Abstract
J Clin Hypertens (Greenwich). 2011;13:710–715. ©2011 Wiley Periodicals, Inc.
The authors investigated whether high‐density lipoprotein (HDL) cholesterol plays a role in arterial stiffening and left diastolic dysfunction in essential hypertension. Carotid arterial stiffness parameter and left ventricular (LV) diastolic function index were evaluated in 217 patients with essential hypertension. The correlations of dyslipidemia, especially low HDL cholesterol, to LV diastolic function and arterial stiffness were investigated in these patients. Arterial stiffness parameter increased with the increasing of E/Em (LV diastolic function index: the ratio of transmitral peak velocity of early filling to peak early diastolic motion velocity of mitral annulus) (r=0.26, P<.01). In univariate regression analysis, HDL cholesterol was inversely associated with arterial stiffness parameter and E/Em (r=−0.23 and r=−0.27, respectively, P<.01). The association of HDL cholesterol with arterial stiffness and LV diastolic function was observed in both men and women. Triglycerides were weakly correlated with arterial stiffness parameter and E/Em, while low‐density lipoprotein and total cholesterol were not. In multiple regression analysis, only low HDL cholesterol was found as an independent predictor for both arterial stiffness and LV diastolic dysfunction. Enhanced arterial stiffness is associated with LV diastolic dysfunction. Low HDL cholesterol may lead to the deterioration of both arterial stiffness and LV diastolic function in patients with essential hypertension.
Both left ventricular (LV) diastolic dysfunction and arterial stiffening, which are common cardiovascular consequences of hypertension, are independent risk factors for cardiovascular morbidity and mortality. 1 , 2
Many well‐known risk factors for diastolic dysfunction, such as hypertension, advanced age, and atherosclerosis, are also associated with an increase in arterial stiffness. 3 These could prompt an etiologically homologous disease of artery and myocardium. Some studies have defined the association between arterial stiffness and ventricular structural or functional effects. 4 , 5 However, the common mechanism of ventricular‐vascular stiffness is still unclear.
While abnormalities in glucose and insulin metabolism have been found to accelerate the deterioration of arterial stiffness and LV diastolic function, 6 , 7 dyslipidemia is also the metabolic abnormality observed most frequently in hypertensive patients. However, the influence of serum lipids on both arterial stiffness and ischemia‐independent cardiac functional changes is still not fully elucidated. 8 In the presented study, the major aim was to investigate the correlation of dyslipidemia, especially low high‐density lipoprotein (HDL) cholesterol, to arterial stiffness and LV diastolic function in patients with essential hypertension.
Methods
Study Population
We conducted a health survey in the Shijingshan community, Beijing, China, where inhabitants were homogeneous Chinese. This survey was funded by the National Natural Science Foundation of China. All patients had signed the consent for the use of collected data without disclosure of personal identity. The study protocol was approved by the local research ethics committee of PLA General Hospital, Beijing, China.
Of the target population, 217 hypertensive patients, who did not receive medication for hypertension, were enrolled at random from the Shijingshan community. Characteristics of the study population are shown in Table I.
Table I.
Clinical Characteristics, Arterial Stiffness, and LV Diastolic Function of the Study Patients
| Characteristic | All (N=217) | Men (n=112) | Women (n=105) |
|---|---|---|---|
| Age, y | 61±12 | 60±10 | 62±13 |
| Body mass index, kg/m2 | 24.7±3.3 | 24.9±2.8 | 24.3±3.8 |
| Systolic blood pressure, mm Hg | 143±16 | 144±15 | 142±13 |
| Diastolic blood pressure, mm Hg | 81±8 | 83±10 | 80±11 |
| Total cholesterol, mmol/L | 5.23±0.88 | 5.16±0.75 | 5.37±0.77a |
| HDL cholesterol, mmol/L | 1.34±0.38 | 1.29±0.27 | 1.42±0.32a |
| LDL cholesterol, mmol/L | 3.30±0.71 | 3.29±0.65 | 3.32±0.61 |
| Triglycerides, mmol/L | 1.38±0.39 | 1.47±0.38 | 1.36±0.37a |
| Fasting plasma glucose, mmol/L | 5.42±0.80 | 5.40±0.76 | 5.43±0.69 |
| E/Em | 10.2±5.75 | 9.8±6.28 | 10.6±3.17 |
| Em/Am | 0.71±0.18 | 0.72±0.16 | 0.68±0.19 |
| Arterial stiffness parameter | 10.8±5.69 | 11.2±4.88 | 9.89±3.43 |
Abbreviations: Em/Am, the ratio of peak early diastolic motion velocity to peak later diastolic motion velocity of mitral annulus (Em to Am ratio); E/Em, the ratio of transmitral peak velocity of early filling to peak early diastolic motion velocity of mitral annulus (E to Em ratio); HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LV, left ventricular. Data are presented as means±standard deviation. a P<.05, vs men.
Patients with secondary hypertension, coronary heart disease, valvular heart disease, atrial fibrillation, congestive heart failure, renal failure (serum creatinine ≥133 μmol/L [1.5 mg/dL]), overt diabetes mellitus, or unsatisfactory echocardiographic finding were excluded from this study. Those treated with lipid‐lowering drugs were also excluded from the study. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg and/or diastolic BP ≥90 mm Hg by repeated measurements.
Arterial Evaluation
The arterial stiffness parameter β on the right carotid artery was evaluated by an ultrasound echo‐tracking system (Aloka α‐10, Tokyo, Japan) with a 7.5‐MHz linear array probe. Stiffness parameter was calculated according to the formula: β=ln [(P s − P d)]/[(D s − D d)/D d], where P s and P d were the systolic and diastolic BP in the brachial artery, respectively. The average of 3 measured systolic and diastolic BPs determined by an automated sphygmomanometer (Omron 705CP; Omron, Kyoto, Japan) was used for analysis. D s and D d are the maximal and minimal diameters of the common carotid artery, respectively. The average of the measured Ds and Dd during 5 cardiac cycles by ultrasonic high‐resolution wall tracking was used for analysis. The collecting image of arterial stiffness measured by echo‐tracking technology on carotid ultrasonography is shown in the Figure.
Figure FIGURE.
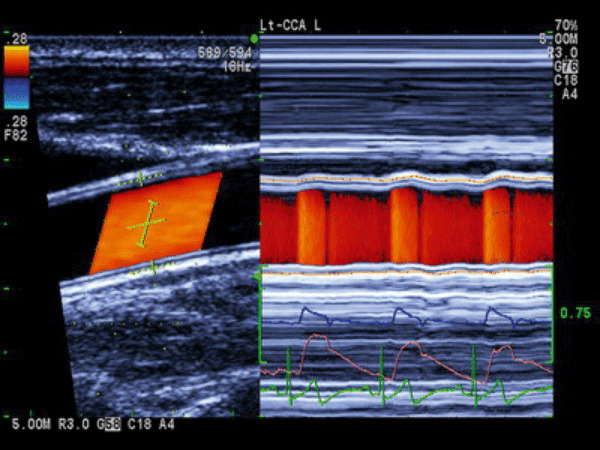
The collecting image of arterial stiffness measured by echo‐tracking technology on carotid ultrasonography. Adjustable gates were positioned at the junctions of the intima and media, and diameter was calculated and displayed in real time as the difference between the displacement waveforms of the anterior and posterior walls.
Echocardiographic Examination
Comprehensive 2‐dimensional and tissue Doppler echocardiography were performed using a cardiac ultrasound unit (Aloka α‐10) with a 1.5‐ to 2.5‐MHz transducer. Standard views and techniques were used according to the guidelines of the American Society of Echocardiography (ASE). 9
Echocardiographic parameters were measured by the consensus of 2 experienced investigators who were blinded to the metabolic data of the patients. To assess LV diastolic function, the diastolic filling of LV (LV inflow) was examined using Doppler echocardiography. The peak velocity of the early diastolic filling wave (E wave) was recorded. Tissue imaging was used to derive peak early diastolic motion velocity (Em) and peak atrial systolic motion velocity (Am) at the 4 margins of the mitral annulus. The LV diastolic filling pattern was obtained with the sample volume at the tips of the mitral valve in the apical 4‐chamber view and recorded at the end‐expiratory phase during quiet breathing. The average values for Em and Am were calculated from 4 margins of the mitral annulus. E/Em and Em/Am were determined as indirect indices of LV filling pressure. 10
Biochemistry Parameters Measurements
Blood biochemistry parameters were determined using a Cobas e 601 analyzer (Roche Diagnostics, Basel, Switzerland). Blood samples were obtained in the morning after overnight (>12‐hour) fasting. Fasting plasma glucose, serum total cholesterol, serum HDL cholesterol, and serum triglycerides were determined by standard laboratory measurements. Serum low‐density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. 11
Statistical Analysis
Data were present as mean±standard deviation or percentage. Unpaired t test was used for comparison between groups. The significance of differences among ≥3 groups was evaluated by an unpaired analysis of variance. Age, sex, body mass index (BMI), smoking, and heart rate were used as covariates in the adjusted analysis. Relationships between variables were analyzed by the univariate linear regression and Pearson’s correlation coefficient. Stepwise multiple regression analysis was performed to identify independent predictors of arterial stiffness and LV diastolic function. Age, sex, BMI, smoking, systolic and diastolic BP, fasting plasma glucose, triglycerides and total, LDL, and HDL cholesterol were included as potential independent variables. Arterial stiffness, LV mass index, and heart rate were added as independent variables in the analysis of diastolic function. All statistical analysis was performed with SPSS 13.0 statistical software package (SPSS Inc, Chicago, IL). A P value <.05 was considered statistically significant.
Results
Clinical Characteristics, Arterial Stiffness, and LV Diastolic Function of the Patients
There were no significant differences between men and women in age, BMI, systolic BP, diastolic BP, heart rate, and fasting plasma glucose (Table I).
Serum levels of total and HDL cholesterol were higher in women compared with men, while that of triglycerides was lower in women. No significant difference was observed in the E/Em ratio, Em/Am ratio, indices of LV diastolic function, and arterial stiffness parameter between sexes.
Univariate Correlation of Lipid Levels With Arterial Stiffness and LV Diastolic Function
Arterial stiffness parameter increased with the increasing of E/Em (r=0.26, P<.01) and Em/Am (r=−0.42, P<.01). No significant correlation was found between total or LDL cholesterol and arterial stiffness or LV diastolic function. However, significant negative correlations of HDL cholesterol with all parameters of arterial stiffness and LV diastolic dysfunction were observed in both men and women. A positive correlation of triglycerides with arterial stiffness was found in women, while a weak correlation with the E/Em ratio was observed only in men (Table II).
Table II.
Correlation Between Lipid Levels and Indices of Stiffness Parameter and LV Diastolic Function
| Stiffness Parameter | E/Em | Em/Am | |
|---|---|---|---|
| Total cholesterol | |||
| All | 0.05 | 0.06 | −0.03 |
| Men | 0.07 | 0.07 | −0.04 |
| Women | 0.03 | 0.04 | −0.02 |
| LDL cholesterol | |||
| All | 0.08 | 0.06 | −0.10 |
| Men | 0.05 | 0.04 | −0.08 |
| Women | 0.10 | 0.09 | −0.12 |
| HDL cholesterol | |||
| All | −0.23a | −0.27a | 0.18b |
| Men | −0.21b | −0.22b | 0.15b |
| Women | −0.26a | −0.31a | 0.20b |
| Triglycerides | |||
| All | 0.16 b | 0.12 | −0.08 |
| Men | 0.06 | 0.14b | −0.11 |
| Women | 0.20b | 0.10 | −0.07 |
Abbreviations: Em/Am, the ratio of peak early diastolic motion velocity to peak later diastolic motion velocity of mitral annulus (Em to Am ratio); E/Em, the ratio of transmitral peak velocity of early filling to peak early diastolic motion velocity of mitral annulus (E to Em ratio); HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. a P<.01. b P<.05.
Comparison of Clinical Findings in Groups of Various HDL Cholesterol and Triglyceride Levels
We subdivided each study group by tertiles of HDL cholesterol and triglycerides and then examined the influence of the lowest HDL cholesterol tertile (≤1.14 mmol/L [44 mg/dL]) or the highest triglyceride tertile (≥1.56 mmol/L [138 mg/dL]) on arterial stiffness and LV diastolic function. All patients were divided into 4 groups according to the lipid levels (Table III): patients without the lowest HDL cholesterol or the highest triglyceride tertile (group 1, n=102); patients with the highest triglyceride tertile alone (group 2, n=36); patients with the lowest HDL cholesterol tertile alone (group 3, n=38); and patients with both the lowest HDL cholesterol and the highest triglyceride tertile (group 4, n=41).
Table III.
Criterion of High‐Density Lipoprotein (HDL) Cholesterol and Triglycerides for the 4 Groups
| HDL Cholesterol Tertile | Triglyceride Tertile | |
|---|---|---|
| Group 1 | ≥1.14 mmol/L (44 mg/dL) | ≤1.56 mmol/L (138 mg/dL) |
| Group 2 | ≥1.14 mmol/L (44 mg/dL) | ≥1.56 mmol/L (138 mg/dL] |
| Group 3 | ≤1.14 mmol/L (44 mg/dL) | ≤1.56 mmol/L (138 mg/dL) |
| Group 4 | ≤1.14 mmol/L (44 mg/dL) | ≥1.56 mmol/L (138 mg/dL) |
There were no significant differences in age, sex (percentage of men), or systolic BP among the 4 groups. BMI was higher in groups 2, 3, and 4 than in group 1. The smoking ratio and heart ratio were higher in group 4 than in group 1. Diastolic BP was higher in group 4 than in the others. All lipid parameters had group‐specific differences as shown in Table III. The fasting plasma glucose level in group 2 and 4 was higher than in groups 1 and 3. The E/Em ratio in groups 3 and 4 (patients who were in the lowest tertile of HDL cholesterol) was significantly higher than in the others, and group 4 had the highest E/Em ratio among the 4 groups. The arterial stiffness parameter and Em/Am ratio in groups 2, 3, and 4 were significantly lower than in group 1, while those in group 4 were lowest and those in group 3 were lower than in group 2 (Table IV).
Table IV.
Comparison of Clinical Findings Among the 4 Groups Divided by HDL Cholesterol and Triglyceride Levels
| Variable | Group 1 (n=102) | Group 2 (n=36) | Group 3 (n=38) | Group 4 (n=41) |
|---|---|---|---|---|
| Age, y | 60±12 | 59±13 | 61±11 | 63±11 |
| Men, % | 49.0 | 58.3 | 57.9 | 46.3 |
| Body mass index, kg/m2 | 23.2±2.9 | 25.0±2.3a | 25.1±3.5a | 25.9±3.1a |
| Heart rate, bpm | 72±10 | 75±9 | 73±11 | 76±10a |
| Smoking ratio, % | 46.9 | 51.7 | 61.4 | 78.2a |
| Systolic blood pressure, mm Hg | 141±9 | 144±10 | 143±11 | 145±16 |
| Diastolic blood pressure, mm Hg | 80±8 | 82±8 | 80±11 | 84±10a |
| Total cholesterol, mmol/L | 5.20±0.68 | 5.49±0.65a | 4.96±0.67b | 5.33±0.82c |
| HDL cholesterol, mmol/L | 1.52±0.29 | 1.40±0.28a | 1.10±0.12a,b | 1.07±0.17a,b |
| LDL cholesterol, mmol/L | 3.27±0.43 | 3.30±0.52 | 3.49±0.71a | 3.45±0.68 |
| Triglycerides, mmol/L | 1.15±0.19 | 2.07±0.78a | 1.25±0.27a,b | 2.15±0.47a,b,c |
| Fasting plasma glucose, mmol/L | 5.38±0.63 | 5.67±0.52a | 5.47±0.89 | 5.81±0.82a |
| E/Em | 7.85±2.05 | 8.05±3.21 | 10.6±3.18a,b | 13.6±3.50a,b,c |
| Em/Am | 0.80±0.15 | 0.74±0.10a | 0.62±0.22a,b | 0.51±0.19a,b,c |
| Arterial stiffness parameter | 8.12±2.04 | 9.35±2.42a | 11.5±3.3a,b | 14.6±6.3a,b,c |
Abbreviations: bpm, beats per minute; E/Em, the ratio of transmitral peak velocity of early filling to peak early diastolic motion velocity of mitral annulus (E to Em ratio); Em/Am, the ratio of peak early diastolic motion velocity to peak later diastolic motion velocity of mitral annulus (Em to Am ratio); HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. Data are presented as means±standard deviation or percentage. a P<.05 vs group 1. b P<.05 vs group 2. c P<.05 vs group 3. Age, sex, body mass index, heart rate, and smoking ratio were used as covariates in the adjusted analysis for E/Em, Em/Am and arterial stiffness.
Multivariate Correlates of Arterial Stiffness Parameter and Diastolic Dysfunction
To confirm whether the effect of low HDL cholesterol on arterial stiffness was independent of other factors (especially high triglycerides, glucose levels, obesity, and sex) and whether its effect on LV diastolic function was independent of LV hypertrophy and arterial stiffness, we investigated possible predictive factors for arterial stiffness and E/Em ratio using a stepwise regression analysis in all patients. As a result, low HDL cholesterol, age, systolic BP, and fasting plasma glucose was an independent determinant of arterial stiffness (Table V). As for the association with LV diastolic function, low HDL cholesterol was a significant predictor of both E/Em ratio and Em/Am ratio (data not shown) independently of other predictive factors, such as age, systolic BP, LV mass index, and arterial stiffness. In addition to total and LDL cholesterol, a high level of triglycerides could not be adopted as an independent determinant of arterial stiffness or LV diastolic dysfunction.
Table V.
Independent Predictors for Arterial Stiffness and LV Diastolic Dysfunction by Stepwise Regression Analysis
| Independent Variable | Standarized Regression Coefficient | Adjusted R 2 | P Value |
|---|---|---|---|
| Arterial stiffness parameter | |||
| Age | 0.307 | 0.418 | <.0001 |
| Systolic BP | 0.253 | ||
| Fasting plasma glucose | 0.169 | ||
| HDL cholesterol | −0.122 | ||
| E/Em | |||
| Age | 0.274 | 0.342 | <.0001 |
| Systolic BP | 0.188 | ||
| Arterial stiffness parameter | 0.151 | ||
| HDL cholesterol | −0.136 | ||
In the model of arterial stiffness as dependent variable, age, sex, body mass index, smoking, systolic and diastolic blood pressure (BP), fasting plasma glucose, total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), and triglycerides were included as potential independent variables. Besides those, arterial stiffness, left ventricular (LV) mass index and heart rate were added as independent variables in the analysis for diastolic function.
Discussion
The presented study showed that the development of LV diastolic dysfunction was accompanied by enhanced arterial stiffness. Metabolic factors may be involved in the progress of both LV diastolic dysfunction and arterial stiffening. Consistent with recent studies, 12 , 13 , 14 low HDL cholesterol instead of total or LDL cholesterol was independently associated with arterial stiffening and LV diastolic dysfunction. While ventricular‐vascular structural and functional changes are increased by normal aging, the presence of cardiovascular risk factors accelerates their changes. 15 Stiffening in both medium and large elastic arteries is associated with multiple cardiovascular risk factors, including hypertension, dyslipidemia, obesity, smoking, diabetes, and aging, all of which have also promoted the development of atherosclerosis in previous studies. 15 , 16
The relationship between arterial stiffness and HDL cholesterol levels has been reported only indirectly in some studies. Ferrier and colleagues 17 reported that atorvastatin treatment reduced artery stiffness in patients with isolated systolic hypertension accompanied with increased HDL cholesterol and reduced total and LDL cholesterol. Moreover, cholesterol‐lowering therapy was efficacious in reducing arterial stiffness in patients with hypercholesterolemia. 18 Brouwers and colleagues 19 studied patients with familial combined hyperlipidemia and found that they had increased arterial stiffness compared with controls. In our study, the significant association of HDL cholesterol with arterial stiffness was observed not only in women but also in men by univariate and multivariate regression analysis.
E/Em and E/Am, which usually reflect LV filling pressure by echocardiographic technology, were used as diastolic function in our study. LV isovolumic relaxation time starts from aortic valve closing to mitral valve opening, which is a process of initiative and energy consumption. The insufficiency of LV relaxation would delay the start of diastolic filling. LV filling pressure increases in proportion to diastolic function and decreases in early and middle diastolic dysfunction. Severe diastolic dysfunction involves myocardial fibrosis and passive filling limitation. When the increasing LV filling pressure was followed by the compensatory left atrial expansion and high pulmonary pressure, LV filling pressures could not be used as an index of diastolic dysfunction. Patients in this study came from the same community and were also in stable condition. In addition, these hypertensive patients did not have severe diastolic dysfunction on echocardiographic examination; therefore, their LV filling pressure accurately reflected their LV diastolic function.
While impaired LV diastolic relaxation is another common cardiac change observed in hypertensive patients, some significant metabolic factors, which are independent of BP and LV mass index, have been associated with this diastolic dysfunction. 20 In fact, abnormalities in glucose and insulin metabolism have been shown to accelerate the deterioration of arterial stiffness and LV diastolic function. 6 , 7 Dyslipidemia is one of metabolic abnormalities. Nonetheless, the correlation between serum lipids and LV diastolic function in essential hypertension was limited. Similar with Horio and colleagues’ study, 21 our findings suggest that only low HDL cholesterol may pose an independent adverse effect on LV diastolic function in hypertensive patients independently of age, sex, BMI, smoking status, BP, total cholesterol, LDL cholesterol, triglycerides, glucose, LV mass index, heart rate, and arterial stiffness. Furthermore, Mizuguchi and associates reported that statin therapy could promote LV diastolic function accompanied with the improvement of blood lipid level, which is consistent with our findings. 22
Our study showed that total and LDL cholesterol had no significant association with impairments of LV diastolic relaxation and arterial stiffness. Brinkley and colleagues believe that plasma oxidized LDL levels (ox‐LDL, a marker of oxidative stress), a key player in the pathogenesis of atherosclerosis, might also play a role in arterial stiffening. 23 In addition, Rietzschel and coworkers proved that ox‐LDL cholesterol was associated with decreases in cardiac function and also a risk marker for early ventricular remodeling independently of vascular alterations. 24 Ox‐LDL cholesterol may be one of the key points in our future research.
In the present study, triglyceride levels showed a weak correlation with arterial stiffness and LV diastolic function, although total and LDL cholesterol did not at all. In addition, arterial stiffening and LV diastolic dysfunction were most advanced in a subgroup with both low HDL cholesterol and high triglycerides. Individuals in this group also had increased BMI, plasma glucose level, and diastolic BP, all of which appeared to be associated with the metabolic syndrome, a cluster of multiple interrelated abnormalities in lipid and glucose metabolism along with hypertension and obesity. 25 , 26 Therefore, the influence of triglycerides was considered only as a part of multiple interrelated metabolic factors.
Clinical Implications and Potential Limitations
Because arterial stiffness may be modifiable, 27 HDL cholesterol might be a potential target for intervention in our future study of diastolic heart failure. HDL cholesterol, as one of complex metabolic factors, has an important role in this “heart‐vessel coupling disease,” 28 but the exact mechanism needs to be explored further. The limitation of the present study lies in its purely cross‐sectional design. We performed only association analysis, but long‐term follow‐up of our patients would be needed to corroborate the proposed value of HDL cholesterol in predicting arterial stiffness and LV diastolic function.
Conclusions
Enhanced arterial stiffness is associated with LV diastolic dysfunction. Low HDL cholesterol may lead to the deterioration of arterial stiffness and LV diastolic properties in patients with hypertension. Low HDL cholesterol would be a risk factor not only for coronary heart disease but also for hypertensive cardiovascular disease.
Acknowledgments
Acknowledgments and disclosures: We thank the staff and participants of this study for their valuable contributions. We also thank the Aloka Corporation (Japan) for free loan of the α‐10 apparatus.
References
- 1. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well functioning older adults. Circulation. 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 2. Senni M, Redfild MM. Heart failure with preserved systolic function: a different natural history? J Am Coll Cardiol. 2001;38:1277–1282. [DOI] [PubMed] [Google Scholar]
- 3. Yambe M, Tomiyama H, Hirayama Y, et al. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res. 2004;27:625–631. [DOI] [PubMed] [Google Scholar]
- 4. Vinereanu D, Nicolaides E, Boden L, et al. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikonomidis I, Tzortzis S, Papaioannou T, et al. Incremental value of arterial wave reflections in the determination of left ventricular diastolic dysfunction in untreated patients with essential hypertension. J Hum Hypertens. 2008;22:687–698. [DOI] [PubMed] [Google Scholar]
- 6. Miyazato J, Horio T, Takishita S, Kawano Y Fasting plasma glucose is an independent determinant of left ventricular diastolic dysfunction in nondiabetic patients with treated essential hypertension. Hypertens Res. 2002;25:403–409. [DOI] [PubMed] [Google Scholar]
- 7. Vinereanu D, Nicolaides E, Tweddel AC, et al. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci. 2003;105:591–599. [DOI] [PubMed] [Google Scholar]
- 8. Palmiero P, Maiello M, Passantino A, et al. Correlation between diastolic impairment and lipid metabolism in mild‐to‐moderate hypertensive postmenopausal women. Am J Hypertens. 2002;15:615–620. [DOI] [PubMed] [Google Scholar]
- 9. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 10. Waggoner AD, Bierig M. Tissue Doppler imaging: a useful echocardiographic method for the cardiac sonographer to assess systolic and diastolic ventricular function. J Am Soc Echocardiogr. 2001;14:1143–1152. [DOI] [PubMed] [Google Scholar]
- 11. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12. Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients >65 years of age. Am J Cardiol. 2006;98:1387–1392. [DOI] [PubMed] [Google Scholar]
- 13. Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chow PC, Ho MH, Lee TL, et al. Relation of arterial stiffness and left ventricular structure and function in adolscent and young adults with pediatric‐onset systemic lupus erythematosus. J Rheumatol. 2007;34:1345–1352. [PubMed] [Google Scholar]
- 15. Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age‐associated increases in vascular thickness andstiffness. J Am Coll Cardiol. 2004;43:1388–1395. [DOI] [PubMed] [Google Scholar]
- 16. Sharrett AR, Ding J, Criqui MH, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2006;186:441–447. [DOI] [PubMed] [Google Scholar]
- 17. Ferrier KE, Muhlmann MH, Baguet JP, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. [DOI] [PubMed] [Google Scholar]
- 18. Yokoyama H, Kawasaki M, Ito Y, et al. Effects of fluvastatin on the carotid arterial media as assessed by integrated backscatter ultrasound compared with pulse‐wave velocity. J Am Coll Cardiol. 2005;46:2031–2037. [DOI] [PubMed] [Google Scholar]
- 19. Brouwers MC, Reesink KD, van Greevenbroek MM, et al. Increased arterial stiffness in familial combined hyperlipidemia. J Hypertens. 2009;27:1009–1016. [DOI] [PubMed] [Google Scholar]
- 20. Schillaci G, Pasqualini L, Verdecchia P, et al. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol. 2002;39:2005–2011. [DOI] [PubMed] [Google Scholar]
- 21. Horio T, Miyazato J, Kamide K, et al. Influence of low high‐density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens. 2003;16:938–944. [DOI] [PubMed] [Google Scholar]
- 22. Mizuguchi Y, Oishi Y, Miyoshi H, et al. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circ J. 2008;72:538–544. [DOI] [PubMed] [Google Scholar]
- 23. Brinkley TE, Nicklas BJ, Kanaya AM, et al. Plasma oxidized low‐density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53:846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rietzschel ER, Langlois M, De Buyzere ML, et al. Oxidized low‐density lipoprotein cholesterol is associated with decreases in cardiac function independent of vascular alterations. Hypertension. 2008;52:535–541. [DOI] [PubMed] [Google Scholar]
- 25. Shinohara K, Shoji T, Kimoto E, et al. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005;12:205–210. [DOI] [PubMed] [Google Scholar]
- 26. Ohnishi H, Saitoh S, Takagi S, et al. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes. 2003;26:437–440. [DOI] [PubMed] [Google Scholar]
- 27. Agata J, Nagahara D, Kinoshita S, et al. Angiotensin II receptor blocker prevents increased arterial stiffness in patients with essential hypertension. Circ J. 2004;68:1194–1198. [DOI] [PubMed] [Google Scholar]
- 28. Kass DA. Age‐related changes in venticular‐arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. [DOI] [PubMed] [Google Scholar]


