Abstract
J Clin Hypertens (Greenwich). 2012;14:250–255. ©2012 Wiley Periodicals, Inc.
The problem of medication adherence is pronounced in hypertensive black men. However, factors influencing their adherence are not well understood. This secondary analysis of the ongoing Counseling African Americans to Control Hypertension (CAATCH) randomized clinical trial investigated the patient, provider, and health care system factors associated with medication adherence among hypertensive black men. Participants (N=253) were aged 56.6±11.6 years, earned <$20,000 yearly (72.7%), and almost one half were on Medicaid (44%). Mean systolic blood pressure was 148.7±15.8 mm Hg and mean diastolic blood pressure was 92.7±9.8 mm Hg. Over one half of participants (54.9%) were nonadherent. In a hierarchical regression analysis, the patient factors that predicted medication adherence were age, self‐efficacy, and depression. The final model accounted for 32.1% of the variance (F=7.80, df 10, 165, P<.001). In conclusion, age, self‐efficacy, and depression were associated with antihypertensive medication adherence in black men followed in Community/Migrant Health Centers. Age is a characteristic that may allow clinicians to predict who may be at risk for poor medication adherence. Depression can be screened for and treated. Self‐efficacy is modifiable and its implications for practice would be the development of interventions to increase self‐efficacy in black men with hypertension.
Black men in the United States have a higher prevalence of hypertension compared with white men. 1 Although effective drug therapy is available to treat hypertension, 2 the rate of medication adherence is unacceptably low in black patients compared with whites. 3 , 4 The problem of poor adherence is even more pronounced in black men. Higher rates of medication nonadherence for black men exist, even in settings where access to care is generally equal regardless of income or race. 5 , 6 , 7 In one cross‐sectional study that examined blood pressure (BP) control differences in hypertensive male veterans using self‐report measures, Bosworth and colleagues 5 found that hypertensive black male veterans were 81% more likely to be nonadherent to their medications when compared with their white counterparts. More objective measures of adherence, such as pharmacy refill records, have also confirmed lower adherence rates for hypertensive black men. Charles and colleagues 3 reported a lower adherence rate for blacks (59.9% vs 74.1%; P<.001) in a study examining racial differences in adherence to cardiac medications among 5269 male veterans followed in a Veteran Affairs (VA) health care system. In a retrospective cohort study using two national VA databases, pharmacy refill data was significantly lower for blacks than whites among a primarily male sample. 6 Similar findings were also reported in another VA study of male veterans. 7
Research exploring black Americans’ knowledge about antihypertensive medication adherence indicates that blacks are typically able to correctly describe the health benefits associated with adherence. 8 , 9 It seems reasonable, then, that a lack of knowledge is not the primary factor responsible for the prevalence of poor medication adherence in this patient population. Instead, a complex set of patient, provider, and health care system factors may be the cause. Patient, provider, and health care system factors that appear to be associated with adherence include self‐efficacy, social support, depression, comorbidity, patient‐provider communication, and health care discrimination. 8 , 10 , 11 , 12 , 13 , 14 , 15 However, the literature examining determinants of medication adherence in hypertensive blacks is small but growing, and there is still much to learn about determinants of antihypertensive medication adherence among black men. The objective of this secondary analysis of an ongoing randomized controlled trial, Counseling African Americans to Control Hypertension (CAATCH), is to determine the patient, provider, and health care system factors associated with medication adherence among hypertensive black men.
Methods
Full details of the CAATCH study design and population have been previously published. 16 Briefly, the purpose of CAATCH is to evaluate the effectiveness of a multi‐level intervention in improving BP control among hypertensive blacks who receive care in Community/Migrant Health Centers (C/MHCs). Using a balanced design, 30 C/MHCs were randomly assigned equally to either the intervention or usual care condition. C/MHCs were matched on the size of the practices before randomization. Assessments are conducted at baseline, 2 weeks post‐baseline (visit 1), and every 3 months thereafter for 12 months (visits 2–5). The primary study end point for CAATCH, assessed at 12 months, is the proportion of patients with adequate BP control. In this secondary analysis, we assessed the patient, provider, and health care system factors associated with medication adherence among hypertensive black men.
The multi‐level intervention was composed of 3 components targeted at patients and 2 components targeted at physicians. The patient components included: (1) interactive computerized self‐paced programmed instruction for educating patients about the causes, complications, and treatment of hypertension; expected side effects of medications; and methods for adoption of lifestyle changes; (2) home BP monitoring; and (3) individual and group behavioral counseling sessions on the adoption of lifestyle modifications conducted by trained study staff, dieticians, and health educators. The physician intervention includes 2 components: (1) monthly case‐rounds with continuing medical education based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) hypertension treatment guidelines and (2) provision of feedback using continuous quality improvement process measures and provision of feedback on participants’ home BP readings.
Sample
The CAATCH study eligibility criteria included: (1) being self‐identified as African American/black and receiving care in participating C/MHC sites; (2) a diagnosis of hypertension (International Statistical Classification of Diseases and Related Health Problems: 401–401.9) on at least 2 previous clinic visits in the previous year; (3) uncontrolled BP (systolic BP [SBP] ≥140 mm Hg or diastolic BP [DBP] ≥90 mm Hg) at the last office visit and at the time of the consent visit; (4) taking at least 1 antihypertensive medication; and (5) at least 18 years of age or older. Patients were excluded if they were unable or unwilling to provide informed consent or not fluent in English. Our current secondary analysis was limited to the men in the sample.
Procedures
Institutional review board approval was obtained prior to data collection. Following consent procedures, a research assistant (RA) completed an eligibility checklist to confirm that the patient met all inclusion criteria. In order to confirm that a patient had uncontrolled hypertension, an average of 3 BP measurements were taken by a trained RA using a validated automated BP monitor BPTru device (VSM MedTech Ltd, Coquitlam, Canada). 17 Patients were seated comfortably for 5 minutes prior to each measurement in accordance with the American Heart Association guidelines for BP measurement. 18
Measures All data for this cross‐sectional study were obtained from the baseline visit prior to the inception of the intervention, thus eliminating any influence it may have had on the current study’s primary measures.
Self‐Reported Medication Adherence We assessed medication adherence via self‐report with the well‐validated scale developed by Morisky and colleagues. 19 Patients responded “yes” or “no” to the following 4 questions: (1) “Have you ever forgotten to take your BP medicine?” (2) “Are you sometimes careless in regards to your medicine?” (3) “Do you skip your medicine when you are feeling well?” and (4) “When you feel badly due to the medicine, do you skip it?” Each negative answer received a score of 0 and each positive answer received a score of 4, with total scores ranging from 0 to 4. A higher score indicated greater nonadherence. A positive answer to any question indicated a problem with adherence. The scale had good internal consistency with a Cronbach’s alpha reported of 0.90 in studies of inner‐city patients with hypertension. 20
Perceived Social Support We assessed perceived social support with the Medical Outcomes Study (MOS) Social Support Survey, a 19‐item measure of various dimensions of social support. 21 Patients rate how often they receive a particular type of support, and response options ranged from “None of the time” to “All of the time.” Items are scored from 1 (none of the time) to 5 (all of the time), and both a total score and separate subscale scores were calculated. Higher scores indicated better social support.
Provider Communication We assessed provider communication with a 13‐item measure derived from a study assessing the effect of physicians’ initial and follow‐up communication styles on the beliefs and behaviors of patients with depression. 22 We chose this measure of provider communication because it is a theoretically based instrument, using concepts from the Health Communication Model. Patients rate their perception of the quality of their providers’ communication and the extent to which the provider encourages patient participation in the treatment process. The first 11 items are based on a 4‐point Likert‐type response format ranging from not at all to very much. Examples of questions include: “To what degree was your doctor friendly during the visit” and “To what extent did your doctor ask if you had questions and concerns?” The remaining 2 questions require categorical (yes/no) responses and ask whether written information about the medication was given to patients and whether a follow‐up appointment was scheduled. These items are scored as 0 = no or 1 = yes. Given that different metrics were used for the final 2 items, each response on the 13‐item scale was converted to a z score and then summed as a continuous measure to create a composite score. Lower scores indicate more collaborative communication. Cronbach’s alpha coefficients of 0.73 indicate that the scale is internally consistent.
Self‐Efficacy Self‐efficacy was assessed with the Medication Adherence/Self‐Efficacy Scale (MASES), a 26‐item measure of patients’ confidence in their ability to take medicine on time in multiple situations. 23 Using a 4‐point Likert‐type response format ranging from not at all sure to extremely sure, participants rate their degree of confidence in taking their BP medications under a variety of situations that may pose difficulties to them. Situations include “busy at home,”“at work,”“when they cause some side effects,” and “when you do not have any symptoms.” Scores for each item are summed and averaged so that the range of possible scores is 1 to 4, with higher scores reflecting higher self‐efficacy. Cronbach’s alpha of 0.95 indicates that the MASES is internally consistent.
Perceived Discrimination Perceived discrimination was assessed with the question: How many times have you been treated unfairly by people in helping jobs (doctors, nurses, dentists, therapists, and others) because you are black? Responses ranged from 1 (not at all) to 4 (very often).
Depression Depression was assessed with the Patient Health Questionnaire Depression Module (PHQ‐9), a 9‐item instrument used to assess and diagnose depressive symptoms. 24 Items assess anhedonia; impairments in sleep, appetite, and concentration; psychomotor retardation; suicidality; low energy; and depressed mood. Responses ranged from 0 (not at all) to 3 (nearly every day), with total scores ranging from 0 to 27. A score is computed by summing the responses. Higher scores indicate greater severity of depression.
Medical Comorbidity History of comorbidity was documented using the Charlson Comorbidity Index, 25 which is a validated and widely used weighted index designed to evaluate the longitudinal risk of mortality attributable to comorbid disease. The Charlson Comorbidity Index is a 19‐item instrument that is used to calculate increased risk of mortality based on comorbid medical conditions. Categories of comorbidity include heart attack, heart failure, asthma, stroke, cancer, HIV/AIDS, and others. A weight is assigned to each category and these weights are used to calculate increased‐risk 1‐year mortality. Higher scores reflect greater likelihood of mortality.
Data Analysis
Analysis was performed using SPSS version 18 (SPSS, IBM, Armonk, NY). We described patient characteristics with frequency and percentage distributions for categorical variables. For continuous variables, values are reported as mean±standard deviation. We used hierarchical regression to determine the additional impact, if any, each sequential group of independent variables contributed to the explained variance in identifying factors associated with medication adherence. Patient demographic factors (age, insurance, and income) were entered in step one. In steps 2 through 4, we entered the patient, provider, and health care system–level factors, respectively. Separate blocks were used to facilitate identification of factors contributing most to medication adherence. A 2‐tailed P value ≤.05 was considered statistically significant.
Results
The patient flow in the study is shown in the Figure. During the study period, 9068 hypertensive patients were screened for the parent trial, of whom 7733 did not meet study criteria. Of the eligible 1335 patients, 267 were excluded for various reasons (eg, declined to participate, BP controlled at screening visit, did not have time to participate). Of the final 1039 patients enrolled into the CAATCH study, 786 patients were excluded from this cross‐sectional study because they were not men or had incomplete data. Excluded patients did not differ significantly from patients in the final sample in terms of age, insurance status, and income.
Figure FIGURE.
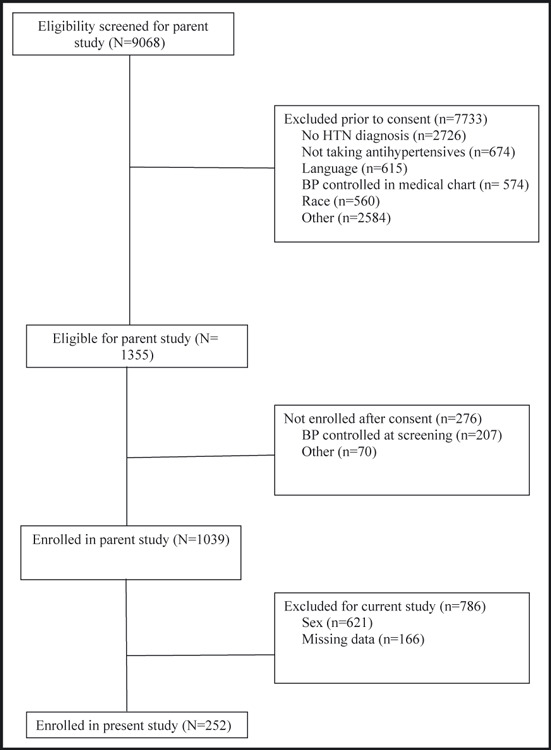
Patient flow through the study. HTN indicates hypertension; BP, blood pressure.
Patient demographics are shown in Table I. Male patients had a mean age of 56.6±11.6 years, were low‐income, and almost one half had Medicaid (44%). Mean SPB and DBP were 148.7±15.8 mm Hg and 92.7±9.8 mm Hg, respectively. Over one half (54.9%) of participants were categorized as nonadherent and reported a mean medication adherence score of 1.06. Most of the male patients (61.7%) reported that they did not perceive health care discrimination.
Table I.
Patient Characteristics (N=253)
| Characteristic | No. (%) | Mean (Standard Deviation) | Range |
|---|---|---|---|
| Age, y | 56.6 (11.6) | 24–90 | |
| Sex | |||
| Male | 252 (100) | ||
| Yearly income, $ | |||
| <10,000 | 116 (45.8) | ||
| 10,001–20,000 | 68 (26.9) | ||
| >20,000 | 60 (23.7) | ||
| Missing | 9 (3.6) | ||
| Insurance | |||
| None | 27 (10.8) | ||
| Private | 38 (15.2) | ||
| Medicare | 64 (25.6) | ||
| Medicaid | 110 (44.0) | ||
| HMO | 10 (4.0) | ||
| Missing | 4 (0.4) | ||
| Blood pressure | |||
| Systolic blood pressure | 148.7 (15.8) | 119–226 | |
| Diastolic blood pressure | 92.7 (9.8) | 66–128 | |
| Adherence | |||
| Yes | 113 (44.7) | ||
| No | 139 (54.9) | ||
| Missing | 1 (0.4) | ||
| Patient measures | |||
| Adherence score | 1.06 (1.2) | 0–4 | |
| Social support | 67.4 (23.6) | 0–100 | |
| Provider communication | 19.5 (7.9) | 10–44 | |
| Self‐efficacy | 2.2 (0.6) | 0.8–3.0 | |
| Depression | 4.6 (4.4) | 0–27 | |
| Medical comorbidity | 2.31 (2.5) | 0–20 | |
| Perceived discrimination | |||
| Never | 161 (61.7) | ||
| Occasionally | 29 (11.1) | ||
| Often | 2 (0.8) | ||
| Very often | 7 (2.7) | ||
| Missing | 64 (23.7) | ||
Factors Associated With Medication Adherence
In step one, patient demographics explained 7.0% of the variance in medication adherence for the men (F=3.24, df 4, 171, P=0.01). In the second step, when patient‐level factors were added to the model, another 24.7% of the variance was explained (F=9.70, df 8, 167, P<.001). In the third step, when provider‐level factors were added to the model, only an additional 0.1% of the variance was explained (F=8.63, df 9,166, P<.001). In the final step, when health care system–level factors were added, another 0.2% of the variance was explained (F=7.80, df 10,165, P<.001). The final model accounted for 32.1% of the variance and was significant (F=7.80, df 10,165, P<.001), although all of the variance was explained by patient‐level factors. Table II shows the full regression model. Patient‐level factors that significantly predicted medication adherence were age, self‐efficacy, and depression.
Table II.
Final Model for Explaining Medication Adherence in Hypertensive Black Men (N=253)
| Variables | Standard Beta | Standard Error | β | t | ρ |
|---|---|---|---|---|---|
| Age | −0.157 | 0.007 | −0.016 | −2.301 | 0.023 |
| Insurance | 0.014 | 0.075 | 0.016 | 0.210 | 0.834 |
| Income | 0.061 | 0.086 | 0.078 | 0.913 | 0.363 |
| Social support | 0.033 | 0.003 | 0.002 | 0.493 | 0.623 |
| Self‐efficacy | −0.458 | 0.143 | −0.975 | −6.833 | <0.000 |
| Communication | −0.046 | 0.010 | −0.007 | −0.690 | 0.491 |
| Perceived discrimination | 0.049 | 0.120 | 0.090 | 0.748 | 0.456 |
| Depression | 0.149 | 0.019 | 0.041 | 2.096 | 0.038 |
| Comorbidity | 0.079 | 0.032 | 0.039 | 1.201 | 0.231 |
Discussion
Our model of patient, provider, and health care system–level factors explained a significant proportion of the variance in medication adherence among hypertensive black men, all of which was captured by patient‐level factors. The patient‐level factors that were significant included self‐efficacy, depression, and age. Patients who exhibited higher self‐efficacy were more likely to report that they were adherent to their antihypertensive medication. Patients with depressive symptoms were more likely to report worse medication adherence. Both of these findings are consistent with other studies among hypertensive blacks. 10 , 11 , 12 , 14 , 15 For example, a cross‐sectional study examining social, clinical, and demographic factors associated with medication adherence in 70 urban hypertensive blacks found that self‐efficacy was significantly correlated with antihypertensive medication adherence. 10 A longitudinal study of 167 hypertensive blacks found that both self‐efficacy and depression were significantly associated with medication adherence. 15 Other investigators have also documented associations between depression and medication adherence in other cross‐sectional studies with hypertensive blacks. 11 , 14 These findings suggest that self‐efficacy and depression are important factors for both black men and women.
We also found that age was a significant predictor of medication adherence in our patient sample of hypertensive black men. Younger men were more likely to be nonadherent to their antihypertensive medication. Previous studies have shown inconsistent results, with some studies finding an association between age and antihypertensive medication adherence and a trend toward younger hypertensive blacks having worse adherence rates 14 , 20 , 26 , 27 and others finding no association. 10 , 12 , 15 , 28 , 29
Patient‐level factors, such as insurance status, income, social support, and comorbidity were not associated with medication adherence among the hypertensive black men in our study. Previous research examining these factors has yielded inconsistent findings, with most studies finding no association among these variables and medication adherence in hypertensive blacks. 10 , 14 , 15 , 20 , 27 , 28 , 29
Perceived health care discrimination was not associated with medication adherence in our study. However, the majority of the sample reported that they did not perceive health care discrimination. Provider communication was also not associated with medication adherence in our patient population. Yet, prior studies have found that provider communication was significantly associated with medication adherence in hypertensive patients. 10 , 14 , 29 Specifically, patients with providers who are empathetic, nonjudgmental, and collaborative are more likely to adhere to their antihypertensive medication. It is important to note that most patients in our study did not have a regular health care provider and their responses may be a result of discontinuous health care as opposed to poor communication. As such, the provider communication scores may reflect patient beliefs about the health care system rather than the actual patient‐provider dialogue. In addition to subjective assessments, we recommend that future studies measure clinical encounters objectively among hypertensive black men. We also recommend that future studies examine the roles patient and physician characteristics may contribute to hypertensive black men’s perceptions of their providers’ communication.
Limitations
Several limitations of our study should be taken into consideration. Our sample consisted of low‐income hypertensive black men. Most of the men were on Medicaid and earned <$20,000 per year. Thus, our results may not be generalizable to other populations. Future research studies should examine factors associated with medication adherence in hypertensive blacks with diverse socioeconomic backgrounds. We also assessed medication adherence with a self‐report questionnaire, and therefore patients may have underestimated or overestimated their rates of adherence. Our sample’s reported nonadherence rate of more than 50%, however, is similar to the nonadherence rates reported by the World Health Organization. 30 We recommend that future research studies measure medication adherence objectively. Finally, the cross‐sectional nature of our study makes it difficult to determine causality.
Conclusions
Our study findings may be clinically useful for providing health care to hypertensive black men. Age was associated with medication adherence, and while it is not modifiable, it is a characteristic that will allow clinicians to predict who may be at risk for medication nonadherence. Depression, another patient‐level factor associated with antihypertensive medication, is common in patients with chronic diseases and is often undertreated. 11 , 31 For clinicians who encounter patients with poor medication adherence, it is important to consider screening them for depression and treating, if necessary. Moreover, medical institutions should increase efforts to train primary care physicians in identifying and understanding the consequences of comorbid depression among individuals with chronic diseases. Finally, self‐efficacy is a characteristic that is modifiable. Implications for practice would be the development of interventions to increase self‐efficacy in hypertensive black men and their confidence in their ability to actively participate in their health care.
Acknowledgments
Acknowledgment: This research was supported by NIH/NHLBI 1 R01 HL078566‐01 and NIH/NCMHD P60MD003421.
References
- 1. Centers for Disease Control and Prevention . A Closer Look at African American Men and High Blood Pressure Control: A Review of Psychosocial Factors and Systems‐Level Interventions. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000.[see comment]. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 3. Charles H, Good CB, Hanusa BH, et al. Racial differences in adherence to cardiac medications. J Natl Med Assoc. 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- 4. Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med. 59‐62 passim, 2002 Oct 2002;112:51–52. [DOI] [PubMed] [Google Scholar]
- 5. Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70.e9–70.e15 [DOI] [PubMed] [Google Scholar]
- 6. Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother. 2009;43:185–193. [DOI] [PubMed] [Google Scholar]
- 7. Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. [DOI] [PubMed] [Google Scholar]
- 8. Lewis LM, Askie P, Randleman S, Shelton‐Dunston B. Medication adherence beliefs of community‐dwelling hypertensive African Americans. J Cardiovasc Nurs. 2010;25:199–206. [DOI] [PubMed] [Google Scholar]
- 9. Martins D, Devang G, Teklehaimanot S, Norris K. High blood pressure knowledge in an urban African‐American community. Ethn Dis. 2001;11:90–96. [PubMed] [Google Scholar]
- 10. Braverman J, Dedier J. Predictors of medication adherence for African American patients diagnosed with hypertension. Ethn Dis. 2009;19:396–400. [PubMed] [Google Scholar]
- 11. Kim MT, Han H‐R, Hill MN, et al. Depression, substance use, adherence behaviors, and blood pressure in urban hypertensive black men. Ann Behav Med. 2003;26:24–31. [DOI] [PubMed] [Google Scholar]
- 12. Krousel‐Wood MA, Muntner P, Joyce CJ, et al. Adverse effects of complementary and alternative medicine on antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc. 2010;58:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogedegbe G, Harrison M, Robbins L, et al. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study.[see comment]. Ethn Dis. 2004;14:3–12. [PubMed] [Google Scholar]
- 14. Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoenthaler A, Ogedegbe G, Allegrante JP. Self‐efficacy mediates the relationship between depressive symptoms and medication adherence among hypertensive African Americans. Health Educ Behav. 2007;36:127–137. [DOI] [PubMed] [Google Scholar]
- 16. Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to control hypertension (CAATCH) trial: a multi‐level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes. 2009;2:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wright JM, Mattu HS, Perry TLJ, et al. Validation of a new algorithm for BPM‐100 electronic oscillometric office blood pressure monitor. Blood Press Monit. 2001;6:161–165. [DOI] [PubMed] [Google Scholar]
- 18. American Heart Association . Blood pressure testing and measurement. 2006; www.americanheart.org. Accessed January 23, 2006.
- 19. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 20. Shea S, Misera D, Ehrlich MH, et al. Correlates of nonadherence to hypertension treatment in an inner‐city minority population. Am J Public Health. 1992;82:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
- 22. Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns. 2000;40:173–185. [DOI] [PubMed] [Google Scholar]
- 23. Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME. Development and evaluation of a medication adherence self‐efficacy scale in hypertensive African‐American patients. J Clin Epidemiol. 2003;56:520–529. [DOI] [PubMed] [Google Scholar]
- 24. Lincoln NB, Nicholl CR, Flannaghan T, et al. The validity of questionnaire measures for assessing depression after stroke. Clin Rehabil. 2003;17:840–846. [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 26. Hekler EB, Lambert J, Leventhal E, et al. Commonsense illness beliefs, adherence behaviors, and hypertension control among African Americans. J Behav Med 2008;31:391–400. [DOI] [PubMed] [Google Scholar]
- 27. Lagu T, Weiner MG, Eachus S, et al. Effect of patient comorbidities on filling of antihypertensive prescriptions. Am J Manag Care. 2009;15:24–30. [PubMed] [Google Scholar]
- 28. Hill MN, Bone LR, Kim MT, et al. Barriers to hypertension care and control in young urban black men.[see comment]. Am J Hypertens. 1999;12(1O Pt 1): 951–958. [DOI] [PubMed] [Google Scholar]
- 29. Turner BJ, Hollenbeak C, Weiner MG, et al. Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharmacoepidemiol Drug Saf. 2009;18:672–681. [DOI] [PubMed] [Google Scholar]
- 30. Sabate E. Adherence to Long‐Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 31. Bogner HR, Cary MS, Bruce ML, et al. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am J Geriatr Psychiatry. 2005;13:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]


