Abstract
J Clin Hypertens (Greenwich). 2012; 14:630–636. © 2012 Wiley Periodicals, Inc.
The possible effects of sesame oil on hemodynamics are unknown. The aim of the study was to investigate the acute and long‐term effects of sesame oil on hemodynamic responses in hypertensive men. The authors enrolled 30 hypertensive men in a two‐phase study. In the first phase, patients consumed 35 g of either sesame oil or control oil. Central and peripheral blood pressure, pulse wave velocity, augmentation index (AI), C‐reactive protein, tumor necrosis factor α, malonydealdehyde, and total antioxidant capacity (TAC) were assessed at fast and 2 hours postprandially. In the second phase, patients consumed 35 g of either sesame oil or control oil daily for 2 months. The above‐mentioned parameters were assessed before and following 15, 30, and 60 days of oil consumption. Sesame oil decreased central and peripheral diastolic pressures 1 hour postprandially (P=.006). Fifteen days of sesame oil intake decreased peripheral systolic blood pressure (P=.016) and heart rate–corrected AI75 (P=.017) and increased TAC (P=.007). This is the first study to demonstrate a favorable acute and long‐term effect of sesame oil on hemodynamics in hypertensive men. Further research is warranted to establish the potential protective role of sesame oil.
In vitro and animal studies have shown favorable effects of sesame oil consumption on cancer, blood lipid levels, 1 and arterial blood pressure (BP). 2 We were able to find only three studies in hypertensive and diabetic hypertensive humans investigating the possible effects of sesame oil on hypertension. 3 , 4 , 5 In these studies, 35 g of sesame oil consumption for 45 days reduced both systolic and diastolic peripheral BP and remarkably elevated antioxidant enzyme activity, thereby reducing oxidative stress. These results confirm animal studies suggesting a remarkable capacity of sesame oil to enhance antioxidant activity. 6 , 7 , 8 Additionally, sesame oil has also been shown to have important anti‐inflammatory effects, as its daily consumption by rats reduced levels of tumor necrosis factor α (TNF‐α) and interleukin 1 and 6. 9 , 10
To the best of our knowledge there is no previous study investigating the acute and long‐term effects of sesame oil on central‐aortic BP and indices of arterial stiffness and wave reflection, established markers of early atherosclerosis and cardiovascular risk. 11 Furthermore, the effect of sesame oil consumption on oxidative stress and inflammatory markers has not been adequately investigated in humans.
Therefore, the aim of the present study was to investigate the acute postprandial and long‐term effects of sesame oil consumption on hemodynamics, inflammatory markers, and oxidative stress in hypertensive patients.
Materials and Methods
Study Population
We recruited 30 recently diagnosed hypertensive men (mean age 52.7±10.4 years; Table I) receiving angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers plus diuretics. Patients with diabetes, coronary artery disease, metabolic syndrome, or other metabolic and renal diseases; taking medication other than those for hypertension; or consuming nutritional supplements were excluded from the study. Patients on specific diets or on diets to reduce body weight were also excluded. All patients gave their informed consent before entering the study. The study was in accordance with the Declaration of Helsinki and the protocol and process for obtaining informed consent was approved by the Alexandra University Hospital ethics committee.
Table I.
Baseline Characteristics of Study Groups
| Variables | Sesame Oil Group (n=17), Mean (SD) | Control Group (n=13), Mean (SD) | P Value |
|---|---|---|---|
| Age, y | 49.8 (8.4) | 56.8 (12) | .074 |
| BMI, kg/m2 | 27.7 (2.4) | 28.5 (2.9) | .473 |
| p‐SBP, mm Hg | 126.8 (17.9) | 132.9 (17.7) | .378 |
| p‐DBP, mm Hg | 78.3 (8.4) | 78.8 (10.8) | .873 |
| c‐SBP, mm Hg | 116.5 (17.1) | 121.4 (10.9) | .436 |
| c‐DBP, mm Hg | 79.4 (8.7) | 77.9 (6.75) | .662 |
| AI, % | 26.7 (8.3) | 28.1 (10.1) | .717 |
| AI75, % | 23.4 (6) | 25.6 (8.4) | .452 |
| PWV, m/s | 9.8 (2.8) | 8.7 (3.7) | .452 |
| CRP, μg/dL | 1.8 (1.8) | 1.9 (2.3) | .986 |
| TNF–α, pg/mL | 0.77 (1.0) | 0.31 (0.51) | .176 |
| MDA, μM | 3.06 (0.9) | 3.8 (2) | .173 |
| TAC, mmol | 0.75 (0.16) | 0.82 (0.16) | .313 |
Abbreviations: AI, augmentation index; AI75, augmentation index heart rate corrected for 75 beats per minute; BMI, body mass index; c‐DBP, central diastolic blood pressure; CRP, C‐reactive protein; c‐SBP, central systolic blood pressure; p‐DBP, peripheral diastolic blood pressure; MDA, malonyldealdehyde;p‐SBP, peripheral systolic blood pressure; PWV, pulse wave velocity; TAC, total antioxidant capacity; TNF, tumor necrosis factor.
Experimental Protocol
The present study was composed of two sequential study phases. During the first phase, patients came to the Vascular Laboratory of Alexandra Hospital, Athens, Greece, after a 12‐hour fast, 12‐hour abstinence from cigarette smoking and medication intake, and 24‐hour abstinence from alcohol and caffeinated beverages. After a 10‐minute resting period in a quiet room with a temperature controlled at 20°C to 25°C in a supine position, ultrasound measurements for assessing arterial stiffness, pulse waves, central and peripheral BP, and blood sample collection were performed. Following, patients were divided into two groups (sesame oil and control) and a light meal was consumed in less than 15 minutes. The meal used for the study was a vegetable soup (containing either 35 g of sesame oil or 35 g of olive or corn oil) and two slices of white bread. Ultrasound measurements were performed 1, 2, and 3 hours following completion of the meal and blood samples were collected 2 hours postprandially.
For the second phase of the study, the patients were divided into two groups. The first group (sesame oil) was instructed to consume 35 g of sesame oil daily for the next 60 days without other alterations in their diet. The second group (control group) was instructed to follow its regular diet. Ultrasound measurements and blood sample collection were repeated in the fasted state at patients’ first visit and after 15, 30, and 60 days from the beginning of the second phase of the study. During the first and fifth week of the study, all patients completed a 3‐day food record in order to assess their diet and to ascertain that they made no alterations, other than the sesame oil consumption.
Aortic Elastic Properties
Carotid‐femoral pulse wave velocity (PWV), an established index of aortic stiffness, is usually calculated from measurements of pulse transit time and the distance traveled between two recording sites. 12 , 13 The equation used to calculate PWV is PWV=distance (meters)/transit time (seconds). All measurements were performed using a validated noninvasive device (Complior, Artech Medical, Pantin, France), which allows online pulse wave recording and automatic calculation of PWV. 12 After the end of the test, two different pulse waves were obtained simultaneously at two sites (at the base of the neck for the common carotid and over the right femoral artery) with two transducers. The distance is defined as: (distance from the suprasternic notch to the femoral artery)−(distance from the carotid artery to the suprasternic notch).
Arterial Stiffness, Wave Reflections, and Central Pressures
Arterial stiffness and wave reflections were evaluated noninvasively by using radial artery applanation tonometry and pulse wave analysis. 14 The main principle of the method is based on: (1) the derivation of the central aortic pressure waveform from the peripheral pressure waveform, by using generalized transfer functions; and (2) the determination of central aortic pressure augmentation, 15 due to the reflected pressure waves. Tonometry of the radial artery was obtained by using a high‐fidelity strain‐gauge transducer placed on the tip of a pencil‐type tonometer (Millar Instruments Inc, Houston, TX). Analysis of the derived aortic pressure waveforms was realized by the Sphygmocor System (ATCOR Medical, Sydney, Australia). Calibration of peripheral pressure recordings was obtained by using the sphygmanometrically measured brachial BP.
Augmentation index (AIx) was used to characterize wave reflections and was calculated as the ratio of the augmentation pressure to the aortic pulse pressure. The magnitude and timing of the arterial wave reflection depend on the elasticity of the arterial walls. Applanation tonometry and pulse wave analysis have been described in detail and validated previously. 16 , 17 The stiffer the arterial system, the greater the augmentation of the central aortic pressure (increased AIx) due to the earlier return of the reflected waves at systole.
Since AIx is influenced by heart rate, which likely differs by patient and may be altered after sesame oil consumption, an index normalized for heart rate of 75 beats per minute (AI75) was used in accordance with Wilkinson and colleagues. 18
Blood Assays
Blood was aliquoted in vacutainers for serum and plasma separation and stored at 4°C for 30 minutes. It was consequently centrifuged at 3500 rpm at 4°C for 10 minutes using a bench centrifuge. The obtained serum and plasma was further aliquoted and stored at −80°C for further analysis.
Blood samples were used for assessment of C‐reactive protein (CRP), TNF‐α, malonyldealdehyde (MDA), and total antioxidant capacity (TAC).
High‐sensitivity CRP (hs‐CRP) was measured by enzyme‐linked immunosorbent assay (ESSAY) (Accubind, Monobind Inc, Lake Forest, CA) with a minimum detectable concentration of 0.2 μg/mL. Intra‐essay and inter‐assay coefficients of variation were 7.8% and 7.9%, respectively. Serum levels of TNF‐α were determined using commercially available high‐sensitivity ELISA kits (Bender Medsystems GmbH, Vienna, Austria). The optical density of each sample was determined using a microplate reader (Expert 96; AsysHitech, Salzburg, Austria). Results were calculated from a standard curve and reported accordingly in picograms per milliliter. The minimum detectable dose was 0.13 pg/mL. The intra‐assay and interassay coefficient of variations were 8.5% and 9.8%, respectively. TAC of plasma was measured by the ferric reducing ability of plasma assay, as previously described. 19 At low pH, the ferric tripyridyltriazine (Fe3+– TPTZ) complex is reduced to the ferrous (Fe2+) form; an intense blue color with an absorption maximum at 593 nm develops. The reaction was monitored for 4 minutes (Spectrophotometer U‐1800; Digilab Hitachi, Tokyo, Japan). Aqueous solutions of known Fe2+ concentrations in the range of 100 μmol/L to 1000 μmol/L were used for calibration. Lipid peroxidation was assessed by thiobarbituric acid reactive substances (TBARS) formation. TBARS was estimated by using a previously described method. 20 Briefly, 0.5 mL of serum was added to 40% trichloroacetic acid and centrifuged for 10 minutes at 2500 rpm to precipitate proteins. From this, 0.5 mL of supernatant was taken and 0.5 mL of 0.67% thiobarbituric acid was added. Similar steps were taken for the standard MDA. The tubes were covered and placed for 1 hour in a boiling water bath. The tubes were then cooled immediately in a chilled water bath and optical density was recorded at 530 nm (Spectrophotometer U‐1800). The results were calculated according to the standard graph.
Statistical Analysis
Continuous variables are presented as mean±standard deviation (SD). All continuous variables were tested for normal distribution by one‐sample Kolmogorov‐Smirnov test and normal probability (Q–Q) plots. Differences of measured variables among specific time points of the study were evaluated by grouped comparisons using independent and paired samples Student t tests (Bonferroni‐corrected P value <.05 was considered statistically significant). Statistical analyses were performed by SPSS 18 for Windows (SPSS Inc, Chicago, IL).
Results
Baseline characteristics of study groups are shown in Table I. Results from the 3‐day food records showed no differences in the first and the fifth week of the long‐term phase of the study in terms of energy, protein, carbohydrates, total fat, monounsaturated fatty acids, polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, alcohol, total fiber, soluble fiber, insoluble fiber, and caffeine consumption (data not shown).
Acute/Postprandial Effects
Peripheral and central diastolic BPs (p‐DBP and c‐DBP, respectively) were reduced 1 hour after the meal in the sesame group (P=.006 for both p‐DBP and c‐DBP) (Table II) but they did not differ during the 2nd and 3rd hour. Peripheral and central systolic BPs (p‐SBP and c‐SBP, respectively) tended to be reduced in the first hour in the sesame group, while AI, heart rate–corrected AI, PWV, CRP, TNF‐α, MDA, and TAC showed no alteration in the immediate postprandial phase following sesame consumption (Table II).
Table II.
Postprandial Effects of Sesame Oil Consumption
| Variables | Group | Baseline | 1 Hour | 2 Hours | 3 Hours | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| p‐SBP, mm Hg | C | 130 (9.7) | 130 (12.6) | 127.1 (12) | 133.8 (8.9) | .983 | .608 | .597 |
| S | 126 (17.9) | 119.7 (16) | 119.2 (19) | 124.3 (15) | .056 | .106 | .057 | |
| p‐DBP, mm Hg | C | 75.6 (6.3) | 75.5 (4.7) | 75.9 (10.7) | 78.5 (7) | .930 | .879 | .382 |
| S | 78.2 (8.7) | 73.5 (7.3) | 74.5 (6.7) | 77.5 (6.2) | .006a | .094 | .431 | |
| c‐SBP, mm Hg | C | 121.4 (11) | 118.4 (11) | 116.7 (12) | 123.1 (9.2) | .459 | .108 | .774 |
| S | 116.5 (17) | 109.7 (16) | 109.4 (17) | 115 (15.1) | .033 | .058 | .066 | |
| c‐DBP, mm Hg | C | 77.9 (6.8) | 77.2 (5.4) | 75.9 (10.6) | 79.5 (6.7) | .640 | .396 | .596 |
| S | 79.4 (8.7) | 74.6 (7.2) | 75.4 (6.7) | 78.5 (6.2) | .006a | .071 | .405 | |
| AI, % | C | 28.1 (10.2) | 26.7 (10.1) | 25.4 (8.5) | 27.7 (8.4) | .433 | .127 | .572 |
| S | 26.7 (8.3) | 24.5 (9.2) | 23.6 (8.2) | 25 (7.9) | .128 | .083 | .141 | |
| AI75, % | C | 25.6 (8.4) | 25.3 (7.7) | 22.0 (6.9) | 24.5 (7.1) | .817 | .021 | .144 |
| S | 23.4 (6) | 22.4 (5.8) | 21.2 (5.3) | 22.5 (5.3) | .362 | .132 | .340 | |
| PWV, m/s | C | 8.1 (4) | – | 9.3 (2.6) | – | – | .391 | – |
| S | 9.8 (2.9) | – | 10.6 (3.1) | – | – | .100 | – | |
| CRP, μg/dL | C | 1.8 (2.2) | – | 1.8 (2.2) | – | – | .620 | – |
| S | 1.8 (1.9) | – | 2.5 (3.5) | – | – | .270 | – | |
| TNF‐α, pg/mL | C | 0.37 (0.55) | – | 0.05 (0.14) | – | – | .146 | – |
| S | 0.68 (1) | – | 0.78 (1) | – | – | .304 | – | |
| MDA, μM | C | 4.2 (2) | – | 4.2 (0.74) | – | – | .078 | – |
| S | 3.04 (1) | – | 2.76 (0.6) | – | – | .248 | – | |
| TAC, mmol | C | 0.85 (0.16) | – | 0.81 (0.15) | – | – | .100 | – |
| S | 0.72 (0.1) | – | 0.75 (0.1) | – | – | .212 | – |
Abbreviations: AI, augmentation index; AI75, augmentation index heart rate corrected for 75 beats per minute; C, control group; c‐DBP, central diastolic blood pressure; CRP, C‐reactive protein; c‐SBP, central systolic blood pressure; MDA, malonyldealdehyde; P1, P value for comparison of baseline with 1‐hour postprandial value; P2, P value for comparison of baseline with 2‐hour postprandial value; P3, P value for comparison of baseline with 3‐hour postprandial value; p‐DBP, peripheral diastolic blood pressure; p‐SBP, peripheral systolic blood pressure; PWV, pulse wave velocity; S, sesame oil group; TAC, total antioxidant capacity; TNF, tumor necrosis factor. All variables are presented as mean (standard deviation). aIndicates a statistically significant value.
Long‐Term Effects
p‐SBP was significantly reduced 15 days after daily sesame oil consumption (P=.016) and c‐SBP tended to be reduced at the same time point (P=.020) (Figure 1). p‐DBP and c‐DBP remained unaltered during the long‐term phase of the study. There was a trend for AI to be reduced 15 days after the beginning of sesame oil supplementation (P=.021) and also a trend for PWV reduction at the last measurement at 60 days (P=.019). Heart‐corrected AI75 was significantly reduced 15 days after daily sesame oil consumption (P=.017) (Figure 2). TAC was elevated in the first 15 days of sesame oil intake (P=.007) (Table III). CRP, TNF‐α, and MDA remained unaltered during the long‐term phase of the study.
Figure 1.
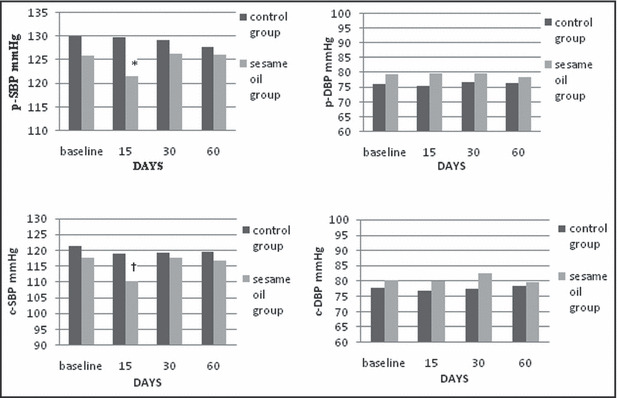
Long‐term effects of sesame oil consumption on peripheral systolic blood pressure (p‐SBP), peripheral diastolic blood pressure (p‐DBP), central systolic blood pressure (c‐SBP), and central diastolic blood pressure (c‐DBP). *Statistically significant compared with baseline values (P=.016). †Tend to be statistically significant compared with baseline values (P=.020).
Figure 2.
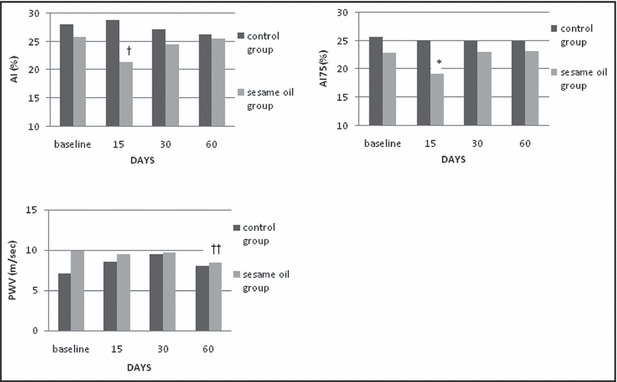
Long‐term effects of sesame oil consumption on augmentation index (AI), heart rate–corrected AI (AI75), and pulse wave velocity (PWV). *Statistically significant compared with baseline values (P=.017). †Tend to be statistically significant compared with baseline values (P=.021). ††Tend to be statistically significant compared with baseline values (P=.019).
Table III.
Long‐Term Effects of Sesame Oil Consumption
| Variables | Group | Baseline | 15 Days | 30 Days | 60 Dys | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| CRP, μg/dL | C | 1.9 (2.3) | 1.8 (1.9) | 1.4 (1.5) | 2.0 (2.5) | .987 | .276 | .650 |
| S | 1.8 (1.8) | 2.4 (2.9) | 1.5 (1.0) | 2.0 (2.3) | .065 | .972 | .176 | |
| TNF‐α, pg/mL | C | 0.31 (0.51) | 0.37 (0.63) | 0.42 (0.55) | 0.54 (0.61) | .789 | .529 | .306 |
| S | 0.77 (1.0) | 0.92 (1.5) | 0.82 (1.3) | 0.96 (1.5) | .441 | .844 | .365 | |
| MDA, μM | C | 3.8 (2.0) | 3.0 (0.52) | 2.7 (0.6) | 3.2 (0.6) | .112 | .039 | .301 |
| S | 3.06 (0.9) | 3.1 (1.1) | 3.1 (1.2) | 2.7 (0.6) | .979 | .910 | .136 | |
| TAC, mmol | C | 0.82 (0.16) | 0.81 (0.2) | 0.81 (0.2) | 0.81 (0.2) | .715 | .875 | .792 |
| S | 0.75 (0.16) | 0.83 (0.2) | 0.78 (0.2) | 0.8 (0.2) | .007a | .229 | .115 |
Abbreviations: C, control group; CRP, C‐reactive protein; MDA, malonyldealdehyde; P1, P value for comparison of baseline with 15 days; P2, P value for comparison of baseline with 30 days; P3, P value for comparison of baseline with 60 days; S, sesame oil group; TAC, total antioxidant capacity; TNF, tumor necrosis factor. All variables are presented as mean (standard deviation). aIndicates a statistically significant value.
Discussion
Sesame oil is a commonly used edible oil. Its effects on vascular function have been investigated almost exclusively in animals. To the best of our knowledge, this is the first study to examine the acute and long‐term effects of sesame oil on arterial stiffness, pulse waves, and markers of inflammation and oxidative stress. Our findings revealed that in the acute phase, 1 hour after consumption of 35 g of sesame oil, peripheral and aortic BP decreased but only diastolic pressures reached statistical significance. On the other hand, in the long‐term phase of the study, p‐SBP was reduced in the first 15 days of sesame oil consumption. At the same time point, arterial stiffness expressed as heart rate–corrected AI, was also reduced and TAC was remarkably elevated, which denotes a significant effect of sesame oil consumption on human hemodynamics and oxidation for the first 15 days of consumption.
This is the first study to show a significant favorable effect of the consumption of only 35 g of sesame oil within 1 hour. Although p‐SBP and c‐SBP were obviously reduced, only diastolic pressures reached statistical significance. Results of the few existing studies of the postprandial effects of various edible oils on BP have shown that a high‐fat meal has either no effect on central and peripheral pressures or that it reduces only peripheral BP. 21 , 22 , 23 In comparison with our findings, it seems that the type of oil used may differentially impact various indices such as central pressures. In the present study, sesame oil reduced aortic DBP, while in previous studies other types of oil had no such effect. 21 It has been shown that antioxidant substances found in sesame oil directly increase nitric oxide (NO) and decrease secretion of endothelin‐1 leading to vasodilation, 24 which may explain the observed postprandial reduction in BPs. Our results from the long‐term study are partly in accordance with the previous human studies of Sankar, 3 , 4 , 5 as there was a significant decrease in peripheral systolic pressure after 15 days of daily consumption. However, we did not find any alteration in DBP, which differs markedly from these studies. Nevertheless, nutritional “interventions” such as edible oils and their constituents, which may lower BP, deserve further investigation as potential nonpharmaceutical agents to reduce cardiovascular risk.
Discrepancies between central and peripheral BP in response to vasoactive substances have been documented previously. 15 , 25 Current practices may underestimate the effects of nutrients and drugs when assessing only peripheral pressure, as central pressures may be more sensitive and in some cases more informative than peripheral BPs. Central (carotid) pressure has been shown to be a better predictor than brachial pressure of the severity of coronary artery disease. 26 Therefore, it is essential to assess central together with peripheral pressures in order to elucidate the effect of sesame oil on hemodynamic parameters in humans.
Assessment of arterial stiffness and pulse waves revealed that heart rate–corrected AI was significantly reduced only 15 days after beginning daily sesame oil consumption. Additionally, both AI and PWV tended to be diminished, although they did not reach statistical significance. The reduction in AI could be attributed to improvement in arterial stiffness, vasodilatation, or both. In vitro studies have shown that sesamin, an important antioxidant found in sesame oil, may enhance NO production and inhibit endothelin‐1 synthesis, 24 inducing vasodilation, which explains in part our finding. Furthermore, NO as well as endothelin‐1 have been directly related not only to vasodilation but also to wave reflections, PWV, and arterial stiffness, 27 , 28 rendering NO and endothelin‐1 synthesis as major possible mechanisms involved in the AI changes observed in the present study. Additionally, the long‐term pressure effect of sesame oil consumption on hemodynamics (central and peripheral systolic pressures) could be well justified, as sesame oil decreased peripheral resistance, expressed as heart rate–corrected AI, at the same time point (15 days of daily consumption) and consequently it decreased wave reflections and left ventricular afterload, thereby decreasing systolic BP.
Furthermore, within the first 15 days of the long‐term phase of the study, there was a significant increase in TAC in the sesame group, suggesting improvement in antioxidant status. These findings are in accordance with several studies in rats showing that sesame oil may reduce oxidative stress. 29 , 30 It is possible that this favorable effect may have offered protection to NO from oxidation and inactivation, which, in turn, enhanced vasodilation and reduction of peripheral resistance and BP. However, these findings are not evident beyond 15 days of sesame oil consumption. This might be due to the relatively low dose of sesame oil used in this study or to an adjustment of the body to the effects of this type of oil. Nevertheless, future studies need to further elucidate the effects of sesame oil on hemodynamic parameters in hypertensive patients, as there are no data concerning optimal doses, timing of consumption in relation to meals, and duration of long‐term consumption.
Finally, we did not detect any alterations in TNF‐α levels in the acute or the long‐term study. Remarkable decreases in TNF‐α by sesame oil constituents have been described previously 9 , 10 in rats; however, this effect may be species‐specific.
Conclusions
Although many in vitro and animal studies have examined the effect of sesame oil in health, less is known about its effects on human hemodynamics. This is the first study that investigated acute and long‐term effects of sesame oil consumption on central and peripheral pressures and indices of arterial stiffness and pulse waves in humans. The data suggest that even 35 g of sesame oil is capable of reducing c‐DBP and p‐DBP within the first hour of its consumption. Long‐term daily sesame oil intake decreased SBP, reduced peripheral resistance, and increased the antioxidant capacity of the body. Sesame oil may prove to be a useful dietary component in our efforts to control BP and hemodynamics and to reduce cardiovascular risk.
Acknowledgments
Acknowledgments and disclosures: Supported by grants from the Sealy Center on Aging, University of Texas Medical Branch at Galveston, the Department of Nutrition and Dietetics Graduate Program, Harokopio University Athens, the Hellenic Heart Foundation, and the Haitoglou Bros SA. We are indebted to the patients for their participation in the study. The authors report that there are no conflicts of interest to disclose.
References
- 1. Hirata F, Fujita K, Ishikura Y, et al. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122:135–136. [DOI] [PubMed] [Google Scholar]
- 2. Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007;47:651–673. [DOI] [PubMed] [Google Scholar]
- 3. Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9:408–412. [DOI] [PubMed] [Google Scholar]
- 4. Sankar D, Rao MR, Sambandam G, Pugalendi KV. Effect of sesame oil on diuretics or Beta‐blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med. 2006;79:19–26. [PMC free article] [PubMed] [Google Scholar]
- 5. Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97–104. [DOI] [PubMed] [Google Scholar]
- 6. Nakano D, Itoh C, Takaoka M, et al. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol Pharm Bull. 2002;25:1247–1249. [DOI] [PubMed] [Google Scholar]
- 7. Noguchi T, Ikeda K, Sasaki Y, et al. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke‐prone spontaneously hypertensive rats. Hypertens Res. 2001;24:735–742. [DOI] [PubMed] [Google Scholar]
- 8. Noguchi T, Ikeda K, Sasaki Y, et al. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke‐prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2004;31(suppl 2):S24–S26. [DOI] [PubMed] [Google Scholar]
- 9. Hsu DZ, Chen KT, Chu PY, et al. Sesame oil protects against lead‐plus‐lipopolysaccharide‐induced acute hepatic injury. Shock. 2007;27:334–337. [DOI] [PubMed] [Google Scholar]
- 10. Hsu DZ, Su SB, Chien SP, et al. Effect of sesame oil on oxidative‐stress‐associated renal injury in endotoxemic rats: involvement of nitric oxide and proinflammatory cytokines. Shock. 2005;24:276–280. [DOI] [PubMed] [Google Scholar]
- 11. Ter Avest E, Stalenhoef AF, de Graaf J. What is the role of non‐invasive measurements of atherosclerosis in individual cardiovascular risk prediction? Clin Sci (Lond). 2007;112:507–516. [PubMed] [Google Scholar]
- 12. Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. [DOI] [PubMed] [Google Scholar]
- 13. O’Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. [DOI] [PubMed] [Google Scholar]
- 14. Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. [DOI] [PubMed] [Google Scholar]
- 15. Karatzis E, Papaioannou TG, Aznaouridis K, et al. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: should we consider monitoring central blood pressure? Int J Cardiol. 2005;98:425–430. [DOI] [PubMed] [Google Scholar]
- 16. O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525 (pt 1):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. [DOI] [PubMed] [Google Scholar]
- 20. Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fugmann A, Millgard J, Sarabi M, et al. Central and peripheral haemodynamic effects of hyperglycaemia, hyperinsulinaemia, hyperlipidaemia or a mixed meal. Clin Sci (Lond). 2003;105:715–721. [DOI] [PubMed] [Google Scholar]
- 22. Hall WL, Sanders KA, Sanders TA, Chowienczyk PJ. A high‐fat meal enriched with eicosapentaenoic acid reduces postprandial arterial stiffness measured by digital volume pulse analysis in healthy men. J Nutr. 2008;138:287–291. [DOI] [PubMed] [Google Scholar]
- 23. Masuo K, Mikami H, Ogihara T, Tuck ML. Mechanisms mediating postprandial blood pressure reduction in young and elderly subjects. Am J Hypertens. 1996;9:536–544. [DOI] [PubMed] [Google Scholar]
- 24. Lee CC, Chen PR, Lin S, et al. Sesamin induces nitric oxide and decreases endothelin‐1 production in HUVECs: possible implications for its antihypertensive effect. J Hypertens. 2004;22:2329–2338. [DOI] [PubMed] [Google Scholar]
- 25. Vlachopoulos C, Hirata K, O’Rourke MF. Pressure‐altering agents affect central aortic pressures more than is apparent from upper limb measurements in hypertensive patients: the role of arterial wave reflections. Hypertension. 2001;38:1456–1460. [DOI] [PubMed] [Google Scholar]
- 26. Waddell TK, Dart AM, Medley TL, et al. Carotid pressure is a better predictor of coronary artery disease severity than brachial pressure. Hypertension. 2001;38:927–931. [DOI] [PubMed] [Google Scholar]
- 27. Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res. 2001;51:351–358. [DOI] [PubMed] [Google Scholar]
- 28. McEniery CM, Qasem A, Schmitt M, et al. Endothelin‐1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. [DOI] [PubMed] [Google Scholar]
- 29. Hsu DZ, Chen KT, Li YH, et al. Sesamol delays mortality and attenuates hepatic injury after cecal ligation and puncture in rats: role of oxidative stress. Shock. 2006;25:528–532. [DOI] [PubMed] [Google Scholar]
- 30. Hsu DZ, Chien SP, Li YH, et al. Sesame oil attenuates hepatic lipid peroxidation by inhibiting nitric oxide and superoxide anion generation in septic rats. JPEN J Parenter Enteral Nutr. 2008;32:154–159. [DOI] [PubMed] [Google Scholar]


