Abstract
J Clin Hypertens (Greenwich). 2011;13:662–666. ©2011 Wiley Periodicals, Inc.
Key Points and Practical Recommendations
-
•
Aliskiren, the sole oral renin inhibitor approved by the US Food and Drug Administration, is indicated for the treatment of hypertension, either as monotherapy or in combination, with reductions in blood pressure similar to other agents.
-
•
Early evidence suggests that aliskiren confers additional benefit in patients with diabetic nephropathy. Data are not yet available to determine whether protection will extend to cardiovascular disease.
-
•
No initial dosage adjustment is required in elderly patients or for patients with mild to severe renal impairment; however, clinical experience is limited in patients with significant renal impairment, and with renal artery stenosis.
-
•
It appears rational to combine aliskiren with agents that otherwise increase plasma renin activity, including thiazide diuretics, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers.
-
•
While there is a reactive rise in renin in response to aliskiren, probably larger than that induced by angiotensin receptor blockers and angiotensin‐converting enzyme inhibitors, there is no evidence that this rise is harmful.
-
•
In placebo‐controlled studies, the incidence of edema anywhere in the body was 0.4% with aliskiren compared with 0.5% with placebo. It is unknown whether angioedema rates are higher in blacks with aliskiren.
-
•
Aliskiren is associated with a slight increase in cough, with rates of about one third to one half seen with angiotensin‐converting enzyme inhibitors.
-
•
Increases in serum potassium >5.5 meq/L were infrequent in patients with essential hypertension treated with aliskiren alone (0.9% compared with 0.6% with placebo).
Background
While blockade of the renin‐angiotensin‐aldosterone system (RAAS) has become a cornerstone of antihypertensive therapy with angiotensin‐converting enzyme (ACE) inhibition and angiotensin receptor blockade, renin inhibition has lagged. Its late appearance on the therapeutic horizon relates largely to biochemistry. The development of renin inhibitors was hampered by high cost of manufacture, coupled with low potency and poor bioavailability of early molecules. With the advent of alternative approaches to manufacture, notably molecular modeling via x‐ray crystallography and reconstruction of the active site of renin, potent nonpeptidic oral renin inhibitors are being produced. Their potential in therapy shows significant promise, although clinical experience and outcome data are limited.
Mechanisms of action
Pharmacology
Renin inhibitors are unique in their effects on the RAAS. Both ACE inhibition and angiotensin receptor blockade lead to a reactive rise in plasma renin activity (PRA) and thus to an increase in the angiotensin peptides, both angiotensin I and angiotensin II in the case of angiotensin receptor blockers (ARBs) and angiotensin I with ACE inhibitors. Renin inhibitors, operating at the first and rate‐limiting step of the cascade, render the entire pathway quiescent. Because renin is specific for the substrate angiotensinogen, renin inhibitors do not cause stimulation of bradykinin or prostaglandins. In addition, renin inhibitors reduce angiotensin II formed by non‐ACE pathways.
Renin inhibitors may also confer benefit by inhibiting activation of the proenzyme prorenin, long thought to be inactive. Prorenin can be activated by conversion to renin through cleavage of a 43‐amino acid segment from the N‐terminal end. Importantly, when it binds to the (pro)renin receptor it can also undergo nonproteolytic activation via conformational change and exposure of the active site. The discovery and identification of this receptor, primarily on glomerular mesangial cells and vascular smooth muscle cells, has offered new insights into possible pathophysiology. 1 With the binding of renin to this receptor, its catalytic efficiency of converting angiotensinogen to angiotensin I increases 4‐fold. As impressive, prorenin was found to bind the receptor, and when bound, is as active as renin in causing a biologic response. Either renin or prorenin occupation of the receptor results in intracellular signaling processes accompanied by activation of mitogen‐activated protein kinases, transforming growth factor β, and plasminogen activator inhibitor‐1, independent of angiotensin generation. If prorenin contributes to pathophysiology, as data in diabetics have suggested, 2 then renin inhibitors possess an expanded potential for therapy compared with ACE inhibitors or ARBs (Figure). Aliskiren has a steady‐state half‐life in plasma of 23 to 36 hours 3 ; its tissue half‐life is even more prolonged.
Figure FIGURE.
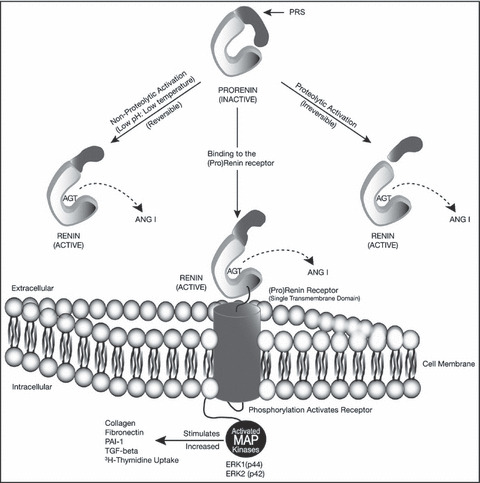
Pro(renin) receptor–binding initiates intracellular signaling 1 of 3 mechanisms to activate prorenin to renin. AGT indicates angiotensinogen; ANG I, angiotensin I; MAP, mitogen‐activated protein; PAI, plasminogen activator inhibitor; PRS, prorenin segment; TGF, transforming growth factor.
Drug Differentiation
To date, aliskiren is the sole oral renin inhibitor approved for use in humans. Earlier molecules, peptide analogs including remikiren and enalkiren, had potency only with parenteral administration given severely limited oral bioavailability. Several laboratories have new orally effective molecules, produced by x‐ray crystallographic procedures, in the pipeline.
Indications and Outcome Studies
Aliskiren is indicated for the treatment of hypertension, either as monotherapy or in combination. Clinical trials in more than 12,000 people have demonstrated that once‐daily aliskiren is effective in treating mild to moderate hypertension, with reductions in blood pressure similar to other agents.
Early placebo‐controlled trials evaluated the impact of aliskiren as monotherapy; a dose‐response relationship was demonstrated up to 300 mg/d. 4 , 5 , 6 No significant further blood pressure (BP) lowering was seen with the 600‐mg dose, which was accompanied by increased gastrointestinal side effects. A series of subsequent clinical trials evaluated the impact of aliskiren against other agents, including hydrochlorothiazide (HCTZ) and ACE inhibitors. These included studies in elderly hypertensive patients 7 , 8 and patients with severe hypertension. 9 They have generally demonstrated a very favorable safety and tolerability profile together with equivalent degrees of BP lowering.
Most hypertensive patients require combination therapy to lower their BP. Aliskiren has been studied in combination with thiazide diuretics, calcium channel blockers (CCBs), ACE inhibitors, and ARBs, and was found to be more effective in combination with each than alone. 10 , 11 , 12 , 13 , 14 , 15 Several long‐term combination therapy trials demonstrated a similar efficacy and safety profile with additive BP‐lowering effects when aliskiren was combined with HCTZ (including one study in obese hypertensives). 11 , 12 Combining aliskiren with thiazides, as with ACE inhibitors and ARBs, blocks the rise in PRA otherwise seen. Aliskiren has also been shown to have additive efficacy with amlodipine. 15 , 16 Interestingly, initial use of combined aliskiren and amlodipine over 24 weeks achieved superior BP reduction and tolerability when compared with sequential add‐on treatment with the same drugs. 15 Dual blockade of the RAAS with aliskiren and ramipril has resulted in lower BP than with either alone. 13 After 6 months of randomized treatment, there was a slower return to median BP level <140/90 mmHg with the aliskiren based regimen (4 weeks) than with ramipril (1 week) following withdrawal of therapy. 13 This sustained BP‐lowering effect has been replicated and is attributed to the long half‐life of the drug. 17 Another study of dual blockade in 1797 patients with mild to moderate hypertension also demonstrated that the combination of aliskiren 300 mg and valsartan 320 mg resulted in a significantly greater BP reduction than either aliskiren or valsartan alone (−17.2/−12.2 mm Hg vs −13/−9 mm Hg and −12.8/−9.7 mm Hg, respectively; P<.0001). 10
The benchmark for selecting antihypertensive therapy in clinical practice has shifted during the past decade. While the primary goal continues to be controlling BP, there is an ever‐increasing burden to show added benefit in terms of improvement in surrogate markers of disease progression, target organ damage, and ultimately in clinical events.
Four cardiorenal surrogate end point studies have been published with aliskiren. The Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) trial demonstrated that the addition of aliskiren to ARB therapy with losartan in patients with hypertension and type 2 diabetic nephropathy for 6 months resulted in a 20% reduction in mean urinary albumin‐to‐creatinine ratio (UACR) (P<.001), when compared with losartan alone. 18 There was a 2/1‐mm Hg (P<.07) greater fall in BP at study end with the renin inhibitor. After adjustment, the reduction in UACR with aliskiren was still 18% greater than with placebo. There was no difference in the overall incidence of adverse events between the two groups. The publication of the AVOID study has raised considerable debate. Therapy with an ARB (or an ACE inhibitor) is considered standard of care in patients with proteinuria and type 2 diabetes mellitus, with many practitioners using the combination to reduce proteinuria. However, reports from the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) question the utility and safety of dual RAAS blockade. 19 The AVOID study comes at a time when alternative approaches to dual RAAS blockade are being sought. Of note, data from the secondary prevention Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) (Table) are awaited to ascertain whether direct renin inhibition with aliskiren reduces cardiovascular and renal morbidity and mortality in patients with type 2 diabetes. 20
Table TABLE.
Summary of Completed BNP Surrogate End Point Studies and Ongoing Clinical End Point Trials
| Study Acronym | Patient Population | Outcome Measure |
|---|---|---|
| ALLAY | Hypertensive with LV hypertrophy | LV mass |
| ALOFT | Hypertensive with class II to IV heart failure | BNP + NT‐proBNP |
| AVOID | Hypertensive type II diabetes with proteinuria | UACR |
| ASPIRE | Post‐MI with systolic dysfunction | LV remodeling |
| ALTITUDE | Type II DM with CVD and/or DM nephropathy | Time to first event—CV death/MI |
| ESRD | ||
| Doubling serum creatinine | ||
| APOLLO | Normal and hypertensive elderly patients | Prevention of CV end points |
| ASTRONAUT | Acute heart failure | Time to first event—CV death |
| Hospitalization | ||
| ATMOSPHERE | Chronic systolic heart failure | Time to first event—CV death |
| Hospitalization |
Abbreviations: BNP, brain natriuretic protein; CV, cardiovascular; DM, diabetes mellitus; ESRD, end‐stage renal disease; LV, left ventricular; MI, myocardial infarction; NT proBNP, N‐terminal proBNP; UACR, urinary albumin creatinine ratio.
The Aliskiren Left Ventricular Assessment of Hypertrophy (ALLAY) trial demonstrated that aliskiren was as effective as losartan in reducing left ventricular mass index (LVMI; P<.001 for noninferiority). However, the combination of aliskiren and losartan was no more effective in reducing LVMI than losartan monotherapy in an obese hypertensive population with documented LV hypertrophy (P=.52). 20 BP was significantly and similarly reduced in all groups, and no added toxicity was reported for the combination. The Aliskiren Study in Post‐MI Patients to Reduce Remodeling (ASPIRE) trial evaluated the impact of aliskiren on left ventricular (LV) remodeling when added to standard therapy with an ACE inhibitor or an ARB in high‐risk post‐myocardial infarction patients with LV systolic dysfunction. 22 The study demonstrated that the addition of aliskiren to standard therapy (that included either an ACE inhibitor or an ARB) provided no further attenuation of LV remodeling and was associated with increased hypotension, hyperkalemia, and elevation in creatinine. The combined results of ALLAY and ASPIRE show that there is no positive impact on LV hypertrophy or LV remodeling with combined aliskiren and ARB or aliskiren and ACE inhibitor therapy.
The addition of aliskiren to standard of care in patients with heart failure, examined in the Aliskiren Observation of Heart Failure Treatment (ALOFT) trial demonstrated a reduction of neurohumoral activation (BNP and NT‐pro‐BNP), previously linked to adverse outcome in patients with heart failure. 23 The rationale for such an approach was supported by the known deleterious impact of activation of RAAS in patients with heart failure and the additional knowledge that while ACE inhibitors and ARBs have proven efficacy in this patient population, sustained increases in PRA persist despite therapy. These data, however encouraging, are not definitive. Wide ranges in standard deviations raise obvious questions regarding reproducibility, and it remains to be proven whether such improvements in neurohumoral activation can be sustained over time and are correlated with a reduction in cardiovascular events. The Aliskiren Trial to Minimize Outcomes in Patients With Heart Failure (ATMOSPHERE) and rationale and design of the multicenter, randomized, double‐blind, placebo‐controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) studies designed to evaluate the impact of aliskiren on heart failure are underway. 24 , 25 Finally the Aliskiren in Prevention of Later Life Outcomes (APOLLO) trial will address elderly patients with a systolic BP 130 to 159 mm Hg, no overt cardiovascular disease, and a high cardiovascular risk profile, in order to test the efficacy of the drug in reducing the risk of major cardiovascular end points.
Ultimately, determination of the impact of direct renin inhibition on clinical end points is essential to delineate the most appropriate use of this approach in clinical practice. Results from studies designed to answer this question are expected in 2012 and beyond. 26
Clinical Use
Hypertension
Aliskiren has been approved for the treatment of hypertension and is effective both as monotherapy and in combination with other classes. Aliskiren has a dose‐related effect on lowering BP at 150 mg and 300 mg. Its BP‐lowering efficacy is comparable with other classes, and it is well tolerated. Greater effect on BP has been seen when aliskiren is taken in combination with a thiazide diuretic, CCB, ACE inhibitor, or ARB.
Target Organ Protection
There are theoretical reasons to speculate why treatment with renin inhibition might be superior to other classes in the prevention of target organ damage. As stated above, these include its action at the rate‐limiting step in the cascade, thus rendering the entire pathway quiescent, together with its inhibition of activation of prorenin. Data from the AVOID trial provide early evidence that aliskiren confers additional protective benefit to standard treatment with ARBs in patients with diabetic nephropathy. Whether protection will extend to cardiovascular disease depends on data as yet largely unavailable. Until that time, clinical use may be confined for the most part to the treatment of hypertension, with consideration given to patients with diabetes and nephropathy.
Variations in Response: Special Situations
There are limited data on the use of aliskiren in special situations. No initial dosage adjustment is required in elderly patients or for patients with mild to severe renal impairment. However, clinical experience is limited in patients with significant renal impairment and with renal artery stenosis. One study demonstrated efficacy of aliskiren in lowering BP in obese hypertensive patients when combined with HCTZ. 12
Commentary on Combination
Combination with aliskiren has been shown to be safe and effective with thiazide diuretics, CCBs, ACE inhibitors, and ARBs. Combination allows lower doses and therefore fewer side effects (ie, less edema with amlodipine 5 mg). It appears rational to combine aliskiren with agents that otherwise result in an increase in PRA, including thiazide diuretics, ACE inhibitors, and ARBs. While there is a reactive rise in renin in response to aliskiren, indeed probably larger than that induced by ARBs and ACE inhibitors, there is no evidence that this rise is harmful. Indeed, this represents part of the evidence that renin inhibition leads to more complete blockade of the renin system. 27
Relevant Drug Interactions and Major Adverse Effects
In general, aliskiren is well tolerated, with a safety profile at doses up to 300 mg, similar to that of placebo or ARB. The most consistently reported side effect is diarrhea, with incidence at 300 mg in the range of 2%; gastrointestinal upset is more likely in women and the elderly. There is no clinical experience with the use of aliskiren in pregnant women, but it carries the same warning as other drugs that act directly on the RAAS and can cause injury and even death to the developing fetus. Aliskiren should be discontinued as soon as possible after pregnancy is diagnosed. Whether aliskiren causes angioedema is unknown. A handful of cases have been reported in clinical studies, at a rate of 0.06%. In placebo‐controlled studies, the incidence of edema anywhere in the body was 0.4% with aliskiren compared with 0.5% with placebo. It is also unknown whether angioedema rates are higher with aliskiren in black patients. Aliskiren is associated with a slight increase in cough; rates in comparison studies have been about one third to one half the rates in the ACE inhibitor arms. Increases in serum potassium >5.5 meq/L were infrequent in patients with essential hypertension treated with aliskiren alone (0.9% compared with 0.6% with placebo). However, when used in combination with an ACE inhibitor in diabetics, such increases occurred more frequently (5.5%). Routine monitoring of electrolytes and renal function is indicated in this population. Lastly, when aliskiren was given with furosemide, the blood concentrations of furosemide were significantly reduced.
Disclosure: The authors received no honoraria for their contribution to this issue.
References
- 1. Nguyen G, Delarue F, Burckle C, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luetscher JA, Kraemer FB, Wilson DM, et al. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312:1412–1417. [DOI] [PubMed] [Google Scholar]
- 3. Nussberger J, Wuerzner G, Jensen C, et al. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1–E8. [DOI] [PubMed] [Google Scholar]
- 4. Stanton A, Jensen C, Nussberger J, et al. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42:1137–1143. [DOI] [PubMed] [Google Scholar]
- 5. Gradman AH, Schmieder RE, Lins RL, et al. Aliskiren, a novel orally effective renin inhibitor, provides dose‐dependent antihypertensive efficacy and placebo‐like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. [DOI] [PubMed] [Google Scholar]
- 6. Kushiro T, Itakura H, Abo Y, et al. Aliskiren, a novel oral renin inhibitor, provides dose‐dependent efficacy and placebo‐like tolerability in Japanese patients with hypertension. Hypertens Res. 2006;29:997–1005. [DOI] [PubMed] [Google Scholar]
- 7. Verdecchia P, Calvo C, Mockel V, et al. Safety and efficacy of the oral direct renin inhibitor aliskiren in elderly patients with hypertension. Blood Press. 2007;16:381–391. [DOI] [PubMed] [Google Scholar]
- 8. Duprez DA, Munger MA, Botha J, et al. Aliskiren for geriatric lowering of systolic hypertension: a randomized controlled trial. J Hum Hypertens. 2010;24:600–608. [DOI] [PubMed] [Google Scholar]
- 9. Strasser RH, Puig JG, Farsang C, et al. A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension. J Hum Hypertens. 2007;21:780–787. [DOI] [PubMed] [Google Scholar]
- 10. Oparil S, Yarows SA, Patel S, et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double‐blind trial. Lancet. 2007;370:221–229. [DOI] [PubMed] [Google Scholar]
- 11. Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens. 2007;25:217–226. [DOI] [PubMed] [Google Scholar]
- 12. Jordan J, Engeli S, Boye SW, et al. Direct renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension. 2007;49:1047–1055. [DOI] [PubMed] [Google Scholar]
- 13. Andersen K, Weinberger MH, Egan B, et al. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6‐month, randomized, double‐blind trial. J Hypertens. 2008;26:589–599. [DOI] [PubMed] [Google Scholar]
- 14. Schmieder RE, Philipp T, Guerediaga J, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation. 2009;119:417–425. [DOI] [PubMed] [Google Scholar]
- 15. Brown MJ, McInnes GT, Papst CC, et al. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel‐group trial. Lancet. 2011;377:312–320. [DOI] [PubMed] [Google Scholar]
- 16. Drummond W, Munger MA, Rafique EM, et al. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add‐on therapy in patients not responding to amlodipine monotherapy. J Clin Hypertens (Greenwich). 2007;9:742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oh BH, Mitchell J, Herron JR, et al. Aliskiren, an oral renin inhibitor, provides dose‐dependent efficacy and sustained 24‐hour blood pressure control in patients with hypertension. J Am Coll Cardiol. 2007;49:1157–1163. [DOI] [PubMed] [Google Scholar]
- 18. Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH, Bangalore S, Ram VS. Telmisartan, ramipril, or both in patients at high risk of vascular events. N Engl J Med. 2008;359:426–427. [PubMed] [Google Scholar]
- 20. Parving HH, Brenner BM, McMurray JJ, et al. Aliskiren Trial in Type 2 Diabetes Using Cardio‐Renal Endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant. 2009;24:1663–1671. [DOI] [PubMed] [Google Scholar]
- 21. Solomon SD, Appelbaum E, Manning WJ, et al. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–537. [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, Hee SS, Shah A, et al. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J. 2011;119:530–537. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJV, Pitt B, Latini R, et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circulation Heart Failure. 2008;1:17–24. [DOI] [PubMed] [Google Scholar]
- 24. Krum H, Massie B, Abraham WT, et al. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail. 2011;13:107–114. [DOI] [PubMed] [Google Scholar]
- 25. Gheorghiade M, Albaghdadi M, Zannad F, et al. Rationale and design of the multicentre, randomized, double‐blind, placebo‐controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT). Eur J Heart Fail. 2011;13:100–106. [DOI] [PubMed] [Google Scholar]
- 26. Lee HY, Oh BH. Cardio‐renal protection with aliskiren, a direct renin inhibitor, in the ASPIRE HIGHER program. Expert Rev Cardiovasc Ther. 2009;7:251–257. [DOI] [PubMed] [Google Scholar]
- 27. Danser AH, Charney A, Feldman DL, et al. The renin rise with aliskiren: it’s simply stoichiometry. Hypertension. 2008;51:e27–e28. [DOI] [PubMed] [Google Scholar]


