Abstract
J Clin Hypertens (Greenwich). 2012;00:00–00 ©2012 Wiley Periodicals, Inc.
The effects of sauna alone vs exercise and sauna on ambulatory blood pressure monitoring and central hemodynamic variables were measured in 16 patients with untreated hypertension assigned to a control period, sauna, or exercise and sauna. Exercise and sauna had positive effects on 24‐hour systolic and mean blood pressure in patients with untreated hypertension. Exercise and sauna and sauna alone reduce total vascular resistance, with positive effects lasting up to 120 minutes after heat exposure.
When hypertension diagnosis is confirmed, nonpharmacologic treatments should be instituted as first‐line therapy, whenever appropriate, according to North American 1 and European 2 guidelines. In order to lower blood pressure (BP), but also to control other cardiovascular risk factors, nonpharmacologic treatments include the following lifestyle changes: smoking cessation, body mass reduction (for overweight people), moderate alcohol consumption and salt intake, increase in fruit and vegetable intake, stress management, and regular physical activity. 2 In real life, prescribed physical exercise is often performed at home and/or in fitness centers and can be followed by sauna bathing when available. In healthy normotensive people, reported acute BP variations during sauna bathing have been variable with either nonchange, decrease, or increase. 3 In healthy young patients, cardiovascular system responses to sauna includes heart rate (HR) and cardiac output (CO) increase and peripheral vascular vasodilatation. 3 , 4 In patients with chronic heart failure and coronary risks factors, 2 weeks of repeated sauna bathing demonstrated favorable hemodynamic and endothelial effects as well as improvement in symptoms and reduction of systolic BP (SBP). 5 , 6 , 7 In the same way, repeated sauna therapy was shown to decrease SBP in hypertensive patients in one study; however, methodology was lacking with no control group and patients criteria were not clearly defined. 8 Only one study in young treated hypertensive patients is available on the acute hemodynamic effects of sauna bath. 9 In this study, SBP was found stable during sauna under placebo and diltiazem conditions and decreased under atenolol. Twenty minutes after, SBP was decreased under placebo and atenolol conditions. 9 Twenty‐four–hour ambulatory BP measurements (ABPM) provides a more stable BP reading compared with home and/or patient office measure, allowing for the detection of masked and/or white coat hypertension. 1 , 2 In addition, 24‐hour ABPM offers additional information on BP evolution during the day and night. 1 , 2 To our knowledge, no study has been performed to assess BP levels the hours following sauna bathing by 24‐hour ABPM, particularly in untreated older hypertensive patients. In the same way, the effects of sauna bathing on central hemodynamics during and after heat stress are not documented in older untreated hypertensive patients. The potential beneficial effect of sauna alone or added to aerobic exercise as a lifestyle treatment of hypertension is unknown. The aim of this study was to measure the effects of sauna bathing alone or along with a 30‐minute aerobic exercise session followed by sauna on short‐term BP, central hemodynamics, and 24‐hour ABPM in patients with untreated hypertension.
Patients and Methods
Patients
Sixteen patients were recruited at the Cardiovascular Prevention and Rehabilitation Centre (ÉPIC) of the Montreal Heart Institute. Our center provides a multiphase, multidisciplinary approach to cardiac rehabilitation as well as exercise and risk factor control programs in the primary prevention setting. 10 Inclusion criteria were age older than 18 years, no medication for hypertension, and a systolic BP (SBP) between 130 mm Hg and 160 mm Hg and/or a diastolic BP (DBP) between 85 mm Hg and 99 mm Hg. Exclusion criteria were age younger than 18 years and previous medication for hypertension. The research protocol was approved by the Montreal Heart Institute Ethics Committee, and written informed consent was obtained prior to study entry.
Study Procedures
At baseline, patients were evaluated with measurement of body mass, height, body composition, fasting lipid profile, resting electrocardiography, and resting BP. Body composition was measured by bioelectrical impedance analysis to estimate lean body mass and fat mass. 11 Then, patients were exposed to 3 different conditions in an order randomly assigned according to a crossover procedure: (1) a resting control period (C) without any sauna or exercise; (2) a sauna intervention (S) consisting of an initial 8‐minute period inside the sauna followed by 2 minutes of cold‐water showering and a 10‐minute rest period outside the sauna, followed by a second 8‐minute period inside the sauna; and (3) an exercise and sauna intervention (ES) consisting of a 30‐minute continuous exercise session on ergocycle at an intensity of 75% of maximal HR, followed by the same sauna intervention described in the second intervention. A minimal duration of 3 days without any significant physical activity was observed between two evaluations for the same patient. Acute physiological measurements (BP and cardiac bioimpedance) were measured at baseline and at 15 and 120 minutes after baseline for the 3 conditions and are detailed in Figure 1. Patients were told to rest at the ÉPIC Centre between those 3 time measurements. BP was measured during the 3 conditions (C, S, and ES) with an automated BP monitor. For baseline, 15 and 120 times, the third BP value of 3 successive measurements was taken into account, whereas during saunas, one reading was performed.
Figure 1.
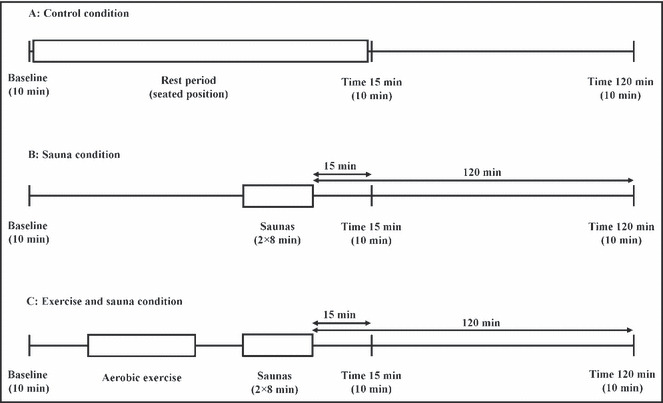
Experimental procedure and physiological measurement times.
Sauna Bathing
The sauna was a standard dry sauna made of cedar wood with dimensions of 4.83 m by 2.44 m, height of 2.08 m, and a bench 51 cm above the ground. The sauna temperature was kept constant between 85°C and 90°C (185°F to 194°F) with a relative humidity of 50% to 60%. 12 Patients were studied during one period of 8 minutes and a second 8‐minute period inside the sauna. After the first 8‐minute period of sauna, the patients left the sauna to have cold‐water showering during 1 to 2 minutes after they had a 10‐minute rest period outside the sauna before coming back in the sauna for the second 8‐minute period. In the sauna, patients wore shorts and sat upright on the bench. They were always accompanied by a nurse and a physician. The patients were allowed to leave the sauna at any time if they felt uncomfortable, but all patients underwent the 2 sauna periods. Patients were instructed not to perform any sauna and/or exercise 3 days before the testing. 12
Exercise Training Session
The exercise training session was performed on an ergocycle. After a warm‐up of 5 minutes, patients performed a 30‐minute continuous exercise session at an intensity of 75% of their maximal HR measured during their last physical stress test evaluation performed in our center. 10 , 13
24‐Hour ABPM
The 24‐hour ABPM (Spacelabs®, model 90207, Issaquah, WA) was performed after each condition (C, S, and ES) just after the last physiological measurement at 120 minutes. An automated sphygmomanometer was established in patients, an appropriate sized cuff was fitted to the nondominant arm, and BP was measured every 20 minutes during the 24‐hour measurement. Patients recorded the times when they went to bed at night and awoke in the morning; such times were then rounded to the nearest hour. The following formula was used to calculate mean BP (MBP): 1/3SBP + 2/3DBP. The differential pressure or pulse pressure was calculated with the usual formula: SBP – DBP.
According to guidelines, the percentage of successful readings should be similar to or more than 70%. 14 The 24‐hour ABPM value was calculated from the average of the total number of BP values measured. Daytime and nighttime ABPM was calculated from the average BP values measured when the patient was awake or sleeping. The noctural BP fall was defined as the degree of fall (%) in noctural MBP relative to the diurnal MBP: 100 × (1–[nighttime MBP/daytime MBP]). Dipping status was expressed as either dipper or nondipper, with dipping defined as a ≥10% nighttime fall in MBP relative to its daytime value. 15
Cardiac Bioimpedance
Cardiac bioimpedance (Manatec, PhysioFlow® Enduro model, Paris, France) was used to measure central hemodynamic modifications before, during, and after the 3 situations (C, S, ES). CO, stroke volume, HR, ventricular ejection time, end‐diastolic volume, and systemic vascular resistance were measured with this device. The theoretic basis for this technique and its application and its validity at rest and during exercise testing have been previously described. 16 , 17
Statistical Analysis
All data were analyzed using StatView software version 5.0 (SAS Institute Inc, Cary, NC) and are presented as mean±standard deviation except where otherwise indicated. Analysis of variance with repeated measure (time and conditions) was performed to compare the physiological measurement (BP and cardiac bioimpendance) at baseline and 15 and 120 minutes after the 3 conditions (C, S, ES). Analysis of variance with repeated measure (time and conditions) was performed to compare the acute physiological responses (BP and cardiac bioimpedance) during sauna bathing alone or during sauna bathing preceded by the 30‐minute exercise training session. Analysis of variance with repeated measure (time and conditions) was performed to compare the 24‐hour ABPM after the 3 conditions (C, S, ES). A post hoc test (Scheffe’s test) was used to localize the differences. P level <.05 was considered statistically significant.
Results
A total of 16 patients older than 18 years (13 men and 3 women; age, 33 to 71 years) were recruited at the ÉPIC Centre. All individuals were ÉPIC Centre members for at least 3 months. There were 8 prehypertensive patients (SBP 120 mm Hg to 139 mm Hg) and 8 stage I hypertensive patients (SBP 140 mm Hg to 159 mm Hg). Anthropometric and clinical data of the 16 patients are presented in Table I.
Table I.
Anthropometric and Clinical Data of 16 Patients With Untreated Hypertension
| Parameters | Mean±SD or No. (%) |
|---|---|
| Age, y | 60.7±9.4 |
| Body mass, kg | 88.5±16.4 |
| Height, m | 1.69±0.08 |
| Men/women, No. | 13/3 |
| Body mass index, kg/m2 | 30.57±4.90 |
| Fat mass, % | 31.4±7.4 |
| Waist circumference, cm | 105.5±14.5 |
| Lean body mass, kg | 60.1±11.1 |
| Glycemia, mmol/L | 5.46±1.17 |
| Total cholesterol, mmol/L | 5.17±1.98 |
| HDL cholesterol, mmol/L | 1.36±0.32 |
| LDL cholesterol, mmol/L | 3.19±1.09 |
| Total cholesterol/HDL | 3.94±1.26 |
| Triglycerides, mmol/L | 1.35±0.61 |
| Triglycerides/HDL | 1.13±0.81 |
| Antiplatelet agents | 4 (25) |
| Statins | 7 (44) |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation.
BP Measurements
24‐Hour ABPM Variables for C, S, and ES Conditions. The 24‐hour ABPM measurements are presented in Table II. The 24‐hour SBP, 24‐hour MBP, and daytime SBP were significantly lower after the ES condition (P=.03 and .04) compared with S and C conditions.
Table II.
24‐Hour ABPM for 16 Patients With Untreated Hypertension
| Parameters | Controlled Condition | Sauna Condition | Exercise and Sauna Condition | ANOVA P Value |
|---|---|---|---|---|
| Daytime SBP, mm Hg | 139±12 | 138±11 | 134±12a | .03 |
| Daytime DBP, mm Hg | 84±5 | 83±6 | 82±6 | .29 |
| Daytime MBP, mm Hg | 102±7 | 102±7 | 100±7 | .051 |
| Daytime pulse pressure, mm Hg | 55±9 | 51±16 | 51±8 | .34 |
| Nighttime SBP, mm Hg | 119±9 | 117±10 | 119±9 | .63 |
| Nighttime DBP, mm Hg | 71±7 | 70±7 | 72±9 | .62 |
| Nighttime MBP, mm Hg | 87±7 | 86±8 | 88±8 | .62 |
| Nighttime pulse pressure, mm Hg | 48±8 | 44±12 | 46±8 | .38 |
| Fall in nighttime BP, % | 14±7 | 15±7 | 11±6 | .54 |
| Nondipper proportion | 5 (29) | 3 (17) | 5 (29) | |
| 24‐h SBP, mm Hg | 136±11 | 134±9 | 131±12a | .04 |
| 24‐h DBP, mm Hg | 82±6 | 81±6 | 80±7 | .08 |
| 24‐h MBP, mm Hg | 100±7 | 99±7 | 97±8a | .04 |
| 24‐h pulse pressure, mm Hg | 53±7 | 52±7 | 51±8 | .13 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; ANOVA, analysis of variance; BP, blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SBP, systolic blood pressure; SBP‐DBP, pulse pressure. Values are means±standard deviation or number (percentage). aDifferent from Controlled and Sauna conditions.
Acute BP Measurement at Baseline and at 15 and 120 Minutes for C, S, and ES Conditions. The acute BP responses measured at baseline and 15 and 120 minutes after the sauna time for the 3 conditions are presented in Table III. Compared with the control condition, there was no significant condition effect of either S or ES on BP. SBP was significantly lower after ES at 15 minutes (time effect: P<.01).
Table III.
BP Measurement at Baseline and 15 and 120 Minutes After the Sauna Time for 3 Conditions (Control, Sauna, and Exercise and Sauna) in 16 Patients With Untreated Hypertension
| Condition and Parameters | Baseline | T15 min | T120 min | ANOVA and P Value |
|---|---|---|---|---|
| Control SBP, mm Hg | 134±12 | 135±16 | 142±14a | a: .31 b: .0015 c: .21 |
| Sauna SBP, mm Hg | 136±15 | 136±14 | 139±13 | |
| Exercise and sauna SBP, mm Hg | 134±18 | 126±10b | 135±10 | |
| Control DBP, mm Hg | 85±8 | 83±10 | 84±7 | a: .60 b: .27 c: .75 |
| Sauna DBP, mm Hg | 86±8 | 85±18 | 84±8 | |
| Exercise and sauna DBP, mm Hg | 83±6 | 81±6 | 83±5 |
Abbreviations: ANOVA, analysis of variance; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; T15 min, measurement 15 minutes after the second sauna time; T120 min, measurement 120 minutes after the second sauna time. a=Condition effect; b=Time effect; c=Interaction effect. a P<.05. b P<.01. Values are presented as mean±standard deviation.
Acute BP Measurement at Baseline, During the 2 Sauna Sessions, and at 15 and 120 Minutes for S and ES Conditions. The acute BP responses measured at baseline, during the 2 sauna sessions, and 15 and 120 minutes after the second sauna for S and ES conditions are presented in Figure 2 (panels A and B). There was a significant decrease in SBP (P<.05) during the second minute of the first sauna compared with baseline for both S and ES conditions. There was a significantly lower SBP (P<.05) for ES vs S at 15 minutes (Figure 2). No adverse effects were noted during sauna sessions in our patients.
Figure 2.
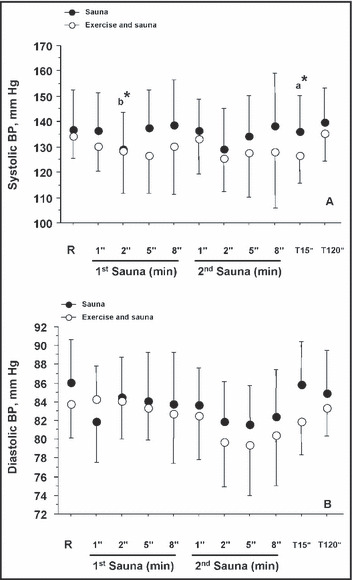
Blood pressure (BP) measurements at baseline, during the saunas, and 15 and 120 minutes after the last sauna time for the 2 conditions (sauna and exercise and sauna) in 16 patients with untreated hypertension. R indicates baseline rest; a, condition effect; b, time effect vs rest baseline values. *P<.05.
Cardiac Bioimpedance Measurements
C, S, and ES Comparison at Baseline and at 15 and 120 Minutes. The cardiac bioimpedance variables measured at baseline and 15 and 120 minutes after the sauna time for the 3 conditions (C, S, ES) are presented in Table IV. Compared with control, HR was higher for S and ES at 15 and 120 minutes (P<.0001). ES HR increased at 15 and 120 minutes (P<.01) compared with baseline. Compared with control, CO was significantly higher at 15 and 120 minutes for S and at 120 minutes for ES (P<.05). Compared with C, stroke volume (SV) was lower at 15 minutes for ES (P=.01). Ventricular ejection time (VET) for ES was lower at 15 minutes compared with S and C (P<.01). ES VET was lower at 120 minutes compared with S and C (P<.0001). VET for S was higher at 120 minutes compared with C (P<.001). End‐diastolic volume was higher at 120 minutes (P<.0001) for the 3 conditions (C, S, ES) compared with baseline values. At baseline, total vascular resistance (TVR) for ES was higher compared with S and C (P<.05). At 15 minutes, TVR was lower for ES and S compared with C (P<.0001), whereas TVR remained lower for ES compared with S (P<.05). At 120 minutes, TVR was lower for ES (P<.0001) and S (P=.0033) compared with C.
Table IV.
Cardiac Bioimpedance Variables Measured at Baseline and 15 and 120 Minutes After the Second Sauna Time for the 3 Conditions (Control, Sauna, and Exercise and Sauna) in 16 Patients With Untreated Hypertension
| Parameters | Baseline | T15 min | T120 min | ANOVA and P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Sauna | Exercise Sauna | Control | Sauna | Exercise Sauna | Control | Sauna | Exercise Sauna | ||
| Heart rate, beats per min | 72±10 | 75±11 | 72±8 | 71±11 | 77±10 | 80±9 | 70±11 | 71±10 | 75±10 | a: <.0001 b: <.0001 c: <.0001 |
| Cardiac output, L/min | 5.0±1.1 | 5.3±1.5 | 5.1±1.4 | 5.0±1.3 | 5.2±1.5 | 5.2±1.3 | 5.0±1.2 | 5.2±1.2 | 5.4±1.7 | a: <.0001 b: .49 c: .10 |
| Stroke volume, mL | 69±17 | 71±21 | 70±20 | 70±18 | 68±20 | 65±16 | 72±19 | 73±18 | 71±20 | a : .06 b: <.0001 c: .15 |
| Ventricular ejection time, ms | 325±38 | 330±51 | 319±55 | 325±56 | 302±61 | 283±66 | 306±60 | 327±50 | 277±82 | a: <.0001 b: <.0001 c: <.0001 |
| End‐diastolic volume, mL | 163±46 | 162±46 | 165±53 | 168±51 | 162±46 | 162±45 | 174±52 | 176±43 | 183±65 | a: .43 b: <.0001 c: .20 |
| Total vascular resistance, dyne·sec/cm5 | 813±246 | 823±239 | 885±537 | 896±430 | 773±255 | 706±193 | 961±363 | 873±301 | 817±267 | a: <.0001 b: <.0001 c: <.0001 |
Abbreviations: ANOVA, analysis of variance; T15 min, measurement 15 minutes after the second sauna time; T120 min, measurement 120 minutes after the second sauna time; a=Condition effect; b=Time effect; c=Interaction effect (condition × time). Values are expressed as mean±standard deviation.
S and ES Comparison at Baseline, During the 2 Sauna Sessions, and at 15 and 120 Minutes. The cardiac bioimpedance variables measured at baseline, during the 2 sauna sessions, and at 15 and 120 minutes for S and ES are presented in Figure 3. Compared with baseline, HR for S and ES increased (P<.0001) during the sauna sessions and at 15 minutes (Figure 3A). SV decreased at 15 minutes for S and ES (P<.0001) compared with baseline (Figure 3B). CO for S and ES increased (P<.0001) during the 2 sauna sessions (Figure 3C). VET decreased during the 5th and 8th minute of the 2 saunas and at 15 and 120 minutes (P<.0001) (Figure 3D). End‐diastolic volume for S and ES increased at 120 minutes compared with rest baseline values (P<.0001) (Figure 3E). Compared with rest baseline values, TVR for E and ES decreased (P<.0001) during the 2 saunas (Figure 3F).
Figure 3.
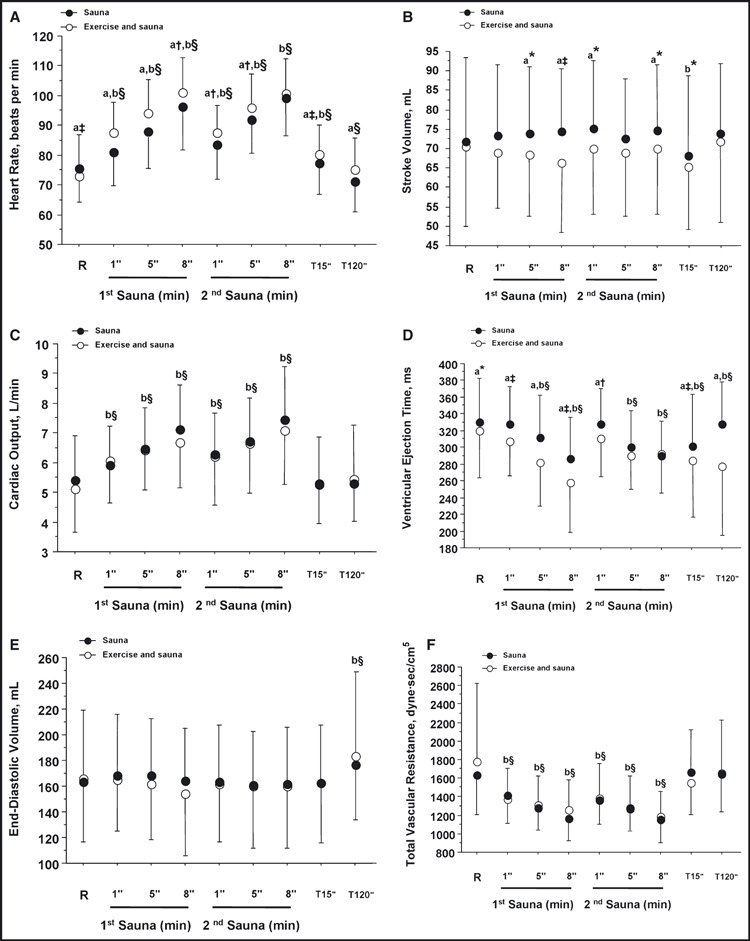
Cardiac bioimpedance variables measured at baseline, during the saunas and 15 and 120 minutes after the last sauna time for the 2 conditions (sauna and exercise and sauna) in 16 patients with untreated hypertension. R indicates baseline rest; a, condition effect; b, time effect; c, interaction effect (condition × time). *P<.05. †P<.01. ‡P<.001. §P<.0001.
Discussion
We demonstrated that exercise followed by sauna resulted in a significant decrease in daytime and 24‐hour SBP in patients with untreated hypertension, while sauna alone had no effects on long‐term BP variables (24‐hour ABPM). In the same patient cohort, sauna alone had no effect on BP measured 15 and 120 minutes after heat exposure, whereas exercise and sauna reduced SBP 15 minutes later. Hemodynamic modifications during sauna included an increase in CO mainly via HR increase, with a concomitant decrease in total peripheral resistance. In parallel, SBP and DBP were relatively stable in hypertensive patients (except with a transitory drop of SBP at the second minute of the first sauna session). Finally, compared with the control condition, hemodynamic modifications were maintained 15 minutes and 120 minutes after sauna (ES and S conditions), with increased CO and reduced total peripheral resistance.
We found a significant transitory hypotensive effect (for SBP) at the second minute of the first sauna session, with no change in DBP. Contradicting results have been found in previous studies concerning SBP modifications during sauna session in healthy patients, with either no changes, decreases, or increases reported, whereas a reduction in DBP is generally found. 3 , 9 , 18 , 19 In patients with hypertension or with coronary heart disease, BP has been found to be stable 9 or decreased 8 during and shortly after sauna. Differences in BP modifications may be due to the temperature of the sauna, the patient’s age (young vs old), cardiovascular conditions (hypertension, coronary heart disease), and the patient’s pharmacologic treatments. After sauna, no BP differences were found in the short‐ (15 and 120 minutes) or long‐term (24‐hour ABPM), whereas a significant increase in SBP was noted for the control condition at 120 minutes. Two previous studies in patients with heart failure and with coronary risks factors have suggested a therapeutic role for sauna. 6 , 7 Two weeks of daily sauna sessions improved SBP and endothelial function in patients with heart failure or coronary risk factors, 6 , 7 whereas DBP was improved only in patients with coronary risk factors. 7 However, only 32% (8 of 25 patients) had hypertension in the study by Imamura and colleagues. 7 In the same way, similar hemodynamic benefits were reported in hypertensive patients after 1 to 3 years of balneotherapy in comparison with kinesiotherapy but without random design. 20 Repeated sauna therapy improves endothelial function by increasing endothelial nitric oxide synthase activity and upregulating endothelial nitric oxide synthase expression by increasing shear stress. 6 , 7 The significant decrease in BP after 2 weeks of sauna treatment is probably due to improved endothelium‐dependent vasodilation. 7 The lack of effects on BP in our study may be due to the fact that we studied only one sauna session, which may represent an insufficient stimulus to improve BP. However, chronic effects of sauna bathing on BP and hemodynamics in untreated hypertensive patients remain to be investigated.
Concerning hemodynamic parameters, a 34% increase in HR was observed at the end of the first sauna session (from 74 to 99 beats per min), in agreement with a previous study in younger hypertensive patients. 19 Another study in young normotensive patients reported a higher HR increase during a sauna session (65% HR increase). 18 In the same way, CO increased from 5.25 L/min to 6.90 L/min (31% increase) in our patients. Previous studies reported either a 50% to 70% CO increase 21 , 22 or no significant increase 23 in normotensive patients. Stroke volume was not modified in our study during sauna sessions, in agreement with previous studies. 3 , 22 The TVR decreased significantly from 1702 to 1212 dynes.sec.cm2 (29% decrease), slightly less than in previous studies, which ranged from a 32% decrease in hot water immersion 24 to 40% to 70% in the sauna. 21 , 22 Mechanisms of these cardiovascular adaptations have been well described, with heat stress being associated with a sympathetic nervous system activation resulting in HR CO increase with reduction of peripheral vascular resistance. Additionally, cathecolaminergic secretion and renin‐angiotensin‐aldosterone system activation in response to sweating with loss of sodium and reduced plasma volume are observed. 4
When an exercise session preceded sauna bathing (ES condition), a hypotensive effect was observed for SBP during sauna, in the short‐term (15 and 120 minutes after), and in the long‐term (reduced 24‐hour SBP, MBP, and daytime SBP). However, no changes were observed for DBP, in disagreement with one previous study in normotensive adults. 25 In this study, post‐exercise sauna BP was compared with post‐exercise BP alone, and sauna was responsible for a DBP decrease. 25 Concerning hemodynamic parameters, some differences were observed for ES. During the ES condition, stroke volume was lower and HR was higher, in agreement with the hypothesis of increased sympathetic activity in response to hypovolemia in order to maintain CO BP while vascular resistances were decreasing. These hemodynamic changes could be determinants of BP decrease when physical activity preceded sauna. Those results agreed with the additional hypotensive effects of physical exercise, in particular in hypertensive patients. 26
Limitations and Strengths
Our study includes several limitations. First, our sample was composed of a relatively low number of untreated hypertensive patients (16), mainly men. Additionally, BP inclusion criteria were relatively large (SBP 130 mm Hg to 160 mm Hg and DBP 85 mm Hg to 99 mm Hg) because those BP ranges are associated with an increased risk of cardiovascular disease, 27 and our baseline office BP values were situated in the prehypertension range. 1 We finally included masked hypertensive patients, with subnormal office BP but elevated ambulatory BP. The strengths of this study include the crossover concept that included a control condition where patients were in control of their own behavior, which is lacking in previous studies. In addition, we studied untreated hypertensive patients and BP measurement (short‐ and long‐term) following sauna.
Conclusions
In untreated hypertensive patients, exercise followed by a sauna session had positive effects on short‐term SBP (at 15 minutes) and 24‐hour ABPM SBP, whereas sauna alone had no effects. Sauna increased CO mainly via HR, with a concomitant decrease in total peripheral resistance and relative BP stability. Finally, a reduction of total peripheral resistance was maintained 15 and 120 minutes after sauna (ES and S conditions) in untreated hypertensive patients. Sauna baths were safe and well tolerated in those patients, but additional studies are required to document whether regular sauna baths could be beneficial as a nonpharmacologic intervention to improve BP and hemodynamics in untreated hypertensive patients.
Acknowledgments and disclosures: The authors would like to thank the ÉPIC members who participated in this study and the ÉPIC Centre research staff (nurses and technicians) for their help in the realization of this study. Dr Mathieu Gayda is funded by the ÉPIC Centre and Montreal Heart Institute Foundations. The experiment complies with the current laws of Canada and was approved by the Montreal Heart Institute Ethics Committee. The authors declare no conflicts of interest.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, De Backer G, Dominiczak A, et al. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 3. Hannuksela ML, Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110:118–126. [DOI] [PubMed] [Google Scholar]
- 4. Kukkonen‐Harjula K, Kauppinen K. Health effects and risks of sauna bathing. Int J Circumpolar Health. 2006;65:195–205. [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto H, Kai H, Nakaura H, et al. Safety and efficacy of repeated sauna bathing in patients with chronic systolic heart failure: a preliminary report. J Card Fail. 2005;11:432–436. [DOI] [PubMed] [Google Scholar]
- 6. Kihara T, Biro S, Imamura M, et al. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:754–759. [DOI] [PubMed] [Google Scholar]
- 7. Imamura M, Biro S, Kihara T, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–1088. [DOI] [PubMed] [Google Scholar]
- 8. Siewert C, Siewert H, Winterfeld HJ, Strangfeld D. [The behavior of central and peripheral hemodynamics in isometric and dynamic stress in hypertensive patients treatment with regular sauna therapy]. Z Kardiol. 1994;83:652–657. [PubMed] [Google Scholar]
- 9. Luurila OJ, Kohvakka A, Sundberg S. Comparison of blood pressure response to heat stress in sauna in young hypertensive patients treated with atenolol and diltiazem. Am J Cardiol. 1989;64:97–99. [DOI] [PubMed] [Google Scholar]
- 10. Gayda M, Brun C, Juneau M, et al. Long‐term cardiac rehabilitation and exercise training programs improve metabolic parameters in metabolic syndrome patients with and without coronary heart disease. Nutr Metab Cardiovasc Dis. 2008;18:142–151. [DOI] [PubMed] [Google Scholar]
- 11. Pietrobelli A, Rubiano F, St‐Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole‐body analysis. Eur J Clin Nutr. 2004;58:1479–1484. [DOI] [PubMed] [Google Scholar]
- 12. Giannetti N, Juneau M, Arsenault A, et al. Sauna‐induced myocardial ischemia in patients with coronary artery disease. Am J Med. 1999;107:228–233. [DOI] [PubMed] [Google Scholar]
- 13. Gayda M, Juneau M, Levesque S, et al. Effects of long‐term and ongoing cardiac rehabilitation in elderly patients with coronary heart disease. Am J Geriatr Cardiol. 2006;15:345–351. [DOI] [PubMed] [Google Scholar]
- 14. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996; 17: 354–381. [PubMed] [Google Scholar]
- 15. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 16. Charloux A, Lonsdorfer‐Wolf E, Richard R, et al. A new impedance cardiograph device for the non‐invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol. 2000;82:313–320. [DOI] [PubMed] [Google Scholar]
- 17. Richard R, Lonsdorfer‐Wolf E, Charloux A, et al. Non‐invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol. 2001;4:202–207. [DOI] [PubMed] [Google Scholar]
- 18. Kauppinen K. Sauna, shower, and ice water immersion. Physiological responses to brief exposures to heat, cool, and cold. Part II. Circulation. Arctic Med Res. 1989;48:64–74. [PubMed] [Google Scholar]
- 19. Kukkonen‐Harjula K, Oja P, Laustiola K, et al. Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur J Appl Physiol Occup Physiol. 1989;58:543–550. [DOI] [PubMed] [Google Scholar]
- 20. Winterfeld HJ, Siewert H, Strangfeld D, et al. [Potential use of the sauna in the long‐term treatment of hypertensive cardiovascular circulation disorders – a comparison with kinesiotherapy]. Schweiz Rundsch Med Prax. 1992;81:1016–1020. [PubMed] [Google Scholar]
- 21. Eisalo A, Luurila OJ. The Finnish sauna and cardiovascular diseases. Ann Clin Res. 1988;20:267–270. [PubMed] [Google Scholar]
- 22. Vuori I. Sauna bather’s circulation. Ann Clin Res. 1988;20:249–256. [PubMed] [Google Scholar]
- 23. Kiss D, Popp W, Wagner C, et al. Effects of the sauna on diffusing capacity, pulmonary function and cardiac output in healthy subjects. Respiration. 1994;61:86–88. [DOI] [PubMed] [Google Scholar]
- 24. Bonde‐Petersen F, Schultz‐Pedersen L, Dragsted N. Peripheral and central blood flow in man during cold, thermoneutral, and hot water immersion. Aviat Space Environ Med. 1992;63:346–350. [PubMed] [Google Scholar]
- 25. Paolone AM, Lanigan WT, Lewis RR, Goldstein MJ. Effects of a postexercise sauna bath on ECG pattern and other physiologic variables. Aviat Space Environ Med. 1980;51:224–229. [PubMed] [Google Scholar]
- 26. Sharman JE, Stowasser M. Australian association for exercise and sports science position statement on exercise and hypertension. J Sci Med Sport. 2009;12:252–257. [DOI] [PubMed] [Google Scholar]
- 27. Vasan RS, Larson MG, Leip EP, et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]


