Abstract
Acid mine drainage-affected environments are interesting microbial niches for the isolation of metal-resistant microorganisms. In this sense, the aim of the present work is to isolate and characterize metal-resistant microorganisms from sediments of an abandoned gold mine located in San Luis (Argentina). For these purposes, the metal removal capacity and the microelemental composition of the biomass exposed to metals were evaluated. Likewise, proteomic techniques were applied to understand the removal and resistance mechanisms. Fusarium tricinctum M6 was isolated and identified as tolerant to Cu(II), Fe(II) and Cr(VI). When faced with 40 μg mL−1 Cu (II), the growth was affected by 60% and the removal capacity was 30–35%. Copper was found uniformly distributed in the biomass (5.23% w/w) and variations in the proportion of other biomass constituent elements were detected. When exposed to Cu(II), F. tricinctum M6 showed differential expression of intra and extracellular proteins involved in different metabolic processes. A large number of proteins with metal ion binding sites were detected both at intra and extracellular levels. The results obtained in the present work indicated bioadsorption of the metal on the cell surface and an important readjustment of the protein expression to counteract the stress produced by Cu(II).
Keywords: Acid mine drainage, Metal-resistance, Fusarium, Intracellular proteomics, Extracellular proteomics
1. Introduction
Despite the benefits that mining activities have for modern human life, throughout history mining has caused significant pollution problems in the sites where operations have been developed. The term Mining Environmental Liabilities (MEL) arose in Latin America and refers to the environmental impacts generated by abandoned mining operations where a regulated and certified mine closure was not carried out (Oblasser and Chaparro Ávila, 2008). One of the most important and documented environmental problems associated with mining activities is the production of Acid Mine Drainage (AMD) (Méndez-García et al., 2015). Acidic water in these environments is produced from oxidative dissolution of sulfide ores that face with oxygen, water, and endogenous microorganisms (Johnson and Hallberg, 2003; Baker and Banfield, 2003). AMD-affected environments present high metal concentrations when compared to other unaffected environments (Fadiran et al., 2014; Bonilla et al., 2018).
Soils and sediments are known as a sink for metals released into the environment from natural or anthropogenic sources (Azarbad et al., 2016). Throughout history, the inherent buffering capacity of soils and sediments when receiving these toxic contaminants has been demonstrated. In these matrices, microorganisms are key biological components for this capacity. Microbial populations of soils and sediments can respond to natural environment changes and cope with the presence of toxic substances, mainly through their genetic malleability (Puglisi et al., 2012).
Long-time exposure to metal contamination leads to the adaptation of microbial communities to survive and persist in contaminated environments. In this sense, microorganisms have developed sophisticated and specific cellular machineries consisting of an extensive network of specialized proteins, transporters and/or involved in gene expression regulation, which respond to both the deficiency and the excess of metals (Ma et al., 2009; Solioz et al., 2010). These cellular mechanisms for maintaining the optimal concentration of metals are known as homeostasis. Microorganisms play an important role in the uptake of metals from the environment by using a multiplicity of homeostatic mechanisms. These mechanisms differ among the different genera and/or microbial species and relatively little is known about them at the molecular level.
The knowledge of the adaptation of different organisms to stress situations, such as the presence of toxic concentrations of metals, is essential to develop strategies for environmental sanitation and treatment of contaminated sites. A very interesting way to study the relation between metals and resistant microorganisms can be Proteomics. Proteome is defined as the total set of proteins expressed by a genome in a cell, tissue or organism at a certain time and condition (Blackstock and Weir, 1999). The proteome is important not only for analyzing the cell behavior, but also for closing the gap between the organisms’ genome and metabolites with a holistic perspective.
Comparison of protein expression profiles obtained under stress conditions caused by the presence of metals, compared to controls without toxic compounds, is useful for the detection of specific changes in the proteome against these conditions (Bonilla et al., 2016). The identification of proteins induced or repressed by the presence of toxic concentrations of a contaminant guide conclusion on the molecular mechanisms involved in the response and removal of metals by resistant microorganisms.
In San Luis (Argentina), there is an abandoned gold mine called “La Esperanza”. After its exploitation, the galleries and facilities were abandoned without an adequate closure process, as established by the current legislation, constituting MEL. According to our previous results, the drainage released from this mine possesses AMD characteristics, showing extreme pH values (2.94 ± 0.03) (Bonilla et al., 2018). These results confirm the persistence of the acidic characteristics of the drainage previously reported by Tripole and Corigliano (2005) and Tripole et al. (2006). The water flows into the La Carolina stream, which is used for recreational and tourist activities. The drainage acidifies the stream and increases its sulfate, Zn, Cd and Te concentrations. In the sediments inside the mine, high concentrations of dangerous metals such as Cr, Fe and Cu are detected, some of the most common metals found in environments affected by mining activities, which show biological toxicity when present in high concentrations. Likewise, both the prokaryotic and the eukaryotic community structures in the area are significantly affected. (Bonilla et al., 2018). For these reasons, this work proposes the AMD-affected environment located in San Luis (Argentina) as an interesting microbial niche for the exploration of metal-resistant microorganisms, capable to grow under simple laboratory conditions and with potential application in bioremediation processes, to study their resistance mechanisms at the molecular level.
2. Experimental
2.1. Isolation and selection of metal-resistant microorganisms
For the isolation of metal-resistant microorganisms, a total of 28 sediment samples were collected in sterile flasks from the La Carolina stream bed and from inside the abandoned gold mine, following the scheme specified in our previous studies (Bonilla et al., 2018). Samples were kept at 4 °C before processing. The isolation and initial maintenance of the microorganisms were carried out on the modified Yeast Extract-Glucose Medium (EG*) (g L−1: Glucose 10.0, Yeast Extract 1.0, K2HPO4 0.5, KH2PO4 0.5, Agar 15.0).
Isolation was carried out through a sequential enrichment method (Perez Silva et al., 2008), using 1% glucose as the unique carbon source. As selection pressure, Fe(II), as FeSO4.7H2O; Cu(II), as CuSO4.5H2O; and Cr(VI), as K2Cr2O7, were added in increasing concentrations from 1 to 5 μg mL−1. The isolates were kept at 4 °C and were lyophilized for their conservation.
The agar diffusion technique was used to pre-select the microorganisms capable to grow in the presence of Fe(II), Cu(II) and Cr(VI) at different concentrations: 100, 250, 500, 750 and 1,000 μg mL−1 (Villegas et al., 2004). The plates were incubated at 30 °C for 72 h. Tolerance to the metals was determined by measuring the diameter of the growth inhibition halos around the wells. As control, 50 μL of sterile bidistilled water was used. The assay was carried out in triplicate.
2.2. Minimum inhibitory concentration (MIC)
The Minimum Inhibitory Concentration (MIC) of the metals in liquid medium was performed through a cell viability test modified in our laboratory, using Resazurin dye as indicator of cell viability (Borra et al., 2009). In this work, MIC was defined as the metal concentration interval comprised between the maximum concentration in which cell viability was observed and the minimum concentration in which viability was not detected. For the analysis, concentrations of Fe(II), Cu(II) and Cr(VI) from 15 to 500 μg mL−1 were added to 10 mL of culture, containing an initial concentration of 1 × 104 cells mL−1 (Rex, 2008). Cultures were incubated during 120 h at 30 °C and 200 rpm.
At the end of the culture time, the cell pellets were washed twice with sterile bidistilled water, resuspended in 1 mL EG* medium and 5 μL of a Resazurin aqueous solution (1 g L−1) sterilized by filtration were added. Finally, pellets were incubated at 30 °C and 200 rpm for 2 h to observe the color variation results. Each metal concentration was evaluated in triplicate.
2.3. Microbial molecular identification
Fusarium tricinctum M6 was identified by PCR amplification and sequencing of different genomic regions. Two pairs of specific eukaryotic primers were used to carry out the amplification: ITS1 – 5.8 S rDNA complete sequence - ITS2 – 28 S rDNA partial sequence (ITS1 F: 5 ′-TCCGTAGGTGAACCTGCGG-3 ′; ITS4 R: 5 ′ TCCTCCGCTTATTGATATGC-3 ′) and Translation Elongation Factor 1-alpha (EF1 F: 5′-ATGGGTAAGGARGACAAGAC-3 ′; EF2 R: 5′-GGARGTACCAGTSAT-CATGTT-3 ′).
The PCR protocol using primers ITS1/ITS4 consisted of a first denaturation cycle at 95 °C for 5 min, followed by 30 cycles of 95 °C for 60 s, 55 °C for 60 s and 72 °C for 90 s. Finally, an elongation cycle was carried out at 72 °C for 7 min. Furthermore, the protocol for amplification using primers EF1/EF2 consisted of one denaturation cycle at 95 °C for 5 min, followed by 35 cycles of 95 °C for 35 s, 54 °C for 30 s, and 72 °C for 90 s. Finally, an elongation cycle was carried out at 72 °C for 7 min
The DNA concentration in the PCR products was determined using Epoch (Biotek) and the integrity of the samples was evaluated through 1% Agarose gel electrophoresis. The PCR products were sent to Macrogen (www.macrogen.com; South Korea) for their purification and sequencing. The sequences were edited with Molecular Evolutionary Genetics Analysis (MEGA v6.0) and were analyzed with BLASTn using NCBI databases (www.ncbi.nlm.nih.gov). Additionally, the Barcode Of Life Data System (Bold Systems v4; www.boldsystems.org) was used for the identification from the ITS sequences.
2.4. Determination of cell growth and removal capability
One hundred mL of liquid EG* medium, supplemented or not with 40 μg mL−1 Cu(II), were inoculated with an initial concentration of 1 × 106 cells mL−1. Cultures were incubated at 30 °C and 200 rpm during 120 h. Every 24 h, 3 mL of sample was taken periodically. The samples were centrifuged at 11000 xg during 10 min to separate the supernatants from the cell biomass.
The supernatants were used to determine the residual copper concentration through inductively coupled plasma mass spectrometry (ICP-Mass ELAN DRC-e). On the other hand, biomass was used for growth kinetics studies by dry weight techniques. The results were expressed in g L−1. All the assays were carried out in triplicate.
Cultures with Cu(II) added but without the inoculums were included as abiotic controls to verify that the components of the culture medium did not participate in the Cu(II) precipitation.
2.5. Microelemental analysis of microbial biomass
Microelemental analysis and surface mapping of the microbial biomass, in the presence and absence of Cu(II), was carried out by Scanning Electron Microscopy coupled to X-Ray Dispersive Energy Spectrometry (SEM-EDS). The analysis was performed at the UNSL Laboratory for Electron Microscopy and Microanalysis (labmem-UNSL, http://labmem.unsl.edu.ar/).
Cultures obtained in the presence and absence of Cu(II) in the culture medium were centrifuged at 4000 xg during 20 min at 4 °C (Centrifuge U-320R) to separate the cell pellet from the supernatant. The biomass was washed three times with sterile bidistilled water and the pellets were processed immediately, without prior preservation.
Samples were mounted on aluminum stubs and dried at room temperature for 7 days. The samples were sputter-coated with carbon (SPI metallizer), and were observed and analyzed under a Zeiss LEO 1450 VP SEM Scanning Electron Microscope, operated at 20 kV, coupled to a Genesis 2000 energy dispersive spectrometer (EDS).
2.6. Collection of intracellular proteins
Cells obtained from culture media in the presence and absence of Cu (II) after 36 h of incubation were harvested by centrifugation at 4000 xg during 20 min at 4 °C (Centrifuge U-320R). Pellets were washed twice with phosphate buffered saline (mM: NaCl 124; NaH2PO4 10; KH2PO4 3) and they were kept at −20 °C. Then, cells were frozen using liquid nitrogen and physically broken using a mortar and pestle. The powder was recovered with Tris-Sucrose buffer (Sucrose 11.29 g dL−1; Tris-HCl 1.5 M pH 8.8 3.33 mL dL−1) and centrifuged at 8500 xg during 20 min at 4 °C. Supernatants were used as samples of intracellular proteins and were kept at −80 °C. The assays were performed in biological triplicate.
2.7. Collection of extracellular proteins from cell-free supernatants
For collection of extracellular proteins, the supernatants obtained at 36 h in the presence and absence of Cu(II) were filtered with nitrocellulose membranes (Microclar, 0.2 μm). Then, the supernatants were concentrated 20X using Vivaspin Turbo 15 3000 MWCO ultrafiltration devices with polyethersulfone membrane (PES) (Sartorius). Finally, they were stored at −80 °C. The same procedure was carried out with uninoculated EG* liquid medium, to be used as control in the extracellular proteomic analyses.
The total protein content in both intracellular and extracellular samples was determined by Bradford (1976). Finally, the samples were aliquoted by calculating the necessary volume to obtain 300 μg of intracellular proteins and 25 μg of extracellular proteins. The calculated volumes were lyophilized. The collection of the extracellular proteins was performed in triplicate.
2.8. Shotgun proteomic analysis (nanouhplc-esi-ms/ms)
Lyophilized samples containing intra and extracellular proteins were reconstituted in a solution to achieve a final concentration of 50 mM Tris-HCl pH 8.0 and 1 μg μL−1 of protein concentration. Proteins were reduced and alkylated with DTT (Sigma-Aldrich, Saint Louis, MO) and Iodoacetamide (Sigma-Aldrich, Saint Louis, MO), respectively. Insolution digestion was performed with Trypsin (Promega, Madison, WI). Then, the tryptic peptides were concentrated with a centrifugal concentrator (SpeedVac, Thermo Savant). The analysis was carried out by Ultra Performance Liquid Chromatography on a nano scale (nanoUHPLC), coupled to tandem mass spectrometry (nanoUHPLC-ESI-MS / MS) (Supplementary Material 1).
2.9. Bioinformatic analysis
Bioinformatics tools were used for the analysis of the mass data by searching against Swiss-Prot (SP) and NCBI databases at MASCOT v2.7.1 (www.matrixscience.com, local license). Customized databases with orthologous microorganisms from SP and NCBI were used due to the lack of specific databases available at the repository sites. The analysis was carried out against a database customized by the combination of four Fusarium spp.: F. graminearum, F. oxysporum, F. pseudograminearum and Fusarium sp. v20190509, 20190214, 20190510, 20190214, respectively (496,327 sequences, 220,659,562 residues).
The comparative study was carried out using ProteoIQ v2.8 (local license). The lists of unassigned peptides were analyzed with protein-BLAST (https://blast.ncbi.nlm.nih.gov) to recover proteins with a homology percentage between 80% and 100%. Additionally, complementary bioinformatics tools (analysis of Gene Ontology, Pathways, String-db, KEGG, Expasy and The Reactome, for example) were used to organize, visualize and interpret the results and their validation.
The extracellular protein-localizations were analyzed by the sub-CELlular LOcalization predictor (CELLO v2.5, http://cello.life.nctu.edu.tw/) (Yu et al., 2004 and 2006). For intra and extracellular proteomic analyses, biological triplicates and analytical duplicates were performed.
3. Results
3.1. Isolation, characterization and identification of metal-resistant microorganisms
Eight microorganisms were isolated following the methodology with selection pressure and pre-selection based on the qualitative tolerance to Fe(II), Cu(II) and Cr(VI) in solid medium, and the most tolerant microorganism was selected. Cell viability assays were carried out on the selected microorganism in the presence of increasing concentrations of the metals. The isolated was able to grow in the presence of 125 μg mL−1 Cr(VI) in the culture medium, and was also capable to grow in the presence of up to 90 μg mL−1 Fe(II) and 60 μg mL−1 Cu(II). Based on these results, the MIC value (μg mL−1) for Fe was stablished in the range 90 < MIC ≤ 125; in the case of Cr(VI), in the interval 125 < MIC ≤ 250; and for Cu(II), at 60 < MIC ≤ 90.
The molecular identification was carried out through PCR amplification of different genomic regions and sequencing of the PCR products. Primers specific for eukaryotic organisms were selected based on macro and microscopic observations, which indicated that the selected isolate was eukaryotic. Using ITS1/ITS4 primers, a 248 bp sequence was obtained (NCBI Accession Number KY596033). From these data, we determined that the microorganism belonged to the Fusarium genus (99.2% identity with different species of the Fusarium genus). To identify the microorganism at the species level, EF1/EF2 primers were selected, which are widely used for the identification of species belonging to the Fusarium genus (Karlsson et al., 2016). In this analysis, a 625 bp sequence was obtained (NCBI Accession Number MN175470). With this additional information, the microorganism was identified at the genus and species level, and was named as Fusarium tricinctum M6.
3.2. Growth and removal assays
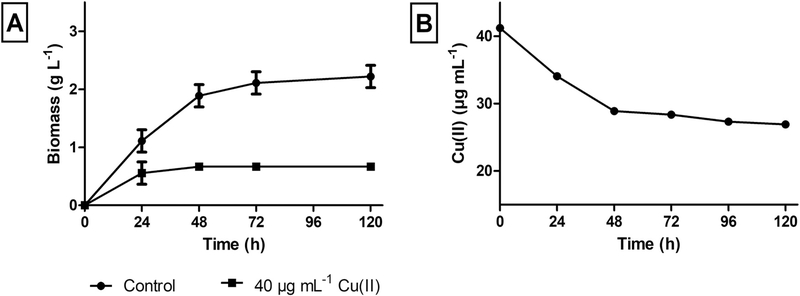
Firstly, a removal study was carried out by facing the microorganism with each metal under study. For this analysis, we worked with a metal concentration corresponding to 50% of the maximum concentration in which cell viability was observed. The evaluation consisted of determining the total metal concentration through ICP-MS at initial time and after 120 h of culture. These data showed that the selected microorganism did not show the ability to remove Cr(VI) or Fe(II) under the evaluated conditions. However, variations were observed when exposed to copper (data not shown). For this reason, a more in-depth study of the growth and removal capacity was performed by confronting the microorganism with Cu(II) in the culture medium (Fig. 1). The Cu(II) concentration was selected taking into account the MIC values previously stablished. For these assays, 40 μg mL−1 Cu(II) were added in the culture medium.
Fig. 1.
Growth evaluation of F. tricinctum M6 in the presence and absence of 40 μg mL−1 Cu(II) in the culture medium (A). Copper remaining concentration in the supernatants of F. tricinctum M6 exposed to the metal (B).
As shown in Fig. 1–A, the presence of Cu(II) inhibited the growth of F. tricinctum M6 in approximately 60% in relation to the reference culture, reaching the stationary phase after 48 h of culture. The cell-free supernatants obtained at different times in the presence of the metal were used to determine the remaining Cu(II) concentration. The maximum Cu(II) removal was obtained after 48 h of culture (34.7%) and it kept constant over time (Fig. 1–B).
3.3. Microelemental analysis of microbial biomass in the presence and absence of Cu(II)
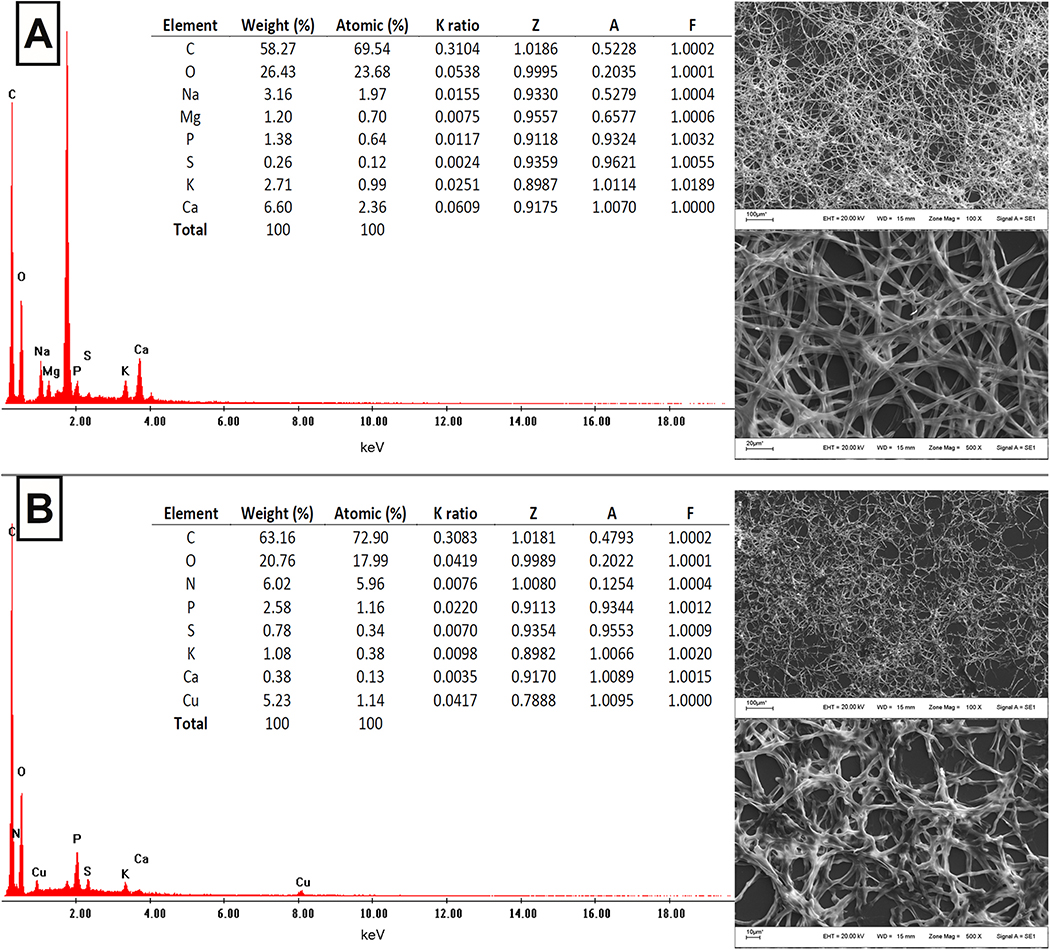
SEM-EDS was applied to evaluate the elemental composition analysis of F. tricinctum M6 in the absence or the presence of Cu(II). The biomass was obtained at 36 h of culture in the exponential growth phase. This study aimed to determine the cellular distribution of the metal and the effect of the Cu(II) presence on other constituents’ elements of the biomass to establish relations among them.
As can be seen in Fig. 2, the SEM images show the toxic effects of Cu (II) on the microbial biomass morphology. For example, significant variations can be observed in the average width of the hyphae. In the biomass obtained in the absence of Cu(II), hyphae showed 3.368 μm as average width, while the hyphae exposed to the metal were about 15% thinner. EDS analyses showed that C, O, P, S, K and Ca were common elements for both conditions. However, in the presence of the metal, the proportion of P was higher (2.58% w/w), while the peaks of K (1.08% w/w) and Ca (0.38% w/w) appeared in lower intensity. In the presence of Cu(II), the Na and Mg peaks disappeared, while N and Cu peaks appear (6.02%, 5.23% w/w, respectively).
Fig. 2.
Microelemental analysis of F. tricinctum M6 in the absence (A) and in the presence (B) of Cu(II) in the culture medium, through SEM-EDS operated at 20 kV.
The results of the microelemental composition of F. tricinctum M6 indicate that the Cu presence affected the homeostasis of other mono and bivalent elements like Na, K, Mg and Ca, may be causing an imbalance that leads to growth inhibition. Likewise, relations between copper and non-metallic elements such as N and P were significant. Moreover, it is important to highlight that a uniform distribution of copper was observed with no accumulation of the metal in the biomass, suggesting that Cu can be adsorbed on the cell membrane.
3.4. Intracellular proteomic analysis
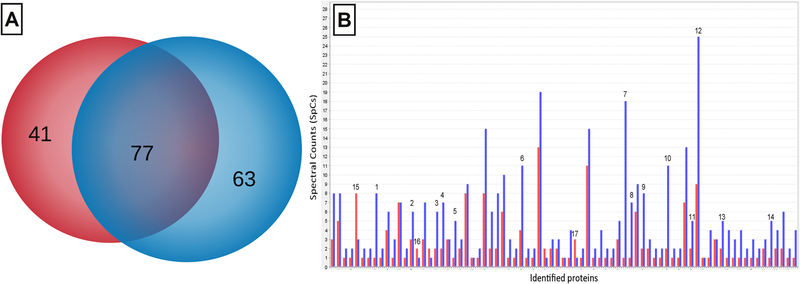
A shotgun proteomic analysis was carried out to evaluate the differential intracellular proteins expression in F. tricinctum M6, both in the presence and absence of Cu(II) in the culture medium. In this study, a total of 181 proteins were identified (Fig. 3). As shown in Fig. 3–A, 63 proteins were detected exclusively in cells obtained in the presence of Cu (II), while 41 were identified in the absence of the metal. Likewise, 77 proteins were detected both in the presence and absence of Cu(II).
Fig. 3.
Intracellular proteomic analysis of F. tricinctum M6. Venn diagram of proteins identified in the presence (blue) and in absence (red) of Cu(II) in the culture medium; the group of proteins shared by both conditions is shown in violet (A). Bar diagram of the semi-quantitative expression of intracellular proteins detected in the presence (blue) and in absence (red) of Cu(II) in the culture medium (B).
A semi-quantitative comparative analysis of the relative abundance was performed on the 77 proteins found in both conditions, using a label-free approach through the normalized spectral counts (SpCs) (Fig. 3B). In this analysis, only 17 proteins showed significant differences in their relative abundances, where 14 were over-expressed and three were down-regulated in the presence of the metal (Table 1).
Table 1.
Intracellular proteins of F. tricinctum M6 identified both in the presence and absence of Cu(II), over-expressed or down-regulated in the presence of the metal.
| Bar Number | Sequence IDa | Sequence nameb | Protein length (AA)c | Protein weight (kDa)d | Log2 scalee |
|---|---|---|---|---|---|
| Over-expressed | |||||
| 1 | A0A0D2Y596 | Elongation factor 3 | 1055 | 117.03 | 1.95 |
| 2 | F6KJZ2 | Calmodulin (Fragment) | 125 | 14.16 | 1.09 |
| 3 | A0A0D2XAN5 | 40 S ribosomal protein S6 | 239 | 27.22 | 1.58 |
| 4 | A0A0D2XC03 | Nucleosome assembly protein 1-like 1 | 404 | 45.43 | 1.79 |
| 5 | A0A1C3YN26 | Phosphoglycerate kinase | 534 | 57.94 | 1.29 |
| 6 | A0A2H3H8F9 | Heat shock 70 kDa protein 4 | 778 | 85.86 | 1.66 |
| 7 | A0A0D2XZN4 | 40 S ribosomal protein S3 | 260 | 28.52 | 3.16 |
| 8 | N4TYJ1 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | 525 | 57.45 | 1.79 |
| 9 | A0A2H3G5U3 | Heat shock protein 60 | 735 | 77.84 | 1.95 |
| 10 | A0A2H3GUK9 | Peroxiredoxin 1 | 212 | 23.80 | 1.45 |
| 11 | A0A2H3FXB5 | 60 S ribosomal protein L9-B | 250 | 28.14 | 1.29 |
| 12 | A0A0P0RS90 | Elongation factor 1-alpha | 460 | 49.82 | 1.48 |
| 13 | A0A0D2YH41 | DnaJ like subfamily C member 2 | 444 | 50.37 | 1.29 |
| 14 | Q4I1N3 | Sulfate adenylyltransferase | 574 | 64.23 | 1.29 |
| Down-regulated | |||||
| 15 | N1RH59 | Ran-specific GTPase-activating protein 1 | 237 | 26.45 | −1.50 |
| 16 | A0A0D2XM79 | Isocitrate dehydrogenase [NADP] | 462 | 51.72 | −1.08 |
| 17 | W9IHZ4 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | 276 | 30.46 | −1.58 |
Accession numbers from UniProtKB/TrEMBL (https://www.uniprot.org).
Function annotations were retrieved from NCBInr (http://www.ncbi.nlm.nih.gov) and UniProt (https://www.uniprot.org/).
Heat map information obtained from ProteoIQ. Log2 > 1 indicates over-expression, while Log2 < − 1 indicates down-regulation.
Proteins over-expressed in the presence of the metal were related to proteins responsible for the protein biosynthesis (Bars 1, 3, 7, 11 and 12), nucleic acid-binding proteins (4), carbohydrate metabolism (5 and 8), redox stress indicator proteins (6, 9, 10 and 13) and calmodulin, a Ca (II)-mediated intracellular signaling protein. In addition to calmodulin, phosphoglycerate mutase also presents bivalent metal ion binding sites, such as Mn(II) (Table 1).
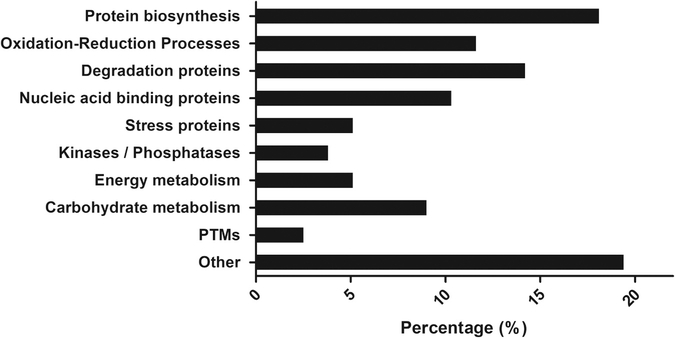
Table 2 shows the proteins identified only in the group exposed to Cu (II), which can be grouped according to their cellular functions obtained from Gene Ontology (GO) in proteins involved in: i) Protein biosynthesis (8 proteins), including translational elongation factors, ribosomal proteins and folding proteins; ii) Oxidation-reduction processes (9); iii) Degradation proteins (11), such as proteases, and proteasome and ubiquitination proteins; iv) Nucleic acid binding proteins (7); v) oxidative stress indicator proteins, such as glutathione reductase; vi) Kinases/Phosphatases (3), which can be important for the enzymatic activation or inactivation; vii) Energy metabolism (4); viii) Carbohydrate metabolism (5), as proteins involved in the glycolytic process and the Krebs cycle; and ix) Proteins responsible for post-translational modifications (2) (Fig. 4).
Table 2.
Intracellular proteins of F. tricinctum M6 identified in the presence of Cu(II) in the culture medium.
| Sequence IDa | Sequence nameb | Protein length (AA)c | Protein weight (kDa)d |
|---|---|---|---|
| Protein biosynthesis | |||
| A0A2H3HCL8 | Elongation factor 1-gamma 1 | 425 | 47.78 |
| A0A0D2XB67 | 40 S ribosomal protein S21 | 87 | 9.63 |
| A0A0D2XA48 | GrpE protein homolog | 244 | 27.43 |
| A0A0D2XWU0 | T-complex protein 1 subunit alpha | 565 | 61.32 |
| A0A0D2Y4M4 | 40 S ribosomal protein S2 | 257 | 27.97 |
| A0A2H3HCC0 | 40 S ribosomal protein S22 | 130 | 14.79 |
| A0A0D2Y2F8 | 40 S ribosomal protein S19 | 150 | 16.58 |
| A0A0D2XJG4 | 60 S ribosomal protein L27a | 149 | 16.79 |
| Oxidation-Reduction Processes | |||
| N1RL11 | NADH-ubiquinone oxidoreductase 29.9 kDa subunit, mitochondrial | 235 | 26.92 |
| N1RDJ1 | L-aminoadipate-semialdehyde dehydrogenase large subunit | 1163 | 129.01 |
| A0A0D2XAA8 | Inosine-5′-monophosphate dehydrogenase | 532 | 56.83 |
| A0A0D2YFU9 | DAO domain-containing protein | 533 | 57.75 |
| A0A0D2XC02 | D-3-phosphoglycerate dehydrogenase 2 | 473 | 51.62 |
| S0EP01 | Probable LEU2-beta-isopropyl- malate dehydrogenase | 1084 | 118.61 |
| I1S2Z0 | PKS_ER domain-containing protein | 368 | 39.83 |
| A0A0D2Y544 | UDPglucose 6-dehydrogenase | 605 | 66.18 |
| A0A0J9VP92 | Uncharacterized protein FOXG_12068 | 501 | 54.50 |
| Degradation proteins | |||
| A0A2H3HD18 | Vacuolar protease A (Fragment) | 429 | 46.42 |
| A0A0D2Y1V9 | 26 S protease regulatory subunit 6 A | 459 | 50.92 |
| A0A0D2XML3 | E3 ubiquitin-protein ligase | 810 | 91.38 |
| A0A0J9UJ20 | 26 S proteasome regulatory subunit N7 | 392 | 42.69 |
| N1RE30 | Cys-Gly metallodipeptidase dug1 | 460 | 50.55 |
| W7MDN2 | Mitochondrial-processing peptidase subunit alpha | 532 | 57.97 |
| A0A0D2×9D5 | 26 S protease regulatory subunit 7 | 440 | 49.07 |
| I1RFS5 | Peptidase alpha subunit | 565 | 61.44 |
| A0A0J9ULK9 | F-box domain-containing protein | 529 | 60.77 |
| C7Z9Q6 | Predicted protein NECHADRAFT_94534 | 577 | 62.78 |
| I1RQ19 | Uncharacterized protein FG06152.1 | 838 | 91.93 |
| Nucleic acid binding proteins | |||
| A0A2H3THI2 | Related to TATA-binding protein associated factor 2 N | 348 | 38.23 |
| A0A0J9WA35 | BZIP domain-containing protein | 212 | 24.35 |
| Q4HTT2 | Histone H2B | 137 | 14.75 |
| K3VN47 | Zn(2)-C6 fungal-type domain- containing protein | 1024 | 116.72 |
| C7YIM9 | Uncharacterized protein BRD2103 | 675 | 74.94 |
| C7YNQ5 | Uncharacterized protein NECHADRAFT_60425 | 604 | 65.40 |
| A0A0J9V1M3 | Uncharacterized protein FOXG_07428 | 841 | 94.08 |
| Stress proteins | |||
| A0A0C4DHU2 | Glutathione reductase | 506 | 54.79 |
| Kinases/Phosphatases | |||
| A0A0D2XQI2 | 3′(2′),5′-bisphosphate nucleotidase | 408 | 43.65 |
| A0A0D2Y8P5 | Adenylate kinase | 256 | 28.26 |
| A0A0J9WPA8 | Protein phosphatase 2 (Formerly 2 A), regulatory subunit A | 514 | 57.03 |
| Energy metabolism | |||
| A0A0D2XEG1 | ATP synthase subunit delta, mitochondrial | 165 | 17.70 |
| A0A0D2XAN8 | V-type proton ATPase subunit B | 511 | 56.65 |
| A0A0D2XHL0 | V-type proton ATPase subunit E | 229 | 25.83 |
| A0A0J9UCJ3 | V-type proton ATPase catalytic subunit A | 788 | 85.90 |
| Carbohydrate metabolism | |||
| A0A0D2Y1Y2 | Fructose-bisphosphate aldolase, class II | 360 | 39.61 |
| A0A0D2XEA1 | Triosephosphate isomerase | 247 | 27.06 |
| A0A0D2XGP1 | Fumarate hydratase, mitochondrial | 529 | 56.80 |
| A0A0D2×829 | Enolase | 438 | 47.28 |
| A0A0D2Y4K0 | Glucose-6-phosphate 1-epimerase | 313 | 33.58 |
| Post-translational modifications (PTMs) | |||
| A0A0D2YKT2 | 1,3-beta-glucanosyltransferase | 375 | 41.05 |
| K3VMJ8 | Uncharacterized protein FPSE_04909 | 513 | 58.50 |
| Other | |||
| N1RHH1 | Tropomyosin-2 | 161 | 18.79 |
| A0A0D2XNF4 | Myo-inositol-1-phosphate synthase | 541 | 59.44 |
| A0A0D2XHT9 | Phospho-2-dehydro-3- deoxyheptonate aldolase | 317 | 34.11 |
| N1REE8 | Rho GDP-dissociation inhibitor | 198 | 22.16 |
| A0A2H3GY22 | Reduced viability upon starvation protein 167 | 434 | 49.42 |
| A0A0D2×9R5 | S-adenosylmethionine synthase | 403 | 44.27 |
| A0A2H3G4L5 | ATP phosphoribosyltransferase | 325 | 35.33 |
| A0A0D2XX43 | pHdomain-containing protein | 834 | 91.54 |
| A0A0J9WG59 | Abhydrolase_3 domain-containing protein | 259 | 28.70 |
| A0A0D2XWU1 | Prolyl-tRNA synthetase | 553 | 62.36 |
| S0DLW9 | Related to putative sterigmatocystin biosynthesis lipase/esterase STCI | 364 | 40.68 |
| A0A0J9WKR9 | Cysteine desulfurase | 401 | 43.75 |
| C7Z6×3 | Predicted protein NECHADRAFT_33672 | 2106 | 231.18 |
Accession numbers from UniProtKB/TrEMBL (https://www.uniprot.org) and NCBI (http://www.ncbi.nlm.nih.gov).
Function annotations were retrieved from NCBInr (http://www.ncbi.nlm.nih.gov) and UniProt (https://www.uniprot.org/).
Fig. 4.
Proteins identified in F. tricinctum M6 exposed to Cu(II), grouped according to their cellular function.
Among the proteins identified in cells grown in the presence of Cu (II), a significant amount of metal ion binding proteins was detected. These proteins generally use metals as cofactors to carry out their cellular functions. Some of these proteins are Inosine-5′-monophosphate dehydrogenase, which uses potassium, and degradation proteins with metalloendopeptidase activity, such as Cys-Gly metallodipeptidase dug1, Mitochondrial-processing peptidase, Peptidase alpha, F-box domain-containing protein and Predicted protein NECHADRAFT_94534. Other proteins that also bind bivalent metal ions are Zn(2)-C6 fungal-type domain-containing protein, Fructose-bisphosphate aldolase, Enolase, S-adenosylmethionine synthase, ATP phosphoribosyltransferase and Cysteine desulfurase. These proteins are involved in a wide variety of cellular processes.
3.5. Extracellular proteomic analysis
The differential expression analysis of extracellular proteins was carried out through shotgun proteomics in cell-free supernatants concentrated at 20X. The supernatants were obtained from cultures of F. tricinctum M6 both in the presence and absence of Cu(II) in the culture medium.
In this study, 32 proteins were detected in cells grown in absence of Cu and 22 proteins in the metal exposed cells. However, when the location of the identified proteins was analyzed by CELLO v2.5 (http://cello.life.nctu.edu.tw/), only eight proteins in the control group and six in the Cu(II) exposed group showed a significant probability to be secreted into the extracellular space (Table 3). These results showed that the rest of the identified proteins probably come from lysis or cell disruption, a process that naturally occurs in the microbial cultures.
Table 3.
Extracellular proteins identified in F. tricinctum M6 cell-free supernatants obtained in the presence and absence of Cu(II) in the culture medium. Predictions obtained from CELLO v2.5.
| Sequence IDa | Sequence nameb | Protein length (AA)c | Protein weight (kDa)d |
|---|---|---|---|
| Control | |||
| A0A395N2×2 | Het-domain-containing protein | 538 | 62.66 |
| A0A090MDG5 | Efflux pump FUS6 | 126 | 14.32 |
| K3VU44 | Beta-glucosidase | 832 | 89.51 |
| C7YSF2 | Endo-chitosanase | 293 | 30.69 |
| W7LIK3 | Uncharacterized protein FVEG_01594 | 795 | 83.27 |
| S0E6U9 | Uncharacterized protein FFUJ_06603 | 787 | 81.86 |
| A0A0D2XFR8 | Uncharacterized protein FOXG_02757 | 787 | 81.98 |
| C7YTA7 | Predicted protein NECHADRAFT_69214 | 294 | 31.91 |
| Exposed to Cu(II) | |||
| A0A428U1G5 | PAD domain-containing protein | 609 | 67.34 |
| A0A098DCZ3 | Beta-xylanase | 563 | 60.66 |
| C7YUT8 | FAD-binding PCMH-type domain-containing protein | 653 | 70.99 |
| W7LGU8 | Uncharacterized protein FVEG_14799 | 124 | 13.95 |
| C7YKT3 | Predicted protein NECHADRAFT_105961 | 135 | 14.56 |
| C7YX48 | Secreted protein NECHADRAFT_95070 | 533 | 59.24 |
Accession numbers from UniProtKB/TrEMBL (https://www.uniprot.org) and NCBI (http://www.ncbi.nlm.nih.gov).
Function annotations were retrieved from NCBInr (http://www.ncbi.nlm.nih.gov) and UniProt (https://www.uniprot.org/).
Some of the extracellular proteins detected in the control cells in F. tricinctum M6 are mainly involved in carbohydrate metabolism. Also, proteins with functions not characterized were detected. When analyzing the proteins of the cells exposed to the metal, proteins with the capability to sequester bivalent ions were identified, such as PAD domain proteins, which are capable of binding Ca(II). Proteins with functions in carbohydrate metabolism, such as Beta-xylanase, or involved in oxidation-reduction processes were identified.
Additionally, proteins able to sequester metal ions + 2 were detected in supernatants of cells exposed to Cu(II) with no probability to be secreted into the extracellular space, according to CELLO v2.5. These proteins include RBR-type E3 ubiquitin transferase (Uniprot accession number A0A3M2SJW4), CCR4-NOT transcription complex subunit 4 (A0A0J9UXE0), Zinc finger protein (A0A2H3RXW3), and uncharacterized proteins CEP52_005114 (A0A428TZU7) and FPSE_03992 (K3VLP0). Notably, these proteins were not identified in the control supernatant of F. tricinctum M6 (data not shown). Therefore, they could play an important role in the homeostasis mechanisms of Cu(II).
4. Discussion
In the present work, through a sequential isolation method with selection pressure, a microorganism tolerant to Fe(II), Cu(II) and Cr(VI) was isolated and identified as F. tricinctum M6. This microorganism showed Cu(II) removal capacity from liquid culture medium. Numerous works reported the ability of Fusarium genus members to tolerate and remove metals. For example, Zhang et al. (2012) isolated F. oxysporum from a Pb and Zn mining area in China and this microorganism resisted the presence of 0.8 mM Cu, showing a similar resistance to F. tricinctum M6. In other work, F. oxysporum MUCL 791 showed Cu(II) removal from aqueous solution (Simonescu and Ferdeş 2012). Likewise, F. oxysporum was used for the treatment of sewage sludge and showed removal capacity against Cd, Sr and Cu (Moursy et al., 2015). Most of these members have been isolated from sites with a high contamination level caused by anthropogenic activities, demonstrating that the contaminated sites are promising natural niches for the isolation of metal-resistant microorganisms.
4.1. Microelemental composition of microbial biomass exposed to Cu(II)
The microelemental composition of F. tricinctum M6 in the presence and absence of Cu(II) was analyzed through SEM-EDS. This study was carried out at the exponential growth phase to analyze the presence, concentration and distribution of copper in the microbial biomass exposed to the metal and, at the same time, to detect variations regarding the control cultures in the absence of the metal. Peaks corresponding to copper were detected when the microorganism was exposed to the metal. Likewise, a uniform distribution of the metal and morphological variations produced by the presence of Cu(II) were observed.
SEM-EDS has been widely used on metal-resistant microorganisms. For example, Lu et al. (2006) confirmed the presence of Pb, Cd and Cu on the surface of Enterobacter sp. J1 and observed morphological changes, obtaining consistent results with those obtained in the present work. Microbial morphological changes in the presence of metal were also detected by Villegas et al. (2009) and Damodaran et al. (2013). Recently, Palanivel et al. (2020) observed cell surface alterations and Cu signal in Pseudomonas stutzeri LA3. These authors proposed that the metal peak detection using SEM-EDS is related to cellular surface adsorption rather than to intracellular bioaccumulation.
In the present work, Cu(II) influenced other constituent elements of the microbial biomass. In the presence of copper, F. tricinctum M6 presented higher proportion of P. The peak of N was also detected, while a lower proportion of K, Ca, and disappearance of Na and Mg were observed, when compared to the control. Some authors claim that ion exchange mechanisms on the cell surface might be involved in the metal removal mechanisms from aqueous solutions. In this sense, Michalak et al. (2011) demonstrated the superficial exchange of alkali and alkaline earth metals by metal ions when exposed Enteromorpha sp. to Cu(II), Mn(II), Zn(II) and Co(II). In the presence of metals, Cu peaks appeared on the surface of the organism, while the Ca and Mg peaks decreased in intensity, and the K and Na peaks disappeared. The importance of the Cu:Na, Cu:Mg, Cu:K and Cu:Ca relation is clearly observed in F. tricinctum M6 exposed to Cu(II). Shinde et al. (2012) also proposed that Ni(II) removal by Yarrowia lipolytica involves an ion exchange mechanism on the yeast cell surface. The disappearance of the K peaks was also reported by Xu et al. (2012) when studying the biosorption of Cd in Penicillium chrysogenum. In a work published by Salvadori et al. (2014), the presence of copper in Rhodotorula mucilaginosa biomass was confirmed and variations in the elemental composition were also observed, since in the presence of the metal, N and P peaks appeared, and Na, Ca and K peaks disappeared.
In the same line of research, Sun et al. (2015) used SEM-EDS in S. cerevisiae exposed to copper and demonstrated an important relation between Cu:Na and Cu:K, suggesting that copper adsorption can be related to the K release from the cell surface. These authors also related the N peak appearance with the complex formation between Cu(II) and nitrogenous compounds of the culture medium. In F. tricinctum M6, an important relation between Cu:K is also observed, as well as the appearance of the N peak, which can be related to what these authors have stated. A similar conclusion was reached by Sheng et al. (2016) who studied Cd accumulation in Lactococcus lactis, indicating that electronegative elements can be responsible for the metal biosorption. Finally, Sivaperumal et al. (2018) exposed the adsorption capacity of Cs by Nocardiopsis sp. 13 H, detecting a microelemental imbalance with disappearance of Mg and appearance of Na, P and K.
The works previously mentioned indicate that the metal presence causes an important variation of the microelemental composition in resistant microorganisms. In this way, it can be proposed that electronegative elements such as P and N may be involved in the uptake of metal ions, while an ion exchange with other metals can also occur on microbial surfaces.
4.2. Proteomic analysis
Using gel-free proteomic approach, the present study carried out the differential expression analysis of intracellular and extracellular proteins of F. tricinctum M6 in the presence and absence of Cu(II). The proteins were obtained at the exponential growth phase, where the highest metabolic activity is observed. It is important to highlight the methodological challenge represented by the unavailability of the sequenced genome of the isolated microorganism, as well as proteomes or transcriptomes specific databases. One of the alternatives to overcome this difficulty is to use available databases of orthologous microorganisms (Bonilla et al., 2020), as done in the present work. The importance of the integration and the comparison of strengths and limitations of proteomic databases is still debated in different fields of study (Subba et al., 2019).
4.2.1. Differential expression of intracellular proteins in the presence of Cu(II)
When exposed to high concentrations of copper, F. tricinctum M6 showed differential expression of intracellular protein profiles. Similarly, proteins related to protein biosynthesis and carbohydrate metabolism have been found in Cyanothece sp. CCY 0110 exposed to Cu(II) (Mota et al., 2015). Chiapello et al. (2015) observed an increased production of chaperones, proteins involved in protein biosynthesis, energy production, carbohydrate metabolism and stress redox in Oidiodendron maius against Cd and Zn. All these data coincide with the results obtained in the present work when confronting the microorganism with Cu(II).
In the presence of copper, stress proteins and proteins for misfolded proteins response were detected in Streptococcus pneumoniae (Guo et al., 2015). In parallel, Chen et al. (2015) detected proteins related to oxidative stress and detoxification in plants exposed to Cu(II). These works agree with the observed in the present study. Likewise, consistent with the presence of oxidative stress, the metal-resistant yeast R. mucilaginosa showed higher expression of proteins related to energy synthesis, protein synthesis and degradation (Ilyas et al., 2016). Proteins related to oxidative stress, such as oxidoreductases, have also been reported in Meyerozyma guilliermondii, a manganese-resistant yeast isolated from acid mine drainage-affected environments (Ruas et al., 2019), and in Acinetobacter calcoaceticus against Cu (Kang et al., 2020).
Over-expression of calmodulin was detected in F. tricinctum M6 exposed to Cu(II). Calmodulin is a protein with important roles in the response to different stresses, including metal stress (Zhang et al., 2016). Notably, this protein can also be activated by the presence of other bivalent metal ions (Mills and Johnson, 1985). The signaling mechanisms activated by metals also affect the expression of kinases and phosphatases (Tiwari and Lata, 2018). In F. tricinctum M6 exposed to Cu(II), E3 ubiquitin ligase was detected, which has been related to abiotic stress in plants in a study carried out by Wu et al. (2016).
The energy metabolism was affected in F. tricinctum M6 exposed to Cu(II). Proteins related to energy metabolism have been reported as important in the response to metals by Zou et al. (2015). In agreement, authors as Feng et al. (2017) state that the tolerance to Cu(II) by Penicillium janthinellum GXCR depends on the energy generation, which is necessary to carry out carbohydrate metabolism. Izrael-Živković et al. (2018) detected proteins involved in energy metabolism, protein biosynthesis and post-translational modifications in P. aeruginosa exposed to Cd(II). Recently, the importance of the energy metabolism to face metals was also observed when exposing Lactobacillus plantarum against lead (Liu et al., 2019) and Streptomyces sp. MC1 to Cr(VI) (Bonilla et al., 2020).
Proteins related to carbohydrate metabolism, and involved in replication, transcription and translation have been reported recently in R. mucilaginosa AN5 (Kan et al., 2019). In a recent study, Streptomyces sp. MC1 exposed to Cr(VI) also showed higher expression of proteins related to protein biosynthesis, proteins involved in oxidation-reduction processes and chaperones with a key role for misfolded protein repair (Bonilla et al., 2020). These works are in concordance with the results obtained with F. tricinctum M6 against Cu(II), where the same protein groups are reported as necessary proteins to face the stress produced by the presence of copper and to maintain the metabolic balance.
Metals are known to be inhibitors of the folding protein process and, at the same time, inhibit the denatured proteins refolding, either naturally or chaperones assisted (Chiapello et al., 2015). This problem enhances the misfolded or defective proteins degradation and causes an increased expression of proteasome and ubiquitination proteins. Likewise, the presence of metals promotes a greater chaperones expression, as observed in this study, which are key components for protein folding and for stress response (Steurer et al., 2018; Kan et al., 2019). To achieve the normal cellular function, cells exposed to Cu(II) can be forced to synthesize more proteins, which explain the increased expression for proteins involved in the protein biosynthesis, and consequently, for proteins involved in nucleic acid modeling and metabolism. The synthesis of new proteins may demand higher energy expenditure by the cells, as observed in F. tricinctum M6. To counteract the oxidative stress caused by the Cu(II) presence, the microorganism shows higher expression of stress response proteins and oxidoreductases. Likewise, an increase expression for kinases and phosphatases is observed, which fulfill functions in the enzymatic activation or inactivation (Tiwari and Lata, 2018).
As mentioned in the Results Section, in the presence of Cu(II) F. tricinctum M6 expressed numerous proteins that possess metal ion binding sites, involved in a wide variety of cellular processes. Metalloproteomics studies the expression of metalloproteins and their changes in the biological time and space. Proteins that control metal homeostasis and proteins regulated by metals bind transiently to the metal ions (Maret, 2010). Therefore, metalloproteomes appear as interesting dynamic structures for metal-resistant organisms (Lancaster et al., 2014).
Chiapello et al. (2015) detected metalloenzymes in O. maius against Cd and Zn, proposing that these proteins may be important for metal tolerance, not only through their enzymatic activity, but also due to their ability to sequester bivalent metal ions. Metal ion binding proteins have also been detected in P. aeruginosa exposed to Cd(II) (Izrael-Živković et al., 2018). In this work, the authors proposed that Cd (II) has the ability to replace the metals used by these proteins as cofactors. Taking into account our results, Cu(II) may also replace the natural cofactors of many proteins at the metal binding sites. In this sense, Farcasanu and Ruta (2018) and Fein et al. (2019) affirm that proteins with metal ion-binding sites present high affinity for a wide variety of metal ions and their promiscuity and increased expression may be key for the metal tolerance in resistant microorganisms.
4.2.2. Differential expression of extracellular proteins in the presence of Cu(II)
The proteins identified in the extracellular space of F. tricinctum M6 exposed to Cu(II) were mainly related to carbohydrate metabolism and oxidation-reduction processes. Likewise, proteins able to chelate metal ions were also identified, such as PAD domain protein, which binds Ca(II).
What happens in the extracellular environment when cells are exposed to metals is still unknown and, to the best of our knowledge, there are only few studies related to this area (Giner-Lamia et al., 2016). For example, the one carried out by Kim et al. (1995), who detected extracellular copper response proteins (CRX) in Methanobacterium bryantii BKYH exposed to Cu(II). Protein secretion was also stimulated by Mg(II) in Vibrio parahaemolyticus (Bhattacharya et al., 2000). Martino et al. (2002) demonstrated that Zn induced the protein secretion in O. maius. In the present work, Beta xylanase was identified in supernatants of F. tricinctum M6 exposed to Cu(II). This enzyme is involved in the carbohydrate metabolism and has previously been associated to the early response to metals in basidiomycetes (Zhao et al., 2015).
As in the intracellular space, proteins with metal binding sites were detected in the F. tricinctum M6 supernatants exposed to copper. These proteins did not show a significant probability to be secreted into the extracellular space, according to CELLO v2.5. Interestingly, Desvaux et al. (2009) have defined Exoproteomics as the study of all the proteins present in the extracellular space, whether secreted or not by the cells. In this way, the authors recognize the importance of all the proteins identified in the supernatants for metal-resistance. It is known that exoproteins found in the presence of metals include many functional categories, the composition of the exoproteome varies under different growth conditions and many of the detected proteins appear as proteins with unknown functions (Giner-Lamia et al., 2016). This clearly provides an interesting framework for studies of the extracellular space.
In the exoprotein content of F. tricinctum M6 in the presence of Cu(II), many of the identified proteins possess metal ion binding sites. These proteins may play a fundamental role in the Cu(II) sequestration and transport, mitigating the toxic effects for the cell. Most of the detected exoproteins show intracellular functions. However, their functions in the extracellular space remain unknown. These results indicate that, when faced with metal stress, the microorganism responds not only by adjusting the intracellular protein profiles, but also by adjusting the protein expression in the extracellular space.
5. Conclusions
The acid mine drainage-affected environment located in La Carolina (San Luis, Argentina) constitutes an interest microbial niche for the isolation of metal-tolerant microorganisms. This fact is an indication of the relation between the environmental conditions and the adaptive capacity of the endogenous microbiota.
The microelemental composition of the biomass exposed to Cu(II) indicates that the surface electronegative elements and the ionic exchange mechanisms play an important role in the copper ions adsorption. Proteomic studies showed that protein expression by F. tricinctum M6 changed to cope with metal toxicity. The differential expression allows the microorganism to counteract the metabolic imbalances that the metal toxicity produces. Likewise, proteins identified in the extracellular space may be crucial for the metal sequestration and transport.
In F. tricinctum M6 exposed to copper, a large number of proteins with bivalent metal ion binding sites at intra and extracellular levels were detected. The evidence regarding the high affinity of these proteins for a wide variety of metal ions would indicate that Cu(II) ions have the ability to replace the metals used by these proteins as cofactors, either activating or inactivating the original protein activity. The results obtained in this work show that there is not only one resistance mechanism in F. tricinctum M6, but a combination of different strategies, including proteomic changes, sequestration of the metal ions and surface adsorption. The study of metal-tolerant organisms is important not only for our mechanistic understanding of selective incorporation and/or metal immobilization, but also for the efforts to harness these skills in bioremediation processes.
5.1. Limitation of the study and future perspective
Future research is needed to in depth into the robustness of the isolated microorganism when faced with the actual environmental conditions of the affected area (low pH values, presence of co-pollutants, temperature, among other). Likewise, the microorganism should be analyzed not only for its application in AMD-affected environments, but also for its application in environments affected by industrial and domestic effluents. The microorganism can also be faced with other bivalent metal ions to confirm the importance of the metal ion-binding proteins for the resistance showed by F. tricinctum M6 against Cu(II). Finally, the microorganism can also be evaluated against metals in a resting-cell approach instead of a growing-cell method.
Supplementary Material
Acknowledgments
The authors thank the financial assistance of the National Agency for Scientific and Technological Promotion, Argentina [PICT 2016 No. 2526 to Dr. Gil]. The authors would also like to thank Messrs. Ryan Johnson and Bill Conn from USD-IT Research Computing for their help in the databases installation and server operation and maintenance, Dr. Walter Lapadula and Dr. Maximiliano Juri Ayub (Molecular Biology Area, FQByF, UNSL) for the helpful collaboration, and Liliana Waicekawsky for the English revision of this article. Bonilla JO thanks CONICET for the awarded doctoral fellowship.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2021.125216.
References
- Azarbad H, van Gestel CA, Niklińska M, Laskowski R, Röling WF, van Straalen NM, 2016. Resilience of soil microbial communities to metals and additional stressors: DNA-based approaches for assessing “stress-on-stress” responses. Int. J. Mol. Sci 17 (6), 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Banfield JF, 2003. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44, 139–152. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Roy SS, Biswas D, Kumar R, 2000. Effect of Mg(2+) ion in protein secretion by magnesium-resistant strains of Pseudomonas aeruginosa and Vibrio parahaemolyticus isolated from the coastal water of Haldia port. FEMS Microbiol. Lett. 185 (2), 151–156. [DOI] [PubMed] [Google Scholar]
- Blackstock WP, Weir MP, 1999. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 17 (3), 121–127. [DOI] [PubMed] [Google Scholar]
- Bonilla JO, Callegari EA, Delfini CD, Estevez MC, Villegas LB, 2016. Simultaneous chromate and sulfate removal by Streptomyces sp. MC1. Changes in intracellular protein profile induced by Cr(VI). J. Basic Microbiol. 56 (11), 1212–1221. [DOI] [PubMed] [Google Scholar]
- Bonilla JO, Kurth DG, Cid FD, Ulacco JH, Gil RA, Villegas LB, 2018. Prokaryotic and eukaryotic community structure affected by the presence of an acid mine drainage from an abandoned gold mine. Extremophiles 22 (5), 699–711. [DOI] [PubMed] [Google Scholar]
- Bonilla JO, Callegari EA, Estevez MC, Villegas LB, 2020. Intracellular proteomic analysis of Streptomyces sp. MC1 when exposed to Cr(VI) by gel-based and gel-free methods. Curr. Microbiol 77 (1), 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra RC, Lotufo MA, Gagioti SM, Barros Fde M, Andrade PM, 2009. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral. Res. 23 (3), 255–262. [DOI] [PubMed] [Google Scholar]
- Bradford MM, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Chen C, Song Y, Zhuang K, Li L, Xia Y, Shen Z, 2015. Proteomic analysis of copper-binding proteins in excess copper-stressed roots of two rice (Oryza sativa L.) varieties with different cu tolerances. PLoS One 10 (4), e0125367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapello M, Martino E, Perotto S, 2015. Common and metal-specific proteomic responses to cadmium and zinc in the metal tolerant ericoid mycorrhizal fungus Oidiodendron maius Zn. Metallomics 7 (5), 805–815. [DOI] [PubMed] [Google Scholar]
- Damodaran D, Balakrishnan RM, Shetty VK, 2013. The uptake mechanism of Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) by mycelia and fruiting bodies of Galerina vittiformis. BioMed Res. Int. 2013, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux M, Hebraud M, Talon R, Henderson IR, 2009. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 17, 139–145. [DOI] [PubMed] [Google Scholar]
- Fadiran AO, Dlamini CL, Thwala JM, 2014. Environmental assessment of acid mine drainage pollution on surface water bodies around ngwenya mine. Swazil. J. Environ. Prot 5, 164–173. [Google Scholar]
- Farcasanu IC, Ruta LL 2018. Metallothioneins, Saccharomyces cerevisiae, and Heavy Metals: A Biotechnology Triad? Chapter 2 pp. 21–39. In: Old Yeasts: New Questions. [Google Scholar]
- Fein JB, Yu Q, Nam J, Yee N, 2019. Bacterial cell envelope and extracellular sulfhydryl binding sites: their roles in metal binding and bioavailability. Chem. Geol 521, 28–38. [Google Scholar]
- Feng X, Xu J, Liang Y, Chen GL, Fan XW, Li YZ, 2017. A proteomic-based investigation of potential copper-responsive biomarkers: Proteins, conceptual networks, and metabolic pathways featuring Penicillium janthinellum from a heavy metal-polluted ecological niche. Microbiologyopen 6 (4), e00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner-Lamia J, Pereira SB, Bovea-Marco M, Futschik ME, Tamagnini P, Oliveira P, 2016. Extracellular proteins: novel key components of metal resistance in cyanobacteria? Front. Microbiol 7, 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Han J, Yang XY, Cao K, He K, Du G, Zeng G, Zhang L, Yu G, Sun Z, He QY, Sun X, 2015. Proteomic analysis of the copper resistance of Streptococcus pneumoniae. Metallomics 7 (3), 448–454. [DOI] [PubMed] [Google Scholar]
- Ilyas S, Rehman A, Coelho AV, Sheehan D, 2016. Proteomic analysis of an environmental isolate of Rhodotorula mucilaginosa after arsenic and cadmium challenge: identification of a protein expression signature for heavy metal exposure. J. Proteom. 141, 47–56. [DOI] [PubMed] [Google Scholar]
- Izrael-Živković L, Rikalović M, Gojgić-Cvijović G, Kazazić S, Vrvić M, Brčeski I, Beškoski V, Lončarević B, Gopčević K, Karadžić I, 2018. Cadmium specific proteomic responses of a highly resistant Pseudomonas aeruginosa san ai. RSC Adv. 8, 10549–10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Hallberg KB, 2003. The microbiology of acidic mine waters. Res. Microbiol. 154, 466–473. [DOI] [PubMed] [Google Scholar]
- Kan G, Wang X, Jiang J, Zhang C, Chi M, Ju Y, Shi C, 2019. Copper stress response in yeast Rhodotorula mucilaginosa AN5 isolated from sea ice, Antarctic. Microbiologyopen 8 (3), e00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Zheng J, Bao J, Wang Z, Zheng Y, He JZ, Hu HW, 2020. Characterization of the copper resistance mechanism and bioremediation potential of an Acinetobacter calcoaceticus strain isolated from copper mine sludge. Environ. Sci. Pollut. Res. 27 (8), 7922–7933. [DOI] [PubMed] [Google Scholar]
- Karlsson I, Edel-Hermann V, Gautheron N, Durling MB, Kolseth A-K, Steinberg C, Persson P, Friberg H, 2016. Genus-specific primers for study of Fusarium communities in field samples. Appl. Environ. Microbiol 82 (2), 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Pihl TD, Reeve JN, Daniels L, 1995. Purification of the copper response extracellular proteins secreted by the copper-resistant methanogen Methanobacterium bryantii BKYH and cloning, sequencing, and transcription of the gene encoding these proteins. J. Bacteriol 24, 7178–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster WA, Menon AL, Scott I, Poole FL, Vaccaro BJ, Thorgersen MP, Geller J, Hazen TC, Hurt RA, Brown SD, Elias DA, Adams MWW, 2014. Metallomics of two microorganisms relevant to heavy metal bioremediation reveal fundamental differences in metal assimilation and utilization. Metallomics 6 (5), 1004–1013. [DOI] [PubMed] [Google Scholar]
- Liu S, Zheng Y, Ma Y, Sarwar A, Zhao X, Luo T, Yang Z, 2019. Evaluation and proteomic analysis of lead adsorption by lactic acid bacteria. Int. J. Mol. Sci 20 (22), 5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WB, Shi JJ, Wang CH, Chang JS, 2006. Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J. Hazard Mater. 134 (1–3), 80–86. [DOI] [PubMed] [Google Scholar]
- Ma Z, Jacobsen FE, Giedroc DP, 2009. Metal transporters and metal sensors: how coordination chemistry controls bacterial metal homeostasis. Chem. Rev 109, 4644–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W, 2010. Metalloproteomics, metalloproteomes, and the annotation of metalloproteins. Metallomics 2 (2), 117–125. [DOI] [PubMed] [Google Scholar]
- Martino E, Franco B, Piccoli G, Stocchi V, Perotto S, 2002. Influence of zinc ions on protein secretion in a heavy metal tolerant strain of the ericoid mycorrhizal fungus Oidiodendron maius. Mol. Cell Biochem. 231 (1–2), 179–185. [DOI] [PubMed] [Google Scholar]
- Méndez-García C, Peláez AI, Mesa V, Sánchez J, Golyshina OV, Ferrer M, 2015. Microbial diversity and metabolic networks in acid mine drainage habitats. Front Microbiol. 6, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak I, Chojnacka K, Marycz K, 2011. Using ICP-OES and SEM-EDX in biosorption studies. Mikrochim Acta 172 (1–2), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JS, Johnson JD, 1985. Metal ions as allosteric regulators of calmodulin. J. Biol. Chem 260 (28), 15100–15105. [PubMed] [Google Scholar]
- Mota R, Pereira SB, Meazzini M, Fernandes R, Santos A, Evans CA, De Philippis R, Wright PC, Tamagnini P, 2015. Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J. Proteom 120, 75–94. [DOI] [PubMed] [Google Scholar]
- Moursy AA, Abdel Aziz OA, Mustafa AZ, 2015. Bioremediation of Irradiated and non-irradiated sewage sludge by Fusarium oxysporium fungi. IOSR J. Eng. 5, 16–23. [Google Scholar]
- Oblasser A, Chaparro Ávila E, 2008. Estudio comparativo de la gestión de los pasivos ambientales mineros en Bolivia, Chile, Perú y Estados Unidos. CEPAL – Serie Recursos Naturales e Infraestructura N° 131. Santiago De. Chile: 2008. ISBN: 978–92-1–323175-3. [Google Scholar]
- Palanivel TM, Sivakumar N, Al-Ansari A, Victor R, 2020. Bioremediation of copper by active cells of Pseudomonas stutzeri LA3 isolated from an abandoned copper mine soil. J. Environ. Manag. 253, 109706. [DOI] [PubMed] [Google Scholar]
- Perez Silva MR, Camacho Pozo MI, Gómez Montes de Oca JM, Ábalos Rodríguez A, Viñas M, Cantero Montero D, 2008. Aislamiento y selección de una cepa bacteriana degradadora de hidrocarburos a partir de suelos contaminados con petróleo. CENIC Cienc. Biológicas 39 (1). ISSN 0253–5688. [Google Scholar]
- Puglisi E, Hamon R, Vasileiadis S, Coppolecchia D, Trevisan M, 2012. Adaptation of soil microorganisms to trace element contamination: a review of mechanisms, methodologies, and consequences for risk assessment and remediation. Crit. Rev. Environ. Sci. Technol 42 (22), 2435–2470. [Google Scholar]
- Rex JH, 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. Clin. Lab. Stand. Inst 28 (16). ISSN 0273–3099. [Google Scholar]
- Ruas FAD, Barboza NR, Castro-Borges W, Guerra-Sá R, 2019. Manganese alters expression of proteins involved in the oxidative stress of Meyerozyma guilliermondii. J. Proteom. 196, 173–188. [DOI] [PubMed] [Google Scholar]
- Salvadori MR, Ando RA, Oller do Nascimento CA, Corrêa B, 2014. Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. PLoS One 9 (1), e87968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Wang Y, Yang X, Zhang B, He X, Xu W, Huang K, 2016. Cadmium tolerant characteristic of a newly isolated Lactococcus lactis subsp. lactis. Environ. Toxicol. Pharm 48, 183–190. [DOI] [PubMed] [Google Scholar]
- Shinde NR, Bankar AV, Kumar AR, Zinjarde SS, 2012. Removal of Ni(II) ions from aqueous solutions by biosorption onto two strains of Yarrowia lipolytica. J. Environ. Manag 102, 115–124. [DOI] [PubMed] [Google Scholar]
- Simonescu CM, Ferdeş M, 2012. Fungal biomass for Cu(II) uptake from aqueous systems. Pol. J. Environ. Stud 21 (6), 1831–1839. [Google Scholar]
- Sivaperumal P, Kamala K, Rajaram R, 2018. Adsorption of cesium ion by marine actinobacterium Nocardiopsis sp. 13H and their extracellular polymeric substances (EPS) role in bioremediation. Environ. Sci. Pollut. Res 25 (5), 4254–4267. [DOI] [PubMed] [Google Scholar]
- Solioz M, Abicht HK, Mermod M, Mancini S, 2010. Response of Gram-positive bacteria to copper stress. J. Biol. Inorg. Chem 15, 3–14. [DOI] [PubMed] [Google Scholar]
- Steurer C, Eder N, Kerschbaum S, Wegrostek C, Gabriel S, Pardo N, Ortner V, Czerny T, Riegel E, 2018. HSF1 mediated stress response of heavy metals. PLoS One 13 (12), e0209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba P, Narayana Kotimoole C, Prasad TSK, 2019. Plant proteome databases and bioinformatic tools: an expert review and comparative insights. OMICS 23 (4), 190–206. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Liu L, Jia B, Zhao F, Huang W, Zhan J, 2015. Copper tolerance and biosorption of Saccharomyces cerevisiae during alcoholic fermentation. PLoS One 10 (6), e0128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Lata C, 2018. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front. Plant Sci. 9, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripole ES, Corigliano MC, 2005. Acid stress evaluation using multimetric indices in the Carolina stream (San Luis –Argentina). Acta Limnol. Bras. 17 (1), 101–114. [Google Scholar]
- Tripole S, Gonzalez P, Vallania A, Garbagnati M, Mallea M, 2006. Evaluation of the impact of acid mine drainage on the chemistry and the macrobenthos in the Carolina stream (San Luis – Argentina). Environ. Monit. Assess 114, 377–389. [DOI] [PubMed] [Google Scholar]
- Villegas LB, Amoroso MJ, de Figueroa LI, 2004. Selection of Tolerant Heavy Metal Yeasts from Different Polluted Sites. In: Environmental Microbiology. Methods Protocols in Biotechnology. Humana Press Inc, pp. 249–256. [Google Scholar]
- Villegas LB, Amoroso MJ, de Figueroa LI, 2009. Responses of Candida fukuyamaensis RCL-3 and Rhodotorula mucilaginosa RCL-11 to copper stress. J. Basic Microbiol. 49 (4), 395–403. [DOI] [PubMed] [Google Scholar]
- Wu X, Gong F, Cao D, Hu X, Wang W, 2016. Advances in crop proteomics: PTMs of proteins under abiotic stress. Proteomics 16 (5), 847–865. [DOI] [PubMed] [Google Scholar]
- Xu X, Xia L, Huang Q, Gu JD, Chen W, 2012. Biosorption of cadmium by a metal-resistant filamentous fungus isolated from chicken manure compost. Environ. Technol 33 (13–15), 1661–1670. [DOI] [PubMed] [Google Scholar]
- Yu CS, Lin CJ, Hwang JK, 2004. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 13, 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CS, Chen YC, Lu CH, Hwang JK, 2006. Prediction of protein subcellular localization. Proteins 64, 643–651. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu S, Zhang L, Nian H, Chen L, 2016. Effect of aluminum stress on the expression of calmodulin and the role of calmodulin in aluminum tolerance. J. Biosci. Bioeng 122 (5), 558–562. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lin L, Chen M, Zhu Z, Yang W, Chen B, Yang X, An Q, 2012. A nonpathogenic Fusarium oxysporum strain enhances phytoextraction of heavy metals by the hyperaccumulator Sedum alfredii Hance. J. Hazard. Mater 229–230, 361–370. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ye JG, Li HB, Yang H, Ke LQ, Liang QL, 2015. Identification of early-response genes involved in cadmium resistance in shiitake mushrooms (Lentinula edodes). Mycol. Prog 14, 114. [Google Scholar]
- Zou HX, Pang QY, Zhang AQ, Lin LD, Li N, Yan XF, 2015. Excess copper induced proteomic changes in the marine brown algae Sargassum fusiforme. Ecotoxicol. Environ. Saf. 111, 271–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.