Abstract
J Clin Hypertens (Greenwich). 2012; 14:293–298. ©2012 Wiley Periodicals, Inc.
The NG_016969.1:g.5003A>G promoter polymorphism (rs168924) in the SLC6A2 norepinephrine transporter gene was found to be predictive of the hypertensive status in a Japanese population, but no data are available for Caucasians. Genotyping for rs168924 was performed in 282 young men with normal blood pressure (BP), grade 1 or 2 hypertension. In addition to casual BP, 24‐hour ABPM and echocardiography were performed. Multiple regression analysis revealed a significant association of rs168924 genotype with diagnosis of hypertension (P=.044), casual systolic BP (SBP) levels (P=.028), and daytime ambulatory SBP (P=.02). The finding that rs168924 was also significantly associated with diastolic posterior wall thickness (P=.041), an echocardiographic index of hypertensive cardiac target organ damage, further supports the notion that the rs168924 SNP in SLC6A2 in fact might influence BP. Unlike previous findings in a Japanese population, in our Caucasian study cohort the presence of the minor rs168924 G allele was associated with lower prevalence of hypertension. J Clin Hypertens (Greenwich). 2012;00:00–00. ©2012 Wiley Periodicals, Inc.
Essential hypertension, defined as chronically elevated blood pressure (BP) occurring in the absence of other predisposing conditions, affects more than 25% of adults worldwide and is a significant risk factor for coronary heart disease, stroke, and kidney disease. 1 The heritability of BP levels has been estimated to be 30% to 35%, 2 indicating that genes play a major role in determining BP and the susceptibility to essential hypertension.
The norepinephrine transporter (NET), encoded by SLC6A2, is primarily responsible for the reuptake of synaptic norepinephrine in both the central and autonomic nervous systems. 3 In the heart, NET recaptures up to 90% of released norepinephrine, making it a critical mediator of cardiac norepinephrine inactivation. 4 In addition, the involvement of NET in neural pathways of BP homeostasis makes the SLC6A2 gene a promising candidate gene for affecting essential hypertension. 5 In fact, a neurogenic origin of disease is evident in a substantial fraction of patients with essential hypertension, as indicated by high norepinephrine spillover from the heart and kidney. 6 This increase in sympathetic activity has been partially related to impaired uptake of norepinephrine. 7 Therefore, functional SLC6A2 single‐nucleotide polymorphisms (SNPs) are expected to affect BP control. 5 Although numerous SLC6A2 SNPs have been identified and some of them have been functionally characterized, 5 , 8 , 9 only the NG_016969.1:g.5003A>G promoter SNP (rs168924) has been associated so far with essential hypertension in a Japanese population. 10 In that study, the minor allele frequency was significantly higher in hypertensive than in normotensive patients. The significance of this finding, however, remained uncertain because rs168924 was not clearly associated with casual systolic or diastolic BP.
We sought to replicate this finding by investigating whether the SLC6A2NG_016969.1:g.5003A>G SNP is associated with essential hypertension in a Caucasian population. The heritable component of BP has been documented in familial and twin studies, suggesting that up to 50% of the variance of BP is attributable to environmental and nongenetic factors. In order to better control environmental and lifestyle factors, comorbidity, and age, all of which can affect BP independently of the genetic background, the study was performed in a large, well‐characterized group of young patients without any renal, hepatic, or cardiovascular disease other than mild‐to‐moderate hypertension. Due to reports of sex differences in human catecholamine transporter function, 11 only men were investigated. To accurately define the influence of the SLC6A2 SNP on BP in a statistical evaluation, we addressed the impact of confounding nongenetic factors (age, body mass index, metabolic factors, smoking, alcohol consumption, physical activity) and adjusted the outcome variable for their influence in a multiple linear regression analysis. By contrast to previous investigations, ambulatory 24‐hour BP recording, which is considered the gold standard for defining BP status, was performed in a large subcohort of our study population. In addition, echocardiography was performed in these individuals for assessing effects on heart structure and function.
Methods
Study Population
Patients were recruited through a BP screening program at the University of Erlangen‐Nuremberg or by advertising in local newspapers in the area of Erlangen‐Nuremberg. Eligible patients were consecutively enrolled. Altogether, 282 young, male, white patients with normal BP or grade 1 or 2 hypertension (according to the European Society of Hypertension/European Society of Cardiology guidelines of 2007 12 ) were included. Office values of 140/90 mm Hg or average daytime ambulatory BP values of 135/85 mm Hg were defined as threshold values for diagnosing hypertension. 12 Further details of the study have been published previously. 13 , 14 Inclusion criteria were ages between 18 and 40 years, Caucasian race, and male sex. Major exclusion criteria included diabetes mellitus, any form of secondary hypertension, grade 3 hypertension, current or previous treatment for arterial hypertension, or any other cardiovascular disease.
The study protocols were approved by the local ethics committee (University of Erlangen‐Nuremberg, Erlangen, Germany), and the study was performed according to good clinical practice guidelines. Informed written consent was obtained before inclusion of patients in the study.
Clinical Parameters
BP and heart rate were measured after 5 minutes of rest in a sitting position with an oscillometric device (Dinamap Pro100V2; Criticon, Norderstedt, Germany), using the appropriate cuff with matched cuff‐size/arm‐size relationship. An average of the last 3 measurements was taken. Estimated glomerular filtration rate (eGFR) was estimated by using the Modification of Diet in Renal Disease formula. 15
Echocardiography and Ambulatory 24‐Hour BP Recording
A total of 209 patients underwent echocardiography and ambulatory 24‐hour BP recording. Two‐dimensional–guided M‐mode echocardiography was performed using an ultrasonoscope (Picker‐Hitachi CS 192; Hitachi, Tokyo, Japan) with a 2.5‐MHz probe. Details are described elsewhere. 16 All echocardiographic readings were independently evaluated by two investigators. The echocardiographic readings were performed blindly with respect to other clinical data and, in particular, to the genotyping of our patients. Relative wall thickness was calculated as 2 times the diastolic posterior wall thickness divided by the end‐diastolic diameter and served as the parameter of concentric left ventricular (LV) hypertrophy. The LV structural and functional parameters were calculated according to recommendations of the American Society of Echocardiography 17 and were corrected according to the suggestions of Devereux and colleagues. 18 Ambulatory 24‐hour BP measurements were taken with an automatic portable device (No. 90207; Spacelab, Redmond, WA). Measurement intervals consisted of every 15 minutes during the day (defined as 7 am–10 pm) and every 30 minutes during the night.
Genotyping by TaqMan PCR
Genomic DNA was extracted from 200 μL of peripheral blood anticoagulated with EDTA with the Flexigene Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction. After extraction, the concentration of DNA was measured photometrically and DNA was diluted to a concentration of 5 ng/μL. Predesigned primer and hybridization probes for SLC6A2 rs168924 (NG_016969.1:g.5003A>G) genotyping were used (assay ID C____581568_10; Applied Biosystems, Weiterstadt, Germany). Primer and hybridization probes amplification was performed in a final volume of 5 μL containing 1 μL of DNA solution (5 ng/μL), 0.1 μL of each primer (100 pmol/μL), 0.1 μL of each probe (100 pmol/μL), 2.5 μL 2× PCR Master Mix (Absolute PCR Master Mix; Abgene, Cambridge, UK). Oligonucleotides comprising the whole amplicon were synthesized (Biomers, Ulm, Germany) for each variant as controls. Reaction mixtures were loaded into 384 well plates and placed in an ABI Prism Sequence Detector 7900 (Applied Biosystems). The polymerase chain reaction (PCR) conditions were as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation (95°C for 15 seconds), annealing, and extension in one step (60°C for 60 seconds). After PCR fluorescence, yield for the two different dyes was measured and presented in a 2‐dimensional allelic discrimination plot (SDS2.1 software; Applied Biosystems). Duplicate samples (10% of all samples) were used as internal controls. The call rate was 100% and the error rate estimated by internal duplicates was 0%. Linkage disequilibrium between rs168924 and rs28386840 was calculated with Haploview (Broad Institute, Cambridge, MA).
Linkage Disequilibrium Analysis
Genotyping of SLC6A2 rs28386840 (NG_016969.1:g.2277A>T) was performed as described above with the following primer and hybridization probes: forward primer, 5′‐GCGTGCTCTGTGCAGTCT‐3′; reverse primer, 5′‐GGAAGGAAACCAGGAGAAAGTAGATT‐3′; VIC‐labeled reporter 1, 5′‐TGAGCACCAGTTTCC‐3′; FAM‐labeled reporter 2, 5′‐TGAGCACCTGTTTCC‐3′ (Applied Biosystems). Linkage disequilibrium between rs168924 and rs28386840 was then calculated with Haploview (Broad Institute).
Statistics
Results are expressed as mean±standard deviation. The rs168924 locus was tested for conformance of genotype frequencies to those expected under Hardy‐Weinberg equilibrium with a chi‐square goodness‐of‐fit test. Allelic association of rs168924 with arterial hypertension or normotension was assessed using Pearson 2×2 contingency table Chi‐square test. The Cochran‐Armitage test for trend was used to assess for the presence of an association between hypertension and rs168924 genotypes. Normal distribution was tested with the Shapiro‐Wilks normality test. For comparisons of baseline characteristics of hypertensive cases and normotensive controls the Student t test was applied when data were normally distributed. Otherwise, the nonparametric Mann‐Whitney U test was used. The impact of rs168924 on BP was tested by multiple linear regression analyses. The multiple linear regression models comprised of rs168924 genotypes as well as clinical parameters (cardiovascular risk factors: age, body mass index, triglycerides, total cholesterol, high‐density lipoprotein [HDL] cholesterol, smoking, alcohol consumption, physical activity, and eGFR) as independent variables. Multiple regression models were used to calculate adjusted BP and heart rate values. Adjustment was made for age, body mass index, triglycerides, total cholesterol, HDL cholesterol, smoking, alcohol consumption, physical activity, and eGFR. Multiple logistic regression analysis was used to investigate the association of rs168924 with diagnosis hypertension. Impact of the rs168924 genotype on adjusted BP and heart rate values was assessed by the Kruskal‐Wallis test for three groups or was analyzed with the Jonckheere‐Terpstra test. For the latter test, a gene‐dose effect, ie, an additive genetic model was assumed and the order of genotypes was predefined as follows: rs168924 AA, AG, and GG. Data were analyzed with SPSS 16.0 for Windows (SPSS Inc, Chicago, IL) and MedCalc 11.6.0.0 (MedCalc Software, Mariakerke, Belgium). Statistical significance was accepted for values of P<.05.
Results
Clinical Characteristics of the Study Population and Association of rs168924 With Essential Hypertension
The clinical characteristics of essential hypertension cases and healthy controls are listed in Table I. There were no significant differences in established cardiovascular risk factors such as age, body mass index, total cholesterol and triglyceride levels, HDL cholesterol, smoking habits, and alcohol consumption between cases and controls.
Table I.
Clinical Characteristics of the Total Study Population
| Variables | Normotension (n=201) | Hypertension (n=81) | P Value |
|---|---|---|---|
| Age, y | 26.9±4.2 | 26.7±4.2 | NS |
| Alcohol, g/wk | 97.3±93.3 | 92.2±110.0 | NS |
| Physical activity, h/wk | 3.5±3.3 | 3.3±3.5 | NS |
| Body mass index, kg/m2 | 24.3±3.4 | 24.5±3.1 | NS |
| Total cholesterol, mg/dL | 186.1±48.2 | 186.5±40.0 | NS |
| HDL cholesterol, mg/dL | 50.4±12.9 | 54.6±15.7 | NS |
| Triglycerides, mg/dL | 127.6±106.95 | 128.2±98.3 | NS |
| eGFR, mL/min/1.73 m2 | 101.9±21.0 | 100.2±17.2 | NS |
| Casual systolic BP, mm Hg | 128.6±11.9 | 146.4±10.0 | <.001 |
| Casual diastolic BP, mm Hg | 79.6±9.8 | 91.6±10.4 | <.001 |
| rs168924 | |||
| AA | 148 (74) | 67 (83) | .116 |
| AG | 49 (24) | 13 (16) | |
| GG | 4 (2) | 1 (1) | |
| rs168924 | |||
| A alleles | 345 (86) | 147 (91) | .113 |
| G alleles | 57 (14) | 15 (9) | |
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; NS, not significant. Data are presented as mean±standard deviation or number (percentage).
The distribution of the rs168924 genotypes in the total study population did not deviate significantly from Hardy‐Weinberg equilibrium (χ2=0.13, P=.72) and was similar to previously reported data in a Japanese population. 10 The minor allele frequency determined in our study (0.131) was similar to that estimated by the HapMap project (Utah residents with ancestry from Northern and Western Europe, 0.155). The rs168924 genotype distribution did not significantly differ between hypertensive and normotensive patients (Armitage’s test for trend P=.116). Frequencies of rs168924 A and G alleles were also not significantly different between cases and controls (relative risk, 0.95; 95% confidence interval [CI], 0.89–1.92; Pearson’s χ2=2.51; P=.113). However, when considering age, body mass index, triglycerides, total cholesterol, HDL cholesterol, eGFR, nicotine, alcohol consumption, and exercise as covariates in a multiple logistic regression analysis, rs188924 was significantly associated with the diagnosis of hypertension (odds ratio, 0.48; 95% CI, 0.23–0.98; P=.044).
Association of rs168924 With BP and Echocardiographic Measures
For a more in‐depth analysis, BP was used as a continuous independent variable, thereby overcoming the limitations of defining cut‐off points for the diagnosis hypertension.
Therefore, we included age, body mass index, triglycerides, total cholesterol, HDL cholesterol, eGFR, nicotine use, alcohol consumption, and exercise as covariates in our analysis. BP and metabolic risk factors potentiate each other, providing the rationale to include these factors in our analysis. Moreover, the prevalence of systolic hypertension increases with age, and excess body weight, reduced physical activity, and excess alcohol intake have been identified as causal factors for hypertension and were therefore also included. The multiple regression analysis revealed a significant association between rs168924 genotype and casual systolic BP (regression coefficient −4.02±1.82, P=.028), but not casual diastolic BP (P=.594). Moreover, comparison of adjusted casual systolic BP values across rs168924 genotypes revealed significant differences (AA 134.7±13.7 mm Hg, AG 131.0±11.5 mm Hg, GG 131.9±5.9 mm Hg; P=.044).
The gold standard for defining BP status is 24‐hour ambulatory BP recording with an automatic device, and echocardiography is a sensitive noninvasive method for assessing cumulative effects of high BP on heart structure and function. A subpopulation of 209 patients from our study cohort underwent 24‐hour ambulatory BP monitoring and echocardiography. Clinical characteristics (Table II) did not differ from the overall study population. As expected, echocardiographic markers of LV hypertrophy (ie, values of posterior and septal wall thickness and LV mass index) were substantially higher in hypertensive patients than in normotensive controls. LV functional parameters (ie, midwall fractional fiber shortening and the ejection fraction) did not differ between the two groups. Multiple regression analysis revealed that daytime ambulatory systolic BP was significantly associated with the rs168924 genotype (regression coefficient −3.51±1.49, P=.020; Figure 1). Genotype‐dependent differences in ambulatory systolic BP were accompanied by significant genotype‐associated changes in the diastolic posterior wall thickness (regression coefficient −0.41±0.20, P=.041).
Table II.
Clinical Characteristics of Individuals In Whom Ambulatory BP and Echocardiography Data Were Available
| Variables | Normotension (n=140) | Hypertension (n=69) | P Value |
|---|---|---|---|
| Age, y | 26.3±4.1 | 26.7±4.4 | NS |
| Alcohol, g/wk | 96.7±93.2 | 92.2±110.0 | NS |
| Physical activity, h/wk | 3.6±3.3 | 3.3±3.5 | NS |
| Body mass index, kg/m2 | 24.3±3.5 | 24.6±3.2 | NS |
| Total cholesterol, mg/dL | 179.4±36.8 | 185.3±37.4 | NS |
| HDL cholesterol, mg/dL | 50.8±13.8 | 54.7±16.2 | NS |
| Triglycerides, mg/dL | 124.9±112.5 | 134.0±78.0 | NS |
| eGFR, mL/min/1.73 m2 | 106.8±23.5 | 105.7±18.7 | NS |
| Diastolic posterior wall thickness, mm | 9.2±1.2 | 9.8±1.4 | .001 |
| Diastolic septal wall thickness, mm | 9.9±1.4 | 10.5±1.5 | .005 |
| Left ventricular mass index, g/m2 | 114.2±20.5 | 121.2±21.9 | .045 |
| Ejection fraction, % | 64.6±6.5 | 65.3±6.5 | NS |
| Midwall fractional fiber shortening, % | 35.7±4.8 | 36.3±5.2 | NS |
| Ambulatory systolic BP: daytime, mm Hg | 124.5±6.5 | 140.5±6.7 | <.001 |
| Ambulatory diastolic BP: daytime, mm Hg | 74.5±5.8 | 84.6±6.8 | <.001 |
| Heart rate: daytime, per minute | 74.1±9.2 | 77.3±9.7 | .02 |
| Ambulatory systolic BP: nighttime, mm Hg | 113.3±7.9 | 123.1±8.6 | <.001 |
| Ambulatory diastolic BP: nighttime, mm Hg | 63.8±6.9 | 69.3±8.5 | <.001 |
| Heart rate: nighttime, per minute | 63.1±10.4 | 63.8±9.9 | NS |
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; NS, not significant. Data are presented as mean±standard deviation.
Figure 1.
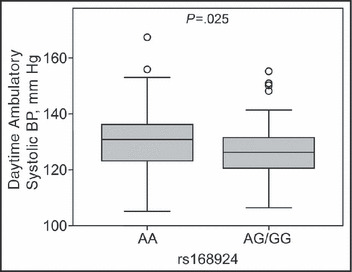
Daytime ambulatory systolic blood pressure (BP) according to the rs168924 A>G genotypes. BP values were adjusted for age, body mass index, triglycerides, total cholesterol, high‐density lipoprotein cholesterol, smoking, alcohol consumption, physical activity, and estimated glomerular filtration rate.
Table III directly compares data from ambulatory BP recordings by genotype. These data did not significantly differ between rs168924 genotypes except daytime systolic BP, which was significantly lower in carriers of the G allele compared with the reference AA genotype. Data suggest a gene dose effect, consistent with a codominant (additive) genetic model, although the small number of individuals with the GG genotype precludes a definite characterization of the genetic model.
Table III.
Ambulatory BP and Heart Rate According to rs168924 Genotype
| AA (n=157) | AG (n=49) | GG (n=3) | P Value | |
|---|---|---|---|---|
| Ambulatory systolic BP: daytime, mm Hg | 130.8±9.4 | 128.1±10.5 | 121.9±8.4 | .020 |
| Ambulatory diastolic BP: daytime, mm Hg | 78.4±7.5 | 78.1±7.1 | 67.8±5.9 | .381 |
| Heart rate: daytime, per minute | 75.3±8.3 | 75.7±8.5 | 71.2±2.9 | .738 |
| Ambulatory systolic BP: nighttime, mm Hg | 117.3±9.0 | 115.1±9.6 | 109.8±4.0 | .106 |
| Ambulatory diastolic BP: nighttime, mm Hg | 66.3±7.2 | 65.6±7.1 | 55.0±2.0 | .153 |
| Heart rate: nighttime, per minute | 63.5±9.3 | 64.1±9.2 | 56.7±8.3 | .792 |
Blood pressure (BP) and heart rate values were adjusted for age, body mass index, triglycerides, total cholesterol, high‐density lipoprotein cholesterol, smoking, alcohol consumption, physical activity, and estimated glomerular filtration rate. Data are presented as mean±standard deviation.
Post‐Hoc Power Calculation
Based on the linear regression model with casual systolic BP as the dependent variable, the number of included predictors, and the observed R2, the sample size was adequate to provide 96% and 88% statistical power at nominal type I error rates of 0.05 and 0.01, respectively.
Linkage Disequilibrium Analysis
To date, the functional role of rs168924 is unknown. In particular, it is unknown whether rs168924 is in linkage disequilibrium (LD) with another functionally significant SNP. A candidate might be the NG_016969.1:g.2277A>T SNP (rs28386840). This SNP in the regulatory region of SLC6A2 within 3‐kb distance from rs168924 alters SLC6A2 promoter activity. 19 Because rs28386840 is not included in the HapMap analysis (Figure 2), it was unknown whether rs28386840 and rs168924 were in LD. Genotyping of our cohort revealed no significant LD between both SNPs (D’=0.587, LOD=1.43, r 2=0.019; Figure 2).
Figure 2.
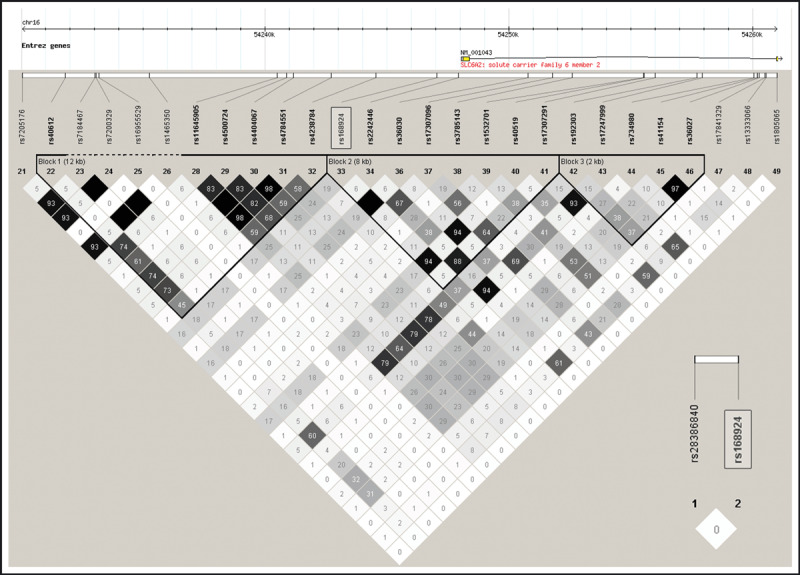
Linkage disequilibrium across the region containing rs168924. Estimates of the square of the correlation coefficient (r 2) were calculated for each pairwise comparison of SNPs based on data from the HapMap‐CEU sample. Insert: Linkage disequilibrium between rs168924 and rs28386840 (r 2) based on data from our study cohort.
Discussion
For our association study, we recruited a homogenous study population with respect to covariates that may—in addition to genetic factors—affect BP, such as age, sex, or antihypertensive medication. In fact, we investigated young, Caucasian men with an early stage of hypertensive heart disease who had never been treated and were not taking any concurrent medication for hypertension. In this respect, our study differs from the study of Ono and colleagues, 10 which was based on cross‐sectional epidemiologic study data. Moreover, we intended to define BP status, in addition to casual BP measurements, more accurately by applying 24‐hour ambulatory BP recording, which is suggested to be the gold standard. Thereby, we sought to better unravel the potential association of a specific genetic factor, namely the rs168924 SNP, with BP.
When established cardiovascular risk factors were considered as covariates by multiple regression analysis, we found an association of the rs168924 SNP with hypertension (odds ratio, 0.48; P=.044) and casual systolic BP (P=.039) at borderline significance. Of note, a significant association of rs168924 with systolic BP was confirmed when an independent measure, namely daytime systolic BP values obtained by ambulatory BP recordings, were analyzed. The finding that rs168924 was also significantly associated with diastolic posterior wall thickness, an echocardiographic index of hypertensive cardiac target organ damage, further strengthens the conclusion that the rs168924 SNP in SLC6A2 in fact influence BP.
Our data are the first to demonstrate a significant correlation of rs168924 with arterial hypertension in Caucasians. Based on previous findings in a Japanese population, 10 we expected the minor G allele to be associated with higher BP. However, in our study population, presence of the minor G allele was associated with lower systolic BP values. The reason for the different findings in our and the previous study is unclear. It can be speculated that the effects of the rs168924 SNP on BP change with age, which was substantially higher in the cohort of the Japanese study. Ethnic differences may also play a role. Of note, Ono and colleagues 10 reported a significant association of rs168924 with the incidence of hypertension, but not with systolic or diastolic BP.
Interpreting the results is complicated by the absence of studies addressing the functional consequences of the rs168924 promoter polymorphism on the transcriptional activity or expression of NET protein. Moreover, it is unknown whether rs168924 is in LD with other SNPs in the SLC6A2 gene or adjacent genes that do actually affect BP. An interesting candidate might be the NG_016969.1:g.2277A>T SNP (rs28386840). This SNP in the regulatory region of SLC6A2 within 3‐kb distance from rs168924 alters SLC6A2 promoter activity and has been associated with attention deficit hyperactivity disorder. 19 A most recent study found a significant association of this SNP with BP response to exercise, 20 while baseline (resting) BP was not associated with rs28386840 in that study. Genotyping of our cohort revealed no significant LD between rs28386840 and rs168924.
If there is indeed an association between rs168924 and BP, why did large genome‐wide association studies (GWAS) fail so far to confirm SLC6A2 as a hypertension susceptibility locus? One reason might have been that GWAS platforms do not yet cover the full spectrum of genetic variability. For example, one platform frequently used in GWAS does not include rs168924 or markers in LD with rs168924, such as rs36030 or rs40519. Another explanation might be related to differences in the study populations and the level by which covariates were controlled (only young male participants without antihypertensive medication use and comorbidities in our study vs cross‐sectional or longitudinal population cohorts in most GWAS).
Conclusions
Based on an in‐depth phenotypic characterization including ambulatory 24‐hour BP recordings and echocardiography, which are not routinely applied to genotype‐phenotype association studies, our data provide evidence that the rs168924 SNP in the SLC6A2 gene in fact modulates BP. The rs168924 G variant was associated with a lower prevalence of arterial hypertension in young Caucasian men.
Acknowledgments
Acknowledgment and disclosure: This work was supported by a grant from the National Genome Research Network (NGFN). None of the authors have any financial conflicts of interest to disclose.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Chang YP, Liu X, Kim JD, et al. Multiple genes for essential‐hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther. 2001;91:35–62. [DOI] [PubMed] [Google Scholar]
- 4. Schömig E, Fischer P, Schonfeld CL, Trendelenburg U. The extent of neuronal re‐uptake of 3H‐noradrenaline in isolated vasa deferentia and atria of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:502–508. [DOI] [PubMed] [Google Scholar]
- 5. Halushka MK, Fan JB, Bentley K, et al. Patterns of single‐nucleotide polymorphisms in candidate genes for blood‐pressure homeostasis. Nat Genet. 1999;22:239–247. [DOI] [PubMed] [Google Scholar]
- 6. Esler M, Rumantir M, Kaye D, et al. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:986–989. [DOI] [PubMed] [Google Scholar]
- 7. Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. [DOI] [PubMed] [Google Scholar]
- 8. Zill P, Engel R, Baghai TC, et al. Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine transporter and analysis in major depression. Neuropsychopharmacology. 2002;26:489–493. [DOI] [PubMed] [Google Scholar]
- 9. Hahn MK, Mazei‐Robison MS, Blakely RD. Single nucleotide polymorphisms in the human norepinephrine transporter gene affect expression, trafficking, antidepressant interaction, and protein kinase C regulation. Mol Pharmacol. 2005;68:457–466. [DOI] [PubMed] [Google Scholar]
- 10. Ono K, Iwanaga Y, Mannami T, et al. Epidemiological evidence of an association between SLC6A2 gene polymorphism and hypertension. Hypertens Res. 2003;26:685–689. [DOI] [PubMed] [Google Scholar]
- 11. Moldovanova I, Schroeder C, Jacob G, et al. Hormonal influences on cardiovascular norepinephrine transporter responses in healthy women. Hypertension. 2008;51:1203. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, De BG, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 13. Ott C, Schwarz T, Hilgers KF, et al. Left‐ventricular structure and function are influenced by angiotensinogen gene polymorphism (‐20 A/C) in young male patients. Am J Hypertens. 2007;20:974–980. [DOI] [PubMed] [Google Scholar]
- 14. Zolk O, Jacobi J, Pahl A, et al. MDR1 genotype‐dependent regulation of the aldosterone system in humans. Pharmacogenet Genomics. 2007;17:137–144. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 16. Schmieder RE, Langenfeld MR, Friedrich A, et al. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation. 1996;94:1304–1309. [DOI] [PubMed] [Google Scholar]
- 17. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 18. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 19. Kim CH, Hahn MK, Joung Y, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention‐deficit hyperactivity disorder. Proc Natl Acad Sci U S A. 2006;103:19164–19169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohli U, Hahn MK, English BA, et al. Genetic variation in the presynaptic norepinephrine transporter is associated with blood pressure responses to exercise in healthy humans. Pharmacogenet Genomics. 2011;21:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]


