethylsulfoxide preparation. Clin Cosmet Investig Dermatol 8: 489, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goto H, Yoshikawa S, Mori K, et al. : Effective treatments for paronychia caused by oncology pharmacotherapy. J Dermatol 43: 670, 2016. [DOI] [PubMed] [Google Scholar]
- 47.Fliegelman MT, Lafayette GO: How we treat paronychia. Postgrad Med 48: 267, 1970. [DOI] [PubMed] [Google Scholar]
- 48.Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. : Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19: 1079, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacouture ME, Maitland ML, Segaert S, et al. : A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 18: 509, 2010. [DOI] [PubMed] [Google Scholar]
- 50.D’Angelo SP, Kris MG, Pietanza MC, et al. : A case series of dose-limiting peripheral edema observed in patients treated with pemetrexed. J Thorac Oncol 6: 624, 2011. [DOI] [PubMed] [Google Scholar]


