Abstract
J Clin Hypertens (Greenwich). 2011;13:836–842. ©2011 Wiley Periodicals, Inc.
Blood pressure (BP) is affected by many environmental factors including ambient temperature, altitude, latitude, noise, and air pollutants. Given their pervasiveness, it is plausible that such factors may also have an impact on hypertension prevalence and control rates. Health care providers should be aware that the environment can play a significant role in altering BP. Although not among the established modifiable risk factors (eg, obesity) for hypertension, reducing exposures when pertinent should be considered to prevent or control hypertension. The authors provide a concise review of the evidence linking diverse environmental factors with BP and suggest an approach for incorporating this knowledge into clinical practice. The authors propose using the term environmental hypertensionology to refer to the study of the effects of environmental factors on BP in clinical and research settings.
In most individuals, the confluence of behavioral factors (eg, sodium intake) and an underlying polygenetic propensity predisposes to hypertension. 1 , 2 All national‐level guidelines recommend that clinicians assess patient attributes proven to impact blood pressure (BP) (eg, diet, obesity) and focus preventive efforts and therapies on these modifiable factors (eg, reduced sodium intake, weight loss) 1 , 2 prior to or in conjunction with pharmacologic therapy. While such lifestyle changes are undeniably important and effective, there has been little to no attention paid to other pertinent and potentially modifiable non–patient‐level issues that can also affect BP, such as factors encountered in the environment. Since small population‐wide increases in BP translate into serious public health burdens, 2 even modest prohypertensive actions of pervasive environmental factors could have a major impact on cardiovascular morbidity and mortality. In addition, the importance of “modifiable” environmental factors is of growing global health importance, as increases in several exposures (eg, air pollution, loud noise) accompany economic development in populous societies that also have few resources for treating established diseases such as hypertension.
We propose the term environmental hypertensionology to describe the study of the effects of environmental factors on BP in clinical practice and/or research. 3 Our objective here is not to provide a complete review of the literature demonstrating associations between the environment and BP; rather, it is to provide a concise overview to familiarize health care providers (particularly hypertension experts) with this topic (Table). Many studies are published across an array of specialty journals, thus hampering awareness (let alone a coherent understanding) of this field. To facilitate this effort succinctly and within a clinically orientated framework, citations are limited to only highly relevant papers wherein more details can be found. Finally, we offer an approach for incorporating this knowledge into clinical practice and suggest pertinent areas for further research.
Table TABLE.
Common Environmental Factors and Blood Pressure
| Factor | BP Effect | Putative Mechanisms |
|---|---|---|
| Temperature Cold Heat Nighttime BP Nighttime temperature | Most common overall effect: inverse association Colder outdoor/indoor ambient temperature related to higher BP Colder temperatures elevate BP variability and aortic pulse pressure Acute heat stress and sauna treatment lower BP Hotter days associated with higher nocturnal BP Hotter nighttime temperature associated with higher daytime BP | Direct thermoregulation‐mediated vasoconstriction HPAA and SNS activation, sodium/volume retention Impaired endothelial‐dependent vasodilatation Reverse of cold mechanisms (above) Possibly reduced sleep duration or quality |
| Season Winter | Most common overall effect: winter season related to higher BP Reduced temperature may be primarily responsible; how‐ever, winter season may have some added independent effects | Cold‐induced mechanisms likely primarily responsible; however, additional chronic alterations may play additive roles: lower vitamin D levels, reduced activity, and weight gain |
| Geography Altitude Latitude | Most common overall effect: higher altitude (>2500 m) and latitude raise BP Ascent to higher altitudes raises BP (variable interindividual responses noted) May be affected by race, acclimatization, rate of climb, or duration of exposure. Long‐term population studies are limited in ability to determine effect and show heter‐ogeneous results on chronic BP levels due to many confounding variables Higher prevalence of hypertension in higher latitudes | Altitude‐induced hypoxemia leading to chemo‐reflex activation along with compensatory responses causing increased SNS and adrenal activity. Long‐term acclimatization may lead to differing responsible responses Other associated factors such as colder temperatures and stress may also play a role. Long‐term increases in red blood cell mass may contribute Effects of lower temperatures and UV light/vitamin D levels Perhaps ancient retained evolutionary changes promoting salt/water retention that are maladaptive to the colder climate with available salt |
| Loud noises | Most common overall effect: exposure to loud noises raises BP Numerous conditions implicated (ambient, occupational, traffic, airports) | Acute SNS activation, HPAA activation Potentially sleep disruption for nocturnal noise |
| Air pollutants Ambient PM Indoor PM SHS | Most common overall effect: exposure to PM raises BP Short‐ and long‐term PM exposures related to higher BP Biomass, cooking, and personal‐level higher PM exposures raise BP SHS exposure raises BP | Acute activation of the SNS via pulmonary autonomic reflexes rapidly raises BP in minutes. Possibly PM constituents reaching the systemic vasculature and promoting vasoconstriction Chronic exposures likely alter vascular tone via endothelia dys‐function or reduced arterial compliance (reduced nitric oxide and higher endothelins) due to PM‐mediated systemic inflammation and oxidative stress Baroreceptor sensitivity may also be impaired by PM inhalation |
Abbreviations: BP, blood pressure; HPAA, hypothalamic pituitary adrenal axis; PM, particulate matter; SHS, secondhand smoke; SNS, sympathetic nervous system.
The Environment and Blood Pressure
Temperature and Season
The prohypertensive actions of exposure to cold ambient temperatures have been known for many years 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 and may largely account for reports of higher BP during winter. 11 , 12 , 13 An inverse short‐term relationship between BP and temperature has been shown to hold true for both indoor and outdoor settings across wide ranges of populations and temperatures. 5 Taken together, these observations suggest that the effects of colder temperatures are both rapid and persistent. Although the mechanisms responsible for raising BP acutely and chronically may overlap (Table), they may not be identical. Other physiologic alterations (eg, reduced vitamin D levels, weight gain, lower activity, changes in diet) could play further roles in the context of chronic seasonal effects. On the other hand, additional meteorologic factors including humidity and barometric pressure have not consistently been associated with BP. In sum, the existing evidence supports a role for colder ambient temperatures leading to higher BP levels both acutely (within 1–7 days) and chronically (between seasons).
The associations between cold exposures and higher BP have typically been based on ambient temperatures averaged over the preceding 24 hours or few days. Acknowledging that brief experimental exposures to cold have been shown to trigger BP elevations within minutes, we explored whether acute alterations (eg, 1‐hour averages) of ambient temperature measured by personal‐level monitors affect BP encountered within a 24‐hour period. Contrary to initial expectations, nighttime ambient temperatures (eg, typically while sleeping from midnight to 6 am on average) showed a positive relationship with BP during the ensuing day. Hotter nights (during the summer or winter) led to higher BP levels measured several hours later in the following afternoon. 10 We speculated that heat‐induced perturbations in sleep quality may have played a role. Previously, it was also shown that hotter daytime temperatures precede increased nocturnal BP levels. 7 These findings suggest that under certain circumstances, brief changes in ambient temperatures might alter BP in differing manners. Although intriguing, these findings require further confirmation. However, it is also important to note that the relationship between temperature and cardiovascular events has often been shown to be “U” shaped with both extreme high and low levels increasing risk. How these temperature‐related cardiovascular risk alterations relate to changes in BP (ie, whether they are causally connected) is not known and requires further research.
Loud Noise
A diverse set of loud conditions associated with modern‐day society have been implicated in raising BP, including roadway traffic, airplanes, and occupational noises. 14 , 15 , 16 , 17 , 18 , 19 Numerous studies show that brief exposure to loud noise significantly increases BP within minutes 16 , 17 and the prohypertensive response (eg, to nighttime aircraft noise) can even occur during sleep. In addition, several studies now demonstrate that living in locations that foster chronic exposure to loud noises (with the most evidence for roadways and airplanes) can increase the risk for overt hypertension. 14 , 15 , 19 Given the variety of conditions implicated and the often reported linear relationship between decibel intensity and BP response, the noise source does not appear fundamentally important. However, there is some suggestion that nighttime (ie, aircrafts) may be even more detrimental than daytime (ie, traffic) loud noise. Men may also be at greater risk for noise‐related development of hypertension. Overall, the existing evidence supports that loud noises can increase BP within minutes as well as chronically promote the development of hypertension for individuals living near significant sources.
Altitude
Fewer studies have evaluated the effects of high altitude on BP, though the published findings generally support a positive relationship. 21 , 22 , 23 , 24 , 25 Individual predisposition may be important, as the effect of altitude appears to vary both within and between individuals,20 and there may even be differences in susceptibility between races. 22 The duration of exposure to high altitude may also play a role. Individuals who have acclimatized over weeks tend to show less of an effect of altitude on BP. Nevertheless, some studies have also shown that high altitude–induced BP elevations can persist for months. 21 There are also confounding effects of lower temperature and light and the increased physical stress sometimes required to survive in more hostile environments. Although the heterogeneous published findings are difficult to completely reconcile, most evidence supports that there is an altitude effect on raising BP, with the most compelling data demonstrating an acute effect in susceptible individuals undergoing short‐term ascents above 2500 meters. 22 , 25 Some people may also be prone to chronic elevations in BP while remaining at higher altitudes, although this issue is less certain. The effects of acclimatization, exposure durations, altitude above sea level required for an effect (and potential exposure‐response relationship), and patient susceptibilities (eg, preexisting hypertension, race) require more investigation. Practical guidelines for managing cardiovascular risk factors among patients ascending to altitude have been provided elsewhere. 25
On the other hand, the literature linking long‐term high altitude exposure, such as that of populations living at higher altitudes, and the prevalence of overt hypertension (or excess cardiovascular events) is more mixed. 24 , 25 Some studies show greater while others lesser rates of hypertension at higher altitudes, which may reflect the effects of multiple confounding ecological, genetic, and lifestyle variables. Indeed, one of the largest studies shows a protective effect on coronary heart disease and stroke. 24
Latitude
Epidemiologic studies suggest that populations show a progressive increase in average BP as distance from the equator increases (ie, higher north). 26 , 27 Latitudinal relationships are complex, as they conflate the effects of geographic clusters of environmental factors (eg, colder weather, reduced UV light, differences in flora and fauna, and light‐dark cycles). 27 All of these factors may individually or collectively affect BP, but an additional intriguing aspect of the relationship of latitude and BP relates to human history. Populations migrating out of Africa had to adapt to changing local environmental conditions, initially through behavioral adjustments and ultimately via genetic adaptations. Such adaptations led to lighter skin and the bulkier body types with shorter limb lengths characteristic of populations of colder climates. When genetic variants favoring heat dissipation, initially selected in hot, arid equatorial climates, encountered the cooler, wetter higher latitude environments, novel alleles were selected and/or the frequencies of previously common alleles drifted to lower prevalence. Evidence favoring the influence of environment on genotype was reported in a recent study, which showed that the frequencies of heat‐adapted alleles in populations are associated with a distinct latitudinal gradient. Furthermore, it is hypothesized that hypertension risk may be highest in individuals who retain a high burden of ancestral alleles favoring salt/water avidity who now live in environments offering free access to salt and water. The aforementioned study also shows a relationship of heat‐adapted alleles and population prevalence of hypertension. 26 This type of environmental influence on genotype has been of particular interest for the issue of salt‐sensitive hypertension, more common in individuals of African descent now living in more northern latitudes. In addition, recent studies have demonstrated a racial difference in renal water handling in which blacks show more avid water conservation and less capacity for free water excretion than whites. Such phenotypic features are consistent with a water‐conserving genotype originating in Africa. Since volume control by the kidney seems to be a critical determinant of BP level, the allelic signatures of the latitudinal forces shaping human genetic history probably play a major role in promoting hypertension.
In addition to favoring sodium‐ and water‐avidity, the need for photoprotection by melanin in equatorial populations results in lesser vitamin D production in dark‐skinned individuals. Vitamin D level is inversely correlated with both hypertension and cardiovascular risk, and low vitamin D levels are more common in higher latitudes, during winter, and in areas of high air pollution. 27 Recent studies have identified an enrichment of genetic variants with latitudinal gradients among vitamin D–related genes, which strongly suggests that the environment has shaped the genetic suite of genes related to vitamin D metabolism. Such genes could affect BP by effects on vitamin D levels or in the cellular action of vitamin D, a pleiotropic effect of a gene affecting both vitamin D and BP regulation. 27
Air Pollutants
A comparatively recent literature illustrates that fine particulate matter (PM) air pollution can also raise BP, as we have recently reviewed elsewhere in greater detail. 28 Fine PM is most often derived from fossil fuel combustion within modern societies, but other sources such as biomass burning for heating and cooking may be important in developing regions. Numerous studies now show that a rapid increase in BP occurs following short‐term (ie, hours to days) PM exposure. Nonetheless, some studies now also suggest that this prohypertensive response might also persist chronically when individuals reside in more polluted regions. A few recent publications even demonstrate that long‐term PM exposure can promote the development of overt hypertension. On the other hand, there is little evidence that other gaseous air pollutants (eg, ozone or nitrogen dioxide) also elevate BP.
Among environmental factors that impact BP, air pollution is likely of paramount public health importance. It (involuntarily) affects the most number of people worldwide and is the 13th leading cause of global mortality (ranking 8th in high‐income nations). 28 Even low levels of PM (5–20 μg/m3), such as those found in wealthier/cleaner countries, are capable of elevating BP. In addition, the public health burden of exposure is disproportionately large among developing nations (eg, China and India) where the growth of hypertension prevalence exceeds the rest of the world (and where >75% of worldwide cardiovascular events occur). Both more ubiquitous and extreme exposures (eg, PM levels >100–150 μg/m3) occur in these regions due to a confluence of factors (eg, traffic congestion, rapid industrialization, fewer regulations). Indoor air quality is also typically worse due to continued biomass burning (still impacting roughly 3 billion people), fewer available household ventilation systems, and the prevalence of secondhand smoke (SHS).
Regarding this latter pollution exposure, we 29 , 30 and others 31 , 32 , 33 , 34 have recently added further evidence that SHS can also raise BP. Recent studies even demonstrate that SHS increases the risk of elevated home BP values and the prevalence of masked hypertension. Controlled experimental exposures 29 , 34 as well as personal particle monitoring within the free‐living environment both link SHS inhalation with acutely higher BP levels. 30 Other studies demonstrate associations with long‐term exposures at home and the workplace with elevated BP. These findings suggest that not only can brief exposures trigger acute BP elevations, but chronically inhaling SHS can increase the risk for overt hypertension. In sum, a growing body of studies demonstrates that common air pollutants (ambient outdoor PM and SHS) are globally important environmental factors capable of elevating BP.
Other Factors
Additional less common exposures shown to increase BP include persistent organic pollutants, metals (ie, lead, cadmium, mercury), and facial cold water exposure (ie, diving reflex). Lead may still be an issue but it is a less widespread problem than in the past as a result of regulations. Although not reviewed in the Table, these uncommon factors should not be entirely overlooked because they may be of importance for certain at‐risk individuals (eg, occupational exposures). Extraordinarily rare exposures shown in case reports to alter BP include moon dust inhalation (elevates BP) and zero‐gravity space flight (lowers BP). Our review did not reveal any additional environmental factor consistently shown to affect BP.
Summary of Effects
The Table summarizes the overall findings of this review and putative biological mechanisms linking environmental factors with BP alterations. It should be noted that not all publications reported similar responses. Some discrepancies can likely be explained by the limited number and small sample sizes of studies and the complexity of the relationships involved. These include differences in susceptibilities (eg, basal BP, comorbidities, medications), residual confounding among unmeasured variables, unaccounted for interactions or colinearity between factors (eg, noise and air pollution, cold and altitude, sunlight and latitude), and variations in intensity of evaluated exposures (eg, different altitudes). The durations of exposures (along with variations in adaptive responses) may also play an important role in determining the reported effects, particularly for high altitude.
Quantitative relationships have not been studied in detail and it is therefore not possible to provide reliable estimates for the absolute change in BP following a discrete level of exposure to each factor. Nevertheless, most publications report average systolic BP elevations in the range of 5 mm Hg to 15 mm Hg following plausible exposures (ie, relevant magnitudes and/or intensities) to the commonly encountered environmental factors listed in the Table. Given that these are the mean changes, certain patients have even larger elevations. The studies also support a linear exposure‐response relation for lower temperatures, higher latitudes, and louder noises—at least across the ranges evaluated. Hence, unusual or extreme exposures may produce even larger elevations in BP. Too few studies are available to draw similar conclusions for the other factors, most notably higher altitudes. PM exposure is uniquely different in this regard. There is a steep linear BP increase in response to very low levels of exposure such as occurs with outdoor ambient air pollution (5–50 μg/m3). 28 The slope of this response remains positive but levels off dramatically prior to reaching the order of magnitude of particles (500–1000 μg/m3) inhaled when exposed to SHS.
Clinical Relevance and Suggested Approach
Our review supports the contention that common environmental factors can elevate BP by a clinically relevant magnitude. It is reasonable to posit that prolonged exposures could increase the incidence of mild hypertension among disposed individuals as well as disrupt BP control in treated patients. It is also plausible that some susceptible patients may experience large and potentially dangerous acute elevations following exposures. In support of this notion, many of these factors (eg, lower temperatures and air pollutants) increase cardiovascular events, although causal linkage commensurate with the magnitude of the environmentally induced BP elevation has not been demonstrated. Other factors (eg, endothelial dysfunction, sympathetic nervous system activation) induced by these exposures may also contribute to the excess cardiovascular risk.
Given the potential importance of this issue to public health, we believe that there should be efforts to increase awareness of environmental hypertensionology among clinicians and scientists. Second, although it is hard to envision how randomized hard‐outcome trials could be undertaken, it should be feasible to develop ways to assess the role of the environmental exposures in BP control, to define the magnitude of the effects, and to seek to understand what factors predispose individuals to particularly robust responses. If reliable predictors of BP responses can be developed, proving patient‐specific education as well as recommendations for prudent steps of intervention can be considered. For the time being, the Figure offers a general approach for health care providers on when and how to incorporate this knowledge into practice.
Figure FIGURE.
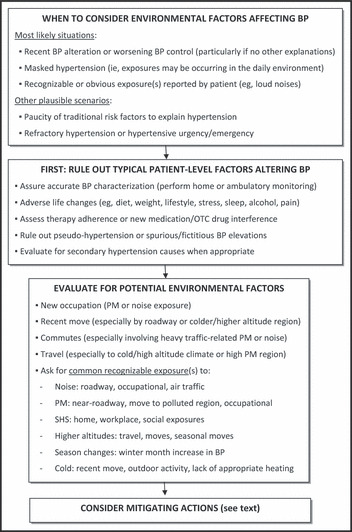
Suggested algorithm for incorporating “environmental hypertensionology” into clinical practice. BP indicates blood pressure; OTC, over‐the‐counter; PM, particulate matter; SHS, secondhand smoke.
Measures to Mitigate Environmental Stressors
Whereas completely mitigating adverse effects of some factors may require impractical major life changes (eg, moving to lower latitudes, altitudes, or local with higher ambient temperatures; seeking a new occupation; relocating to a less‐polluted city), there may be more realistic options that may still be possible for many scenarios. The following suggested behavioral changes may lead to lower BP levels and potentially even provide observable health benefits. In support of this hypothesis is that societal changes in environmental exposures have already proven effective. For example, reductions in SHS exposures via public smoking bans have proven to dramatically lower cardiovascular risk within as little as a few months among nonsmoking individuals. 35 The following suggestions should be construed as actions to consider when the situation is deemed appropriate (Figure).
Occupational exposures to noise and PM (eg, construction) can be reduced by wearing protective ear muffs and high‐efficiency facemasks when possible. Residential exposures to noise and PM can also be blunted by installing household sound proofing and air filtration systems. If adverse effects of residential exposures are deemed serious, major relocations may not be required. Moving ≥400 meters away from large roadways and airports (the most common urban sources) greatly reduces the associated exposures to both noise and air pollution. 36 Exposure to traffic‐related PM while on roadways (eg, during commutes) can also be dramatically reduced by usage of commercially available in‐cabin filtration systems. 36
Other measures might include recommendations for high‐risk patients to avoid voluntary trips to regions with extreme environments (high altitudes 25 or pollution, 36 colder weather). If travel to such locales is necessary, making sure that patients achieve adequate hypertension control prior to departure and monitoring for BP alterations during and after exposures may be prudent. In addition, it has been shown that wearing high‐efficiency particle facemasks can reduce PM inhalation when within highly polluted cities. 37
Although entirely avoiding cold temperatures in certain climates may be more difficult, it may nonetheless be possible that taking steps to reduce prolonged unnecessary or extreme exposures to outdoor cold (and if that is not possible wearing appropriate warm clothing) might be effective in reducing BP elevations. Assuring adequate household heating may also be a consideration. Regardless, it should be strongly recommended to avoid the associated seasonal adverse health changes (eg, less activity and weight gain) during colder weather. Physicians should be aware that BP is typically higher among patients during the winter and play closer attention to hypertension control during colder periods.
Detailed recommendations have been provided elsewhere in regards to preventing the adverse cardiovascular effects of higher altitudes. 25 Proper preparation, acclimatization efforts, and careful monitoring of BP are prudent actions. Returning to lower altitudes typically reverses any BP elevation. Finally, it is possible that physician‐directed medication adjustments due to BP induced by any of these environmental factors may even be required; however, the optimal pharmacologic approach remains untested and therefore must be individualized.
There are a few studies that support the efficacy of these simple interventions listed above. Reducing PM exposure by wearing a facemask lowers BP while walking near roadways in heavily polluted Beijing. 37 Even the simple measure of keeping windows closed to reduce penetrance of urban air pollution indoors or using portable household filters can effectively lower BP. 38 , 39 Since noise magnitude and temperature levels are linearly related to BP, it is rational to propose that practical steps to reduce (even if not completely eliminating) these exposures should be beneficial as well.
Future Research
Several questions, some of which were previously discussed within their respective sections, remain to be fully elucidated. The following is a list of potential areas for future research.
-
•
Evaluate for interactions among common coexposures that may have additive, synergistic, or antagonistic effects on BP (eg, noise and PM in urban settings; colder temperature and higher altitude)
-
•
Elucidate the role of confounding by common coexposures (as above). For example, how much does loud noise coexposure explain the previously presumed PM‐mediated effects on BP of living near roadways? 18 What is the role of lower vitamin D status in relation to latitude‐ or cold‐related BP elevations?
-
•
Better characterize the biological mechanisms involved in raising BP of each factor (particularly in relation to longer vs shorter durations of exposures and counter‐regulatory responses).
-
•
Evaluate the effect of these factors on hypertension control rates and hypertension prevalence in populations throughout the world.
-
•
Investigate the efficacy of practical interventions to reduce these exposures on BP.
Conclusions
Many common environmental factors can have a clinically meaningful effect on BP. Health care providers, hypertension experts in particular, should be aware of these relationships and consider recommending to their patients to reduce pertinent exposures to help control BP. Future hypertension guidelines 1 , 2 should consider addressing the importance of the environment on BP during hypertension management.
References
- 1. Mancia G, Laurent S, Agabiti‐Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Take Force document. J Hypertens. 2009;27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 2. Chobaninan AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Bhatnagar A. Environmental cardiology. Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. [DOI] [PubMed] [Google Scholar]
- 4. Alpérovitch A, Lacombe J‐M, Hanon O, et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals. The Three‐City Study. Arch Intern Med. 2009;169:75–80. [DOI] [PubMed] [Google Scholar]
- 5. Barnett AG, Sans S, Salomaa V, et al. The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12:195–203. [DOI] [PubMed] [Google Scholar]
- 6. Halonen JI, Zanobetti A, Sparrow D, et al. Relationship between outdoor temperature and blood pressure. Occup Environ Med. 2011;68:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Modesti PA, Borabito M, Bertolozzi I, et al. Weather‐related changes in 24‐hour blood pressure profile. Effects of age and implications for hypertension management. Hypertension. 2006;47:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Sun Z. Cardiovascular responses to cold exposure. Front Biosci. 2010;2:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luurila AJ, Kohvakka A, Sundberg S. Comparison of blood pressure response to heat stress in sauna in young hypertensive patients treated with atenolol and diltiazem. Am J Cardiol. 1989;64:97–99. [DOI] [PubMed] [Google Scholar]
- 10. Brook RD, Shin HH, Bard RL, et al. Can personal exposures to higher nighttime and early morning temperatures increase blood pressure? J Clin Hypertens. 2011; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nafstad MC. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO). Eur J Epidemiol. 2006;21:485–491. [DOI] [PubMed] [Google Scholar]
- 12. Woodhouse PR, Khaw K‐T, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens. 1993;11:1267–1274. [PubMed] [Google Scholar]
- 13. Al‐Tamre YY, Al‐Hayali JMT, Al‐Ramadhan EAH. Seasonality of hypertension. J Clin Hypertens. 2008;10:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarup L, Babisch W, Houthuijs D, et al. Hypertension and exposure to noise near airports: the HYENA study. Environ Health Perspect. 2008;116:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barregard L, Bonde E, Öhrström E. Risk of hypertension from exposure to road traffic noise in a population‐based sample. Occup Environ Med. 2009;66:410–415. [DOI] [PubMed] [Google Scholar]
- 16. Chang T‐Y, Lai Y‐A, Hsieh H‐H, et al. Effects of environmental noise exposure on ambulatory blood pressure in young adults. Environ Res. 2009;109:900–905. [DOI] [PubMed] [Google Scholar]
- 17. Haraladbidis AS, Dimakopoulou K, Vigna‐Taglianti F, et al. Acute effects of night‐time noise exposure on blood pressure in populations living near airports. Eur Heart J. 2008;29:658–664. [DOI] [PubMed] [Google Scholar]
- 18. Davies HW, Vlaanderen JJ, Henderson SB, et al. Correlation between co‐exposures to noise and air pollution from traffic sources. Occup Environ Med. 2009;66:347–350. [DOI] [PubMed] [Google Scholar]
- 19. Barregard L. Traffic noise and hypertension. Environ Res. 2011;111:186–187. [DOI] [PubMed] [Google Scholar]
- 20. Hasler E, Suter PM, Vetter W. Race specific altitude effects on blood pressure. J Hum Hypertens. 1997;11:435–438. [DOI] [PubMed] [Google Scholar]
- 21. Sizlan A, Ogur R, Ozer M, et al. Blood pressure changes in young male subjects exposed to a median altitude. Clin Auton Res. 2008;18:84–89. [DOI] [PubMed] [Google Scholar]
- 22. Handler J. Altitude‐related hypertension. J Clin Hypertens. 2009;11:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luks AM. Should travelers with hypertension adjust their medications when traveling to high altitude? High Altitude Med Biol. 2009;10:11–14. [DOI] [PubMed] [Google Scholar]
- 24. Faeh D, Gutzwiller F, Bopp M, et al. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120:495–501. [DOI] [PubMed] [Google Scholar]
- 25. Rimoldi SF, Sartori C, Seiler C, et al. High‐altitude exposure in patients with cardiovascular disease: risk assessment and practical recommendations. Prog Cardiovasc Dis. 2010;52:512–524. [DOI] [PubMed] [Google Scholar]
- 26. Young J, Chang Y‐PC, Kim JD‐O, et al. Differential susceptibility to hypertension is due to selection during the out‐of‐Africa expansion. PLoS Genet. 2005;1:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rostand SG. Ultraviolet light may contribute to geographic and racial pressure differences. Hypertension. 1997;30:150–156. [DOI] [PubMed] [Google Scholar]
- 28. Brook RD, Rajagopalan S. Particulate matter air pollution and blood pressure. J Am Soc Hypertens. 2009;3:332–350. [DOI] [PubMed] [Google Scholar]
- 29. Bard RL, Dvonch JT, Kaciroti N, et al. Is acute high‐dose secondhand smoke exposure always harmful to microvascular function in healthy adults? Prev Cardiol. 2010;13:175–179. [DOI] [PubMed] [Google Scholar]
- 30. Brook RD, Bard RL, Burnett RT, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. [DOI] [PubMed] [Google Scholar]
- 31. Makrisk TK, Thomapoulous C, Papadopoulous DP, et al. Association of passive smoking with masked hypertension in clinically normotensive nonsmokers. Am J Hypertens. 2009;22:853–859. [DOI] [PubMed] [Google Scholar]
- 32. Yarlioglues M, Kaya MG, Ardic I, et al. Acute effects of passive smoking on blood pressure and heart rate in healthy females. Blood Press Monit. 2010;15:251–256. [DOI] [PubMed] [Google Scholar]
- 33. Seki M, Inoue R, Ohkubo T, et al. Association of environmental tobacco smoke exposure with elevated home blood pressure in Japanese women: the Ohasma study. J Hypertens. 2010;8:1814–1820. [DOI] [PubMed] [Google Scholar]
- 34. Heiss C, Amabile N, Lee AC, et al. Brief secondhand smoke exposure depresses endothelial progenitor cell activity and endothelial function. J Am Coll Cardiol. 2008;51:1760–1771. [DOI] [PubMed] [Google Scholar]
- 35. Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke‐free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brook RD, Rajagopalan S, Pope CA III, et al. Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 37. Langrish JP, Mills NL, Chan JKK, et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part Fibre Toxicol. 2009;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin L‐Y, Lin C‐Y, Lin Y‐C, et al. The effects of indoor particles on blood pressure and heart rate among young adults in Taipei, Taiwan. Indoor Air. 2009;19:482–488. [DOI] [PubMed] [Google Scholar]
- 39. Lin LY, Chen HW, Su TL, et al. The effects of indoor particle exposure on blood pressure and heart rate among adults: an air filtration‐based intervention study. Atmospher Environ. 2001. doi: 10.1016/j.atmosenv.2011.05.014. [DOI] [Google Scholar]


