Abstract
J Clin Hypertens (Greenwich). 2011;13:621–627. ©2011 Wiley Periodicals, Inc.
Mercury has a high affinity for sulfhydryl groups, inactivating numerous enzymatic reactions, amino acids, and sulfur‐containing antioxidants (N‐acetyl‐L‐cysteine, alpha‐lipoic acid, L‐glutathione), with subsequent decreased oxidant defense and increased oxidative stress. Mercury binds to metallothionein and substitute for zinc, copper, and other trace metals, reducing the effectiveness of metalloenzymes. Mercury induces mitochondrial dysfunction with reduction in adenosine triphosphate, depletion of glutathione, and increased lipid peroxidation. Increased oxidative stress and reduced oxidative defense are common. Selenium and fish containing omega‐3 fatty acids antagonize mercury toxicity. The overall vascular effects of mercury include increased oxidative stress and inflammation, reduced oxidative defense, thrombosis, vascular smooth muscle dysfunction, endothelial dysfunction, dyslipidemia, and immune and mitochondrial dysfunction. The clinical consequences of mercury toxicity include hypertension, coronary heart disease, myocardial infarction, cardiac arrhythmias, reduced heart rate variability, increased carotid intima‐media thickness and carotid artery obstruction, cerebrovascular accident, generalized atherosclerosis, and renal dysfunction, insufficiency, and proteinuria. Pathological, biochemical, and functional medicine correlations are significant and logical. Mercury diminishes the protective effect of fish and omega‐3 fatty acids. Mercury inactivates catecholaminei‐0‐methyl transferase, which increases serum and urinary epinephrine, norepinephrine, and dopamine. This effect will increase blood pressure and may be a clinical clue to mercury‐induced heavy metal toxicity. Mercury toxicity should be evaluated in any patient with hypertension, coronary heart disease, cerebral vascular disease, cerebrovascular accident, or other vascular disease. Specific testing for acute and chronic toxicity and total body burden using hair, toenail, urine, and serum should be performed.
There is increasing concern regarding the overall health effects of chronic exposure to various heavy metals in the environment. This is particularly true of mercury and less so with other heavy metals such as cadmium, lead, aluminum, iron, and arsenic. The cardiovascular consequences of mercury toxicity have not been carefully evaluated until recently. This paper will critically review the cardiovascular consequences of mercury toxicity in humans as it relates to hypertension, generalized atherosclerosis, coronary heart disease (CHD), myocardial infarction (MI), cardiac arrhythmias, heart rate variability, sudden death, cerebrovascular accidents (CVA), carotid artery disease, renal dysfunction, and total mortality.
Types of Mercury
Mercury exists in three basic forms: elemental, inorganic, and organic (Table I). 1 , 2 , 3 , 4 , 5 Dental amalgams are the most common source for elemental mercury vapor, which is a stable monoatomic gas. Inorganic mercury, which is a divalent compound, is the toxic species found in human tissue after conversion from the other forms. Organic mercury in the form of methyl and ethyl mercury is primarily from fish, sea mammals, and thimerosal vaccines. Although dental amalgams have historically been the major source of human exposure, fish and sea mammals are becoming an increasing environment source of potential mercury toxicity. 1 , 2 , 4 , 5
Table I.
Mercury Types
| 1. Elemental | Mercury vapor (Hg°), a stable monoatomic gas | Dental amalgams |
| 2. Inorganic | Divalent mercury (Hg2+) | Toxic species in human tissue after conversion |
| 3. Organic | Methyl mercury (CH3Hg+) | Fish, sea mammals |
| Ethyl mercury (CH3CH3Hg+) | Thimersol vaccines |
Mercury Biotransformation and Biomethylation
Mercury from various sources, including elemental mercury from earth sources or inhaled mercury vapor, methyl and ethyl mercury are converted by biomethylation to inorganic divalent mercury, the toxic form in human organs and tissues (Figure 1). 4 Divalent mercury is soluble and stable in water and undergoes biomethylation to methyl mercury, which is found in high concentrations in certain fish and sea mammals. It is this source that is becoming the major source of human exposure to mercury.
Figure 1.
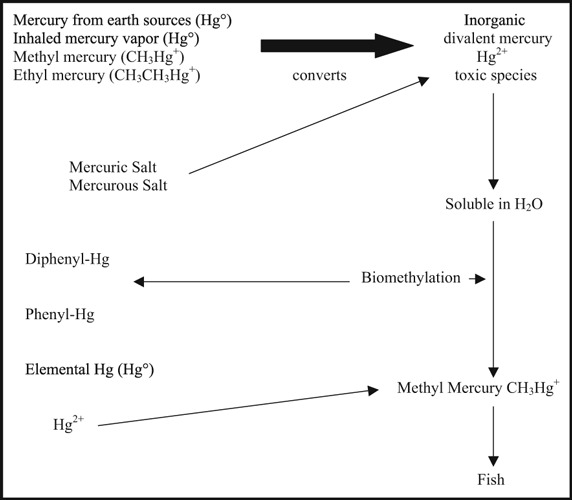
Mercury biotransformation and biomethylation.
The Environmental Protection Agency has determined the safe daily intake of mercury to be <0.1 μg/kg/d. 4 However, 12% of women have hair mercury above the level at which stopping consumption of highly contaminated fish would be advisable (1.0 μg/g). 4 It is estimated that one dental amalgam filling releases about 3 μg to 17 μg of mercury vapor per day. The typical amalgam is composed of 50% mercury, 25% silver, and 25% tin, copper, and nickel. 4 , 6 , 7 Fish and sea mammals provide about 2 μg/d to 3 μg/d depending on the type and amount consumed. 1 , 2 , 4 , 5 The long‐lived large predatory fish such as swordfish, tilefish, shark, and king mackerel contain about 1 μg of methyl mercury per gram. Pike, whale, bass, tuna, and trout are about 0.1 μg to 0.5 μg of mercury per gram. Nine vaccines that contain thimerosol (50% mercury) as a preservative would give an estimated exposure of 62 μg of organic mercury. 1 , 2 , 4 , 5 All other sources of mercury provide about 0.3 μg/d. 1 , 2 , 4 , 5
Important Facts About Mercury
Mercury is the most dangerous of all the heavy metals. 8 It will modify the distribution and retention of other heavy metals. 9 , 10 , 11 Mercury has no known physiologic role in human metabolism, and the human body has no mechanisms to actively excrete mercury. 12 Mercury thus accumulates during life so that the average 165‐lb person has a total body burden of about 13 mg of mercury. 8 Mercury has a high affinity for sulfhydryl groups, various enzymes and amino acids, N‐acetyl cysteine (NAC), alpha lipoic acid (ALA), and glutathione (GSH), which provide about 10% to 50% of the plasma protein antioxidant capacity. 8 , 12 , 13 Both NAC and ALA, as well as cysteine, are precursors for glutathione, which is the most potent intracellular antioxidant and protects against oxidative stress, inflammation, and cardiovascular disease. 3 , 4 , 5 , 8 , 9 , 12 This mercury‐induced reduction in oxidant defense and increase in oxidative stress increase the risk for CVD and CVA. Selenium antagonizes some of the adverse effects of mercury by forming a seleno‐mercury complex in tissue that is less toxic. 9 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Higher intake of selenium reduces mercury‐related CVD and CVA.
Physiologic Basis of Mercury Toxicity
Mercury induces mitochondrial dysfunction and oxidative stress. 21 , 22 The primary mitochondrial dysfunction occurs at the ubiquinone‐cytochrome B region and with NADH dehydrogenase causing displacement of Fe++ and Cu+ ions in the a3Cub center of cytochrome C (Figure 2). This results in depolarization and auto‐oxidation of the inner mitochondrial membrane with lipid peroxidation and severe mitochondrial dysfunction. Physiologic consequences include increased hydrogen peroxide, depletion of mitochondrial glutathione by more than 50%, increased lipid peroxidation markers such as TBARS by more than 70%, oxidation of pyridine nucleotides such as NAD(p)H, and altered calcium homeostasis. 21 , 22 This severe mitochondrial dysfunction increases oxidant stress and reduces oxidant defenses, which has enormous health implications.
Figure 2.
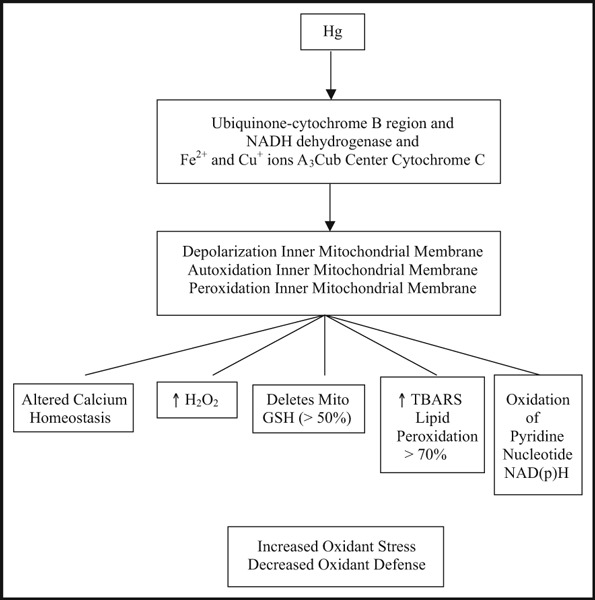
Pathophysiologic basis of mercury toxicity, mitochondrial dysfunction, and oxidative stress.
The primary three sources of mercury‐induced lipid peroxidation include the Fenton reaction, affinity for sulfhydryl groups, and selenium deficiency. 8 Mercury serves as a direct catalyst in Fenton‐type reactions and as an indirect catalyst via iron stimulation, which increases the production of radical oxygen species and superoxide anion. 8 Mercury’s high affinity for SH, such as glutathione, NAC, and ALA, which comprise much of the antioxidant capacity of plasma, reduces both membrane and plasma antioxidant defense. Finally, insoluble complexes of mercury with selenium reduces selenium availability, which is a necessary cofactor for glutathione peroxidase (GPx) activity to break down hydrogen peroxides and various other toxic peroxidation products, which further increases risk for CVD and CVA. Plasma and intracellular antioxidant capacity are both reduced. 8
Vascular Biologic Effects of Mercury
Numerous toxic effects of mercury have been demonstrated in vitro and in animal and human studies (Table II). Mercury increases free‐radical production 3 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ; inactivates antioxidant defenses 3 , 23 , 24 ; binds to thiol‐containing molecules3,23–25,30; binds to selenium forming seleno‐mercury complexes, reducing selenium availability for GPx activity 3 , 23 , 24 , 27 , 31 ; inactivates glutathione, catalase, and superoxide dismutase 25 , 26 , 27 , 30 ; increases lipid peroxidation 28 , 32 , 33 ; increases oxidation of LDL (oxLDL); and increases plasma oxLDL complexes. 8 Thrombosis is potentiated by increased platelet aggregation, 33 increases in Factor VIII, platelet factor 4, 2 , 4 and thrombin with reductions in protein C. 33 , 34 Endothelial cell formation and migration are reduced, which decreases vascular endothelial repair, decreases nitric oxide, and causes endothelial dysfunction. 35 Apoptosis is increased, 25 monocyte function and phagocytosis are impaired, 25 immune function is reduced, 25 and vascular inflammation is increased with elevations of tumor necrosis factor α and interleukin 6. 25 There is increased production and release of superoxide anion from human neutrophils and monocytes, 23 , 25 depolarization of the inner mitochondrial membrane with severe mitochondrial dysfunction, 21 , 22 and disruption of plasma membrane lipid integrity by translocation of phosphytidyl serine (PS). 25 Mercury stimulates proliferation of vascular smooth muscle cells 36 and inactivates paraoxonase, an extracellular antioxidative enzyme related to high‐density lipoprotein (HDL), CHD, and MI risk. 37 , 38 The clinical consequences of these and other pathophysiologic mechanisms explains the wide variety of cardiovascular diseases caused by mercury including CHD, MI, arrhythmias, abnormal heart rate variability, generalized atherosclerosis, sudden death, CVA, carotid artery stenosis, renal dysfunction, and hypertension. 4 , 5 , 6 , 7 , 8 , 9 , 13 , 15 , 19 , 26 , 28 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Mercury activates phospholipase A2 (PLA‐2) and induces formation of arachidonic acid metabolites such as total prostaglandins, thromboxane B2, and 8‐isoprostane in vascular endothelial cells and activates vascular endothelial cell phospholipase D. 26 , 51 , 52 , 53 , 54 Many of the cardiovascular consequences of mercury are mitigated by concomitant intake of fish containing omega‐3 fatty acids and by the intake of selenium. 8 , 45 , 46 , 47 , 48 , 49 , 50 Even very low levels of chronic mercury exposure promote endothelial dysfunction as a result of increased inflammation, oxidative stress, reduced oxidative defense, reduction in nitric oxide (NO) bioavailability, which increases the risk of CVD and CVA. 26
Table II.
Vascular Biologic Effects of Mercury
| 1. Increased free radical production and increase in oxidative stress |
| 2. Inactivation of antioxidant defenses |
| 3. Mitochondrial dysfunction |
| 4. Binds to thiol‐containing molecules (sulfhydryl groups) |
| 5. Binds to SE forming Se‐Hg complex‐mercury selenide, which decreases Se available for cofactor with glutathione peroxidase |
| 6. Inactivates glutathione, catalase, superoxide dismutase |
| 7. Increases lipid peroxidation in all organs |
| 8. Increases oxidation of low‐density lipoprotein and oxidation of low‐density lipoprotein immune complexes |
| 9. Increased platelet aggregation and thrombosis |
| 10. Increased coagulation and thrombosis: increases Factor VIII, platelet factor 4, and thrombin and reduces protein C |
| 11. Inhibit endothelial cell formation and migration and decreases endothelial repair |
| 12. Decreases nitric oxide bioavailability |
| 13. Endothelial dysfunction |
| 14. Increase apoptosis |
| 15. Reduced monocyte function and phagocytosis |
| 16. Immune function is impaired |
| 17. Increased vascular inflammation with increase tumor necrosis factor α and interleukin 6 |
| 18. Stimulation of vascular smooth muscle cells |
| 19. Inactivation of paroxonase and other high‐density lipoprotein proteins and enzymes |
| 20. Translocaion of membrane phosphytidyl serine |
| 21. Activates phospholipase A2 |
| 22. Activates phospholipase D |
In summary, the overall vascular effects of mercury include oxidative stress–decreased oxidative defense, inflammation, thrombosis, VSM proliferation and migration, endothelial dysfunction, reduced NO bioavailability, dyslipidemia, immune dysfunction, and mitochondrial dysfunction (Table III). All of these abnormalities have the potential to increase the risk for hypertension, CVD, and CVA.
Table III.
Summary of the Overall Vascular Biologic Effects of Mercury
| 1. Oxidative stress |
| 2. Inflammation |
| 3. Thrombosis |
| 4. Vascular smooth muscle proliferation and migration |
| 5. Endothelial dysfunction |
| 6. Dyslipidemia (oxidation of high‐density lipoprotein and paraxonase) |
| 7. Immune dysfunction |
| 8. Mitochondrial dysfunction |
Clinical Vascular Consequences of Mercury Toxicity
The clinical consequences of mercury toxicity include hypertension, 13 , 27 , 39 , 40 , 55 , 56 , 57 , 58 , 59 , 67 , 68 , 69 , 70 CHD, 5 , 15 , 41 , 60 , 61 MI, 19 , 39 , 41 , 60 , 61 reduction in heart rate variability, 70 increase in carotid intima‐media thickness (IMT) 69 and carotid obstruction, 13 CVA, 39 , 68 generalized atherosclerosis, 4 renal dysfunction, and proteinuria, 4 and an overall increase in total and cardiovascular mortality. 4 , 68 Gomez and colleagues 68 followed 3998 workers in mercury mines exposed to inorganic mercury from 1895 to 1994 and found a 2.78‐time increased incidence of hypertension, 1.17‐time increased risk of stroke and 1.51‐time increased risk in total cardiovascular mortality, but no increase in CHD. In a study of Faroese whaling men, both toenail and hair mercury levels were significantly associated with increased carotid IMT and hypertension. 69 Evidence from these and other epidemiologic and clinical studies suggest that people with high levels of urine, hair, blood, and toenail mercury have an increased risk of cardiovascular diseases. 5 , 8 , 50 , 56 , 59 , 60 , 67 , 68 , 69
Coronary Heart Disease and Myocardial Infarction
In rabbits exposed to inhaled mercury vapor, the cardiovascular and cardiac pathology includes bradycardia, thrombosis in small and medium caliber arteries, focal necrosis with thickening of the endocardium of the perivalvular regions, papillary muscles and valves, endothelial proliferation with inflammatory foci and focal edema, inflammation, and fibrosis of the ascending aorta. 42
In a case control study in 9 counties of 684 men with their first MI, there was a significant association of toenail mercury content, adipose tissue DHA, and first MI. 5 There was a 15% higher toenail mercury content as assessed by neutron activation analysis (NAA) in the men with their first MI compared with the control group (95% confidence interval [CI], 5–25). The risk‐adjusted odds ratio [OR] for MI was 2.16 in highest vs the lowest quintile (P=.006, 95% CI, 1.09–4.29). The adipose DHA was directly proportional to the mercury toenail content (P<.001), and the DHA content was inversely correlated to MI with an OR of 0.59 in the highest vs the lowest quintile (P=.02; 95% CI, 0.30–1.19). This important study concluded that there exists a positive monotonic increase in the risk of MI with mercury toenail content above the 0.25 μg/g level, which was even steeper when adjusted for the DHA adipose tissue content. Mercury diminishes the cardiovascular protection of fish consumption. Another study substantiated these results in which the highest quartile of DHA with the lowest quartile of mercury was associated with a 67% reduction in CHD (P<.016). 43
In another large nested case control study of 33,733 male health care professionals between the ages of 40 to 75 years (Health Professionals Follow‐Up Study), however, no association between mercury toenail content assessed by NAA and CHD was found. 9 Yet, if dentists were excluded, there was a nonsignificant correlation of toenail mercury and CHD. Also, patients with the highest tertile of mercury and the lowest serum selenium level had a significant increase in CHD.
Other human studies have shown mixed results. 6 , 8 , 39 , 40 , 44 Mercury miners showed no relationship between CHD and serum mercury levels. 27 However, another study of European mercury miners showed a significant relationship of mercury exposure to total mortality (increase 8%), hypertension (increase 46%), CHD (increase 36%), renal disease (increase 55%), and CVA (increase 36%). 39 A Finnish study found a significant relationship between hair mercury, 24‐hour urine mercury and cardiovascular events. 8 In patients with hair mercury in the highest tertile (>2.0 μg/g) and increased 24‐hour urinary mercury, CHD, and MI risk was increased 2‐fold (P=.005), cardiovascular death increased by 2.9 times (P=.014), and circulating oxLDL and immune complexes to oxLDL increased significantly (P=.01). The Gothenburg Study showed no relationship between serum mercury content and the number of amalgam fillings and CHD or MI. 6 The National Health and Nutrition Examination Survey (NHANES) from 1999 to 2002 found levels of DHA and EPA and other nutrients in fish, even with elevated mercury levels, helped to offset the risk of CHD and MI. 62 The fish intake resulted in lower levels of C‐reactive protein and higher serum HDL cholesterol as well. 62 The risk of hypertension over 10 years was highly correlated in a group of chemical factory workers exposed to mercury vapor. 63
Stroke and Carotid Atherosclerosis
High hair mercury content increases carotid IMT and carotid atherosclerosis. 13 A study of 1014 men between the ages of 42 to 60 years found an increase in mean carotid IMT over 4 years related directly to hair mercury content (P=.0007). 13 Each increase in 1 μg in hair mercury content equaled a 0.008‐mm increase in carotid IMT, a 7.3% increase over the mean. There was a 0.042 mm per 4 years in the highest quintile vs the lowest quintile, which correlated with a 32% greater increase (P<.05). In addition, mercury hair content was proportional to blood pressure (BP), fibrinogen levels, waist/hip ratio, and low HDL cholesterol (all significant at P=.0002). Many studies on the risk of fish intake, mercury, and stroke have been inconclusive. Different stroke types have often not been separated. In a population‐based cohort, mercury levels and relative content of fatty acids were determined in erythrocyte membranes in the population consuming one meal per week as fish. 64 In women, there was a nonsignificant decrease in stroke risk with increasing fish intake (OR, .90). The risk for stroke in men rose with increasing fish intake (OR, 1.24). The corresponding risk for mercury in men was 0.99 and for the sum of proportions of EPA and DHA was 1.08. This study suggested that the risk for stroke between sexes differs with increasing fish intake, EPA, and DHA consumption, but there was no association between stroke risk and mercury at these lower levels of one meal of fish per week. There are many basic mechanisms by which mercury can increase the risk for stroke as discussed earlier in this paper. The increases in both BP and pulse pressure, 13 , 39 , 40 , 44 , 55 , 56 , 57 , 58 , 59 , 60 , 67 the increased thrombotic risk related to increased platelet aggregation, 33 increase in Factor VIII, thrombin, and platelet factor 4 23 and reduction in protein C 33 , 34 as well as endothelial dysfunction from reduced NO bioavailability 35 may account for much of the observed elevation in CVA risk with mercury. One recent study showed that mercury increases thrombotic risk by enhancement of procoagulant activity in erythrocytes by protein thiol depletion–mediated phosphatidyl serine exposure and microvesical generation. 65
Hypertension
The association of mercury toxicity and hypertension in humans is convincing. 13 , 39 , 40 , 44 , 55 , 56 , 57 , 58 , 59 , 60 , 67 Mercury miners were found to have significant increases in systolic BP (P<.01) that correlated with lipid peroxidation and overall oxidative stress (P<.01). 27 European mercury miners had a 46% greater incidence of hypertension vs aged‐matched controls. Other studies have shown significant correlations with hair mercury content, hypertension, and carotid IMT. 13 In a study of 251 persons in the Brazilian Amazon, BP was significantly associated with total hair mercury levels. The OR for elevated systolic BP with total hair mercury >10 μg/g was 2.91 (1.26–7.28). 56 In 101 participants in the Wisconsin Sleep Cohort study, those in the upper quartile of blood mercury were 1.9 times more likely to be hypertensive (P=.023) and those in the upper quartile of hair mercury were 4 times more likely to be hypertensive (P=.02), but there was no change in brachial artery flow–mediated vasodilation or the middle cerebral artery reactivity to CO2. 57 In 732 Inuit adults, blood mercury level was correlated with systolic BP and pulse pressure (P=.0004) and diastolic BP (P=.069). 58 In a comparative population study, long‐term methyl mercury exposure, as measured by hair mercury levels, was associated with a risk of hypertension of 1.4 to 1.6 times in 833 patients. 59 In a sample of 1240 women aged 16 to 49 who participated in the NHANES 1999 to 2000, Vupputuri and colleagues 60 found a significant increase in systolic BP with increasing levels of blood total mercury, but only among non‐fish consumers. There was a 1.83‐mm Hg increase in systolic BP for each 1.3 μg/L increase in total blood mercury (95% CI, 0.36, 3.30; interaction P=.02).
Pederson and colleagues 67 found an increase in pulse pressure using 24‐hour ambulatory BP monitoring (54 mm Hg vs 50 mm Hg, P<.0001) that was related to blood mercury levels (ρ=.272, P<.01) in a group of Greenlanders consuming more fish than a group of Danes. Mercury is also significantly associated with reduced heart rate variability in addition to increased pulse pressure and hypertension. 70 Reduced heart rate variability may predispose to ventricular fibrillation and sudden cardiac death, as well as being associated with angina, MI, CHD, CHF, and all‐cause mortality. 70
In acute and probably chronic mercury intoxication, mercury binds to the sulfhydryl group S‐adenosyl methionine and inactivates this enzyme, which is a necessary cofactor for catecholaminei‐0‐methyl transferase, the enzyme needed to convert norepinephrine, epinephrine, and dopamine by methoxylation. 40 This results in a clinical syndrome that resembles a pheochromocytoma crisis with malignant hypertension in acute mercury intoxication and significant increases in urinary catecholamines in chronic mercury toxicity. This can be a helpful clinical clue to mercury‐induced hypertension. It would be important to measure baseline and provoked 24‐hour urine mercury levels in patients with hypertension with a history or clinical evidence of possible mercury exposure. Measurement of timed baseline and provoked urine collections for heavy metals is cost‐effective, at about $150 for most laboratories, and is reimbursed by insurance with proper coding. Mercury also induces renal dysfunction and proteinuria, which contribute to sodium retention and hypertension. 26 , 39 , 40 , 45 Studies have shown an increase in renal insufficiency in mercury miners by 55%. 39 Mercury concentrates in the renal tubules and in the glomerulus and results in proteinuria, fibrosis, chronic renal dysfunction, and renal insufficiency. 26 , 44
Summary
Mercury has a high affinity for sulfydryl groups, which inactivate numerous enzymatic reactions, amino acids, and sulfur‐containing antioxidants (NAC, ALA, GSH) with decreased oxidant defense and increased oxidative stress. Mercury binds to metallothionein and substitute for zinc, copper, and other trace metals, reducing the effectiveness of metalloenzymes. Mercury also induces mitochondrial dysfunction with reduction in ATP, depletion of glutathione, and increased lipid peroxidation. Oxidative stress and decreased oxidative defense are common (especially with mercury). Selenium and fish high in omega‐3 fatty acid content antagonize mercury toxicity. The overall vascular effects of mercury include increases in oxidative stress and inflammation, reduction in oxidative defense, thrombosis, vascular smooth muscle dysfunction, endothelial dysfunction, dyslipidemia, and immune and mitochondrial dysfunction. The clinical consequences of mercury toxicity include hypertension, CHD, MI, cardiac arrhythmias, sudden death, reduced heart rate variability, increased carotid IMT and carotid artery obstruction, CVA, generalized atherosclerosis, and renal dysfunction, insufficiency, and proteinuria. Pathological, biochemical and functional medicine correlations are significant and logical. Mercury diminishes the protective effect of fish and omega‐3 fatty acids. Mercury inactivates catecholamine‐0‐methyl transferase, which increases serum and urinary epinephrine, norepinephrine, and dopamine. This effect will increase BP and may be a clinical clue to mercury toxicity. Mercury toxicity should be evaluated in any patient with hypertension, CVD, CHD, CVA, or other vascular disease and who have a clinical history of exposure or clinical evidence on examination of mercury overload. Specific testing for acute and chronic toxicity and total body burden using hair, toenail, urine, and serum should be performed. The 24‐hour urine measurements should be done with baseline and provoked samples.
References
- 1. Keating MH, Mahaffey KR, Schoemy R, et al. Mercury Study Report to Congress. Vol. I. Executive Summary. EPA‐452/R‐97‐003. Washington, D.C.: Environmental Protection Agency, December 1997. [Google Scholar]
- 2. Committee on the Toxicological Effects of Methylmercury, Board on Environmental Studies and Toxicology, Commission on Life Sciences . Toxicological Effects of Methylmercury. Washington, D.C.: National Research Council, 2000. [Google Scholar]
- 3. Magos L. Physiology and toxicology of mercury. Met Ions Biol Syst. 1997;34:321–370. [PubMed] [Google Scholar]
- 4. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury – current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. [DOI] [PubMed] [Google Scholar]
- 5. Guallar E, Sanz‐Gallardo MI, van’t Veer P, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. [DOI] [PubMed] [Google Scholar]
- 6. Ahlqwist M, Bengtsson C, Lapidus L, et al. Serum mercury concentration in relation to survival, symptoms, and diseases: results from the prospective population study of women in Gothenburg, Sweden. Acta Odontol Scand. 1999;57:168–174. [DOI] [PubMed] [Google Scholar]
- 7. Bergdahl IA, Schutz A, Ahlqwist M, et al. Methylmercury and inorganic mercury in serum – correlation to fish consumption and dental amalgam in a cohort of women born in 1922. Environ Res. 1998;77:20–24. [DOI] [PubMed] [Google Scholar]
- 8. Salonen JT, Seppanen K, Nyyssonen K, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. [DOI] [PubMed] [Google Scholar]
- 9. Yoshizawa K, Rimm EB, Morris JS, et al. Mercury and the risk of coronary heart disease in men. N Engl J Med. 2002;347:1755–1760. [DOI] [PubMed] [Google Scholar]
- 10. Chmielnicka J, Bem EM, Kaszubski P. Organ and subcellular distribution of cadmium in rats exposed to cadmium, mercury, and selenium. Environ Res. 1983;31:266–272. [DOI] [PubMed] [Google Scholar]
- 11. Komsta‐Szumska E, Chmielnicka J. Effects of zinc, cadmium or copper on mercury distribution in rat tissues. Toxicol Lett. 1983;17:349–354. [DOI] [PubMed] [Google Scholar]
- 12. International Programme on Chemical Safety (IPCS) . Methylmercury. Environmental Health Criteria 101. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 13. Salonen JT, Seppanen K, Lakka TA, et al. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population‐based prospective 4‐year follow‐up study in men in eastern Finland. Atherosclerosis. 2000;148:265–273. [DOI] [PubMed] [Google Scholar]
- 14. Parizek J, Ostadalova I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia. 1967;23:142–143. [DOI] [PubMed] [Google Scholar]
- 15. Ganther HE, Goudie C, Sunde ML, et al. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–1124. [DOI] [PubMed] [Google Scholar]
- 16. Ganther HE, Sunde ML. Effect of tuna fish and selenium on the toxicity of methylmercury: a progress report. J Food Sci. 1974;39:1–5. [Google Scholar]
- 17. Stoewsand GS, Bache CA, Lisk DJ. Dietary selenium protection of methylmercury intoxication of Japanese quail. Bull Environ Contam Toxicol. 1974;11:152–156. [DOI] [PubMed] [Google Scholar]
- 18. Sumino K, Yamamoto R, Kitamura SA. Role of selenium against methylmercury toxicity. Nature. 1977;268:73–74. [DOI] [PubMed] [Google Scholar]
- 19. Singhal RK, Anderson ME, Meister A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987;1:220–223. [DOI] [PubMed] [Google Scholar]
- 20. Seppanen K, Kantola M, Laatikainen R, et al. Effect of supplementation with organic selenium on mercury status as measured by mercury in pubic hair. J Trace Elem Med Biol. 2000;14:84–89. [DOI] [PubMed] [Google Scholar]
- 21. Lund BO, Miller DM, Woods JS. Studies on Hg(II)‐induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem Pharmacol. 1993;45:2017–2024. [DOI] [PubMed] [Google Scholar]
- 22. Shenker BJ, Guo TL, Shapiro IM. Low‐level methylmercury exposure causes human T‐cells to undergo apoptosis: evidence of mitochondrial dysfunction. Environ Res. 1998;77:149–159. [DOI] [PubMed] [Google Scholar]
- 23. Jansson G, Harms‐Ringdahl M. Stimulating effects of mercuric‐ and silver ions on the superoxide anion production in human polymorphonuclear leukocytes. Free Radic Res Commun. 1993;18:87–98. [DOI] [PubMed] [Google Scholar]
- 24. Clarkson TW. The toxicology of mercury. Crit Rev Clin Lab Sci. 1997;34:369–403. [DOI] [PubMed] [Google Scholar]
- 25. Insug O, Datar S, Koch CJ, et al. Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology. 1997;124:211–224. [DOI] [PubMed] [Google Scholar]
- 26. Wiggers GA, Pecanha FM, Briones AM, et al. Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am J Physiol Heart Circ Physiol. 2008;295:H1033–H1043. [DOI] [PubMed] [Google Scholar]
- 27. Kobal AB, Horvat M, Prezelj M, et al. The impact of long‐term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J Trace Elem Med Biol. 2004;17:261–274. [DOI] [PubMed] [Google Scholar]
- 28. Park ST, Lim KT, Chung YT, et al. Methylmercury‐induced neurotoxicity in cerebral neuron culture is blocked by antioxidants and NMDA receptor antagonists. Neurotoxicology. 1996;17:37–45. [PubMed] [Google Scholar]
- 29. Miller DM, Lund BO, Woods JS. Reactivity of Hg(II) with superoxide: evidence for the catalytic dismutation of superoxide by Hg(II). J Biochem Toxicol. 1991;6:293–298. [DOI] [PubMed] [Google Scholar]
- 30. Naganuma A, Koyama Y, Imura N. Behavior of methylmercury in mammalian erythrocytes. Toxicol Appl Pharmacol. 1980;54:405–410. [DOI] [PubMed] [Google Scholar]
- 31. Cuvin‐Aralar ML, Furness RW. Mercury and selenium interaction: a review. Ecotoxicol Environ Saf. 1991;21:348–364. [DOI] [PubMed] [Google Scholar]
- 32. Rungby J, Ernst E. Experimentally induced lipid peroxidation after exposure to chromium, mercury or silver: interactions with carbon tetrachloride. Pharmacol Toxicol. 1992;70:205–207. [DOI] [PubMed] [Google Scholar]
- 33. Lin TH, Huang YL, Huang SF. Lipid peroxidation of rats administered with methyl mercuric chloride. Biol Trace Elem Res. 1996;54:33–41. [DOI] [PubMed] [Google Scholar]
- 34. Wierzbicki R, Prazanowski M, Michalska M, et al. Disorders in blood coagulation in humans occupationally exposed to mercuric vapors. J Trace Elem Exp Med. 2002;15:21–29. [Google Scholar]
- 35. Kishimoto T, Oguri T, Abe M, et al. Inhibitory effect of methylmercury on migration and tube formation by cultured human vascular endothelial cells. Arch Toxicol. 1995;69:357–361. [DOI] [PubMed] [Google Scholar]
- 36. Lu KP, Zhao SH, Wang DS. The stimulatory effect of heavy metal cations on proliferation of aortic smooth muscle cells. Sci China B. 1990;33:303–310. [PubMed] [Google Scholar]
- 37. Gonzalvo MC, Gil F, Hernandez AF, et al. Inhibition of paraoxonase activity in human liver microsomes by exposure to EDTA, metals and mercurials. Chem Biol Interact. 1997;105:169–179. [DOI] [PubMed] [Google Scholar]
- 38. Salonen JT, Malin R, Tuomainen TP, et al. Polymorphism in high density lipoprotein paraoxonase gene and risk of acute myocardial infarction in men: prospective nested case‐control study. BMJ. 1999;319:487–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boffetta P, Sallsten G, Garcia‐Gomez M, et al. Mortality from cardiovascular diseases and exposure to inorganic mercury. Occup Environ Med. 2001;58:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres AD, Rai AN, Hardiek ML. Mercury intoxication and arterial hypertension: report of two patients and review of the literature. Pediatrics. 2000;105:E34. [DOI] [PubMed] [Google Scholar]
- 41. Barregard L, Sallsten G, Jarvholm B. Mortality and cancer incidence in chloralkali workers exposed to inorganic mercury. Br J Ind Med. 1990;47:99–104. [PMC free article] [PubMed] [Google Scholar]
- 42. Wojciechowski J, Kowalski W. Cardiac and aortic lesions in chronic experimental poisoning with mercury vapors. Pol Med Sci Hist Bull. 1975;15:255–260. [PubMed] [Google Scholar]
- 43. Rissanen T, Voutilainen S, Nyyssonen K, et al. Fish oil‐derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Circulation. 2000;102:2677–2679. [DOI] [PubMed] [Google Scholar]
- 44. Kobal AB, Flisar Z, Miklavcic V, et al. Renal function in miners intermittently exposed to elemental mercury vapour. Arh Hig Rada Toksikol. 2000;51:369–380. [PubMed] [Google Scholar]
- 45. Connor WE. Importance of n‐3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:171S–175S. [DOI] [PubMed] [Google Scholar]
- 46. Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART). Lancet. 1989;2:757–761. [DOI] [PubMed] [Google Scholar]
- 47. GISSI‐Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico) . Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 48. Marckmann P, Gronback M. Fish consumption and coronary heart disease mortality: a systemic review of prospective cohort studies. Eur J Clin Nutr. 1999;53:585–590. [DOI] [PubMed] [Google Scholar]
- 49. MacIntosh DL, Williams PL, Hunter DJ, et al. Evaluation of a food frequency questionnaire‐food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol Biomarkers Prev. 1997;6:1043–1050. [PubMed] [Google Scholar]
- 50. Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6‐year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–497. [PubMed] [Google Scholar]
- 51. Mazerik JN, Mikkilineni H, Kuppusamy VA, et al. Mercury activates phospholipase a (2) and induces formation of arachidonic acid metabolities in vascular endolthelial cells. Toxicol Mech Methods. 2007;17:541–557. 55. [DOI] [PubMed] [Google Scholar]
- 52. Mazerik JN, Hagele T, Sherwani S, et al. Phospholipase A 2 activation regulates cytotoxicity of methylmercury in vascular endothelial cells. Int J Toxicol. 2007;26:553–569. [DOI] [PubMed] [Google Scholar]
- 53. Hagele TJ, Mazerik JN, Gregory A, et al. Mercury activates vascular endothelial cell phospholipase D through thiols and oxidative stress. Int J Toxicol. 2007;26:57–69. [DOI] [PubMed] [Google Scholar]
- 54. Peltz A, Sherwani SI, Kotha SR, et al. Calcium and calmodulin regulate mercury‐induced phospholipase D activation in vascular endothelial cells. Int J Toxicol. 2009;28:190–206. [DOI] [PubMed] [Google Scholar]
- 55. Al‐Saleh I, Shinwari N, Mashhour A, et al. Cadmium and mercury levels in Saudi women and its possible relationship with hypertension. Biol Trace Elem Res. 2006;112:13–29. [DOI] [PubMed] [Google Scholar]
- 56. Fillion M, Mergler D, Sousa Passos CJ, et al. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ Health. 2006;5:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bautista LE, Stein JH, Morgan BJ, et al. Association of blood and hair mercury with blood pressure and vascular reactivity. WMJ. 2009;108:250–252. [PMC free article] [PubMed] [Google Scholar]
- 58. Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54:981–986. [DOI] [PubMed] [Google Scholar]
- 59. Yorifugi T, Tsuda T, Kashima S, et al. Long‐term exposure to methylmercury and its effects on hypertension in Minamata. Environ Res. 2010;110:40–46. [DOI] [PubMed] [Google Scholar]
- 60. Vupputuri S, Longnecker MP, Daniels JL, et al. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res. 2005;97:195–200. [DOI] [PubMed] [Google Scholar]
- 61. Moszczynski P. Mercury and the risk of coronary heart disease. Przegl Lek. 2006;63(Suppl. 7):84–87. [PubMed] [Google Scholar]
- 62. Virtanen JK, Rissanen TH, Boutilainen S, et al. Mercury as a risk factor for cardiovascular diseases. J Nutr Biochem. 2007;18:75–85. [DOI] [PubMed] [Google Scholar]
- 63. Smith KM, Barraj LM, Kantor M, et al. Relationship between fish intake, n‐3 fatty acids, mercury and risk of CHD (National Health and Nutrition Examination Survey 1999–2002). Public Health Nutr. 2009;12:1261–1269. [DOI] [PubMed] [Google Scholar]
- 64. Skoczynska A, Jedrejko M, Martynowicz H, et al. The cardiovascular risk in chemical factory worker exposed to mercury vapor. Med Pr. 2010;61:381–391. [PubMed] [Google Scholar]
- 65. Wennberg M, Berdahl IA, Stegmayr B, et al. Fish intake, mercury, long chain n‐3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr. 2007;98:1038–1045. [DOI] [PubMed] [Google Scholar]
- 66. Lim KM, Kim S, Kim K, et al. Low‐level mercury can enhance procoagulant activity of erythrocytes: a new contributing factor for mercury‐related thrombotic disease. Environ Health Perspect. 2010;118:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pedersen EB, Jergensen ME, Pedersen MB, et al. Relationship between mercury in blood and 24‐h ambulatory blood pressure in Geenlander and Danes. Am J Hypertens. 2005;18:612–618. [DOI] [PubMed] [Google Scholar]
- 68. Garcia Gomez M, Boffetta P, Caballero K, et al. Cardiovascular mortality in mercury miners. Med Clin (Barc). 2007;128:766–771. [DOI] [PubMed] [Google Scholar]
- 69. Choi AL, Weihe P, Budtz‐Jorgensen E, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valera B, Dewailly E, Poirier P. Cardiac autonomic activity and blood pressure among Nunavik Inuit adults exposed to environmental mercury: a cross‐sectional study. Environ Health. 2008;7:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


