Abstract
J Clin Hypertens (Greenwich). 2012;14:216–221. ©2012 Wiley Periodicals, Inc.
The initial description of Page kidney, a form of renin‐mediated hypertension, included athletes with renal subcapsular hematoma after flank trauma. Subsequently, nontraumatic etiologies were identified. In this study, the authors compare traumatic and nontraumatic causes of Page kidney. All cases with hypertension attributable to renal hematoma at our institution from 1960 to 2010 were reviewed. Twenty‐six patients (9 trauma, 17 nontrauma), with a mean age of 36.7 years, were included. Trauma patients were younger (P<.001), had lower systolic blood pressures (P=.011), and higher baseline estimated glomerular filtration rate (eGFR), (P=.027) at presentation. No differences in presenting features, imaging, urinalysis, or pathology are noted. Nontrauma cases required more antihypertensive medications (P=.001) and had higher nephrectomy rates. eGFR improved in all, but more in, trauma cases (P=.05). Through the analysis of 26 cases of Page kidney, two distinct groups were identified. Trauma patients tended to be younger, male, have less renal impairment and lower systolic blood pressure. Nontrauma patients required more antihypertensive medications and had a higher nephrectomy rate. New‐onset hypertension occurred independent of etiology, calling for close surveillance of blood pressures.
In 1939, Page 1 described a canine experiment in which he wrapped kidneys in cellophane and noted the induction of hypertension by perinephritis and compression of renal parenchyma. Subsequently, in 1955 he presented the first clinical case of Page kidney in an American football player. The patient experienced blunt renal injury with subcapsular hematoma and renin‐mediated hypertension, which normalized after nephrectomy. 2 Further observational studies confirmed the etiology of this clinical entity, with measurements of renin lateralization to the affected kidney 3 and by demonstrating responses to both saralazine infusion 4 , 5 and angiotensin‐converting enzyme (ACE) inhibitors. 6 Page kidney is believed to occur secondary to microvascular ischemia due to external compression and activation of the renin‐angiotensin‐aldosterone system, leading to hypertension.
Since this original description, more than a hundred cases have been published in the literature, including some cases retrospectively identified from case reports pre‐dating Page’s discovery. Page kidney has been reported in all age groups, including pediatric, 7 , 8 adolescent, 9 and adult patients in association with multiple etiologies, including blunt trauma, iatrogenic intervention (including surgical complications 10 , 11 , 12 such as following ureteral surgery and post‐renal biopsy [both allograft 13 , 14 , 15 , 16 , 17 , 18 and native 19 kidney]), domestic violence, 20 and parenchymal renal disease. 21 , 22 The largest published series to date is an international literature review. 19 , 23
The etiology and clinical presentation of Page kidney is believed to be changing. A number of explanations have been postulated, including the increased availability and quality of imaging; improvements in protective equipment worn by athletes; the increasing number of investigations being ordered by health care providers; and the increasing number of invasive procedures. It has been postulated that Page kidney that occurs as a result of a complicated renal allograft biopsy may be a different entity, as it is generally associated with acute hypertension and a concomitant decline in renal function, typically not present in a native Page kidney. 19
In this study, we aimed to review patients with Page kidney at our institution by analyzing presenting features, clinical findings, diagnostic techniques, and treatment interventions. In addition, we aimed to compare the blood pressure and renal function outcomes among the patients with different Page kidney etiologies.
Materials and Methods
A retrospective chart review of all patients seen at our institution between 1960 and 2010 was performed. As not all cases may have been assigned the specific diagnosis code of Page kidney, the Mayo Medical Index was searched using multiple keywords including intra‐renal hematoma, hypertension, and Page kidney. The retrieved medical records were screened initially for the possible inappropriate use of diagnostic codes; two independent investigators reviewed the remainder. All charts were further reviewed with predefined inclusion criteria, including confirmed renal hematoma, either by diagnostic imaging or postoperative surgical pathology, accompanied by a new or worsening hypertension attributable to the renal hematoma.
For the purposes of this study, hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and a diastolic blood pressure (DBP) ≥90 mm Hg. Worsening hypertension was defined as an increase in either SBP or DBP ≥10 mm Hg, or as a need to increase either the dose or the number of antihypertensive medications immediately after the insult. Trauma cases were defined as Page kidney occurring in association with traumatic events, such as motor vehicle accidents, football/hockey injuries, and falls/accidents at home (Figure 1). Nontrauma cases included both spontaneous cases (defined as renal hemorrhage occurring in association with renal pathology, such as tumor, arteriovenous malformation, cyst rupture, glomerulonephritis, or vasculitis) and iatrogenic cases (native kidney biopsy). Nontrauma cases were included only if kidney compression was noted on pathology or clinical imaging. Allograft cases were excluded. Successful response to treatment was defined as SBP <140 mm Hg and DBP <90 mm Hg, withdrawal of antihypertensive therapy, or an improvement in the Modification of Diet in Renal Disease glomerular filtration rate (MDRD GFR) of ≥10%.
Figure 1.
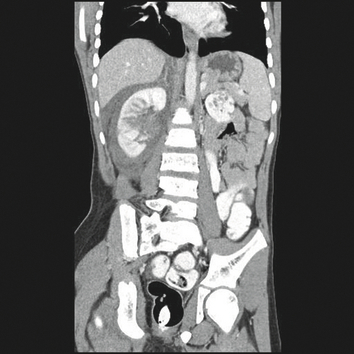
A case of an 11‐year‐old boy who experienced a right renal laceration in a snow‐sledding accident. This noncontrast‐enhanced 3‐dimensional reconstruction image shows a large right perinephric hematoma. Hypertension was noted on presentation with Page kidney and he was commenced on angiotensin‐converting enzyme inhibitor therapy. Seven months after the accident, his blood pressure normalized and antihypertensive medication was discontinued.
Data extraction included patient demographics, clinical presentations, comorbid conditions, blood pressures, serum creatinine at presentation and after therapeutic intervention, diagnostic modalities used to document hematoma and therapeutic interventions. Statistical analysis was performed using PASW for Mac version 18.0 (SPSS, IBM, Armonk, NY). Quantitative data were reported using mean, median, standard deviation, and range, where appropriate. Further analysis for associations among variables was carried out using chi‐square, Student t test, and analysis of variance, where appropriate. A P value ≤.05 was considered statistically significant.
Results
The initial search yielded 116 potential patients treated at our institution during the study period. Following an in‐depth review, as described above, 26 patients fit the criteria for a diagnosis of Page kidney. An additional six cases of Page kidney occurring in patients with renal allografts were identified, but excluded from analyses.
Baseline Variables
A wide age range of patients were included in the analysis, with a mean age of 36.7±20.4 years. The population was predominantly male (53.8% [n=14]). The etiology was traumatic in 34.6% (n=9) of cases, with the remainder (65.4% [n=17]) without any associated trauma. Sports injuries accounted for 5 of the trauma cases, with the remaining 4 occurring as a result of motor vehicle accidents. Nontrauma‐associated cases occurred due to several reasons, including native kidney biopsies (n=5), ruptured renal cyst (n=3), hypernephroma (n=2), incidental diagnosis of hypertension (n=2), polyarteritis nodosa (n=2), arteriovenous malformation (n=1), renovascular disease (n=1), and spontaneous intrarenal hemorrhage (n=1). Trauma patients were, on average, 28.41 years younger than nontrauma patients (P<.001). Prior to presentation with Page kidney, 8 of 26 patients (31%) had hypertension, seen exclusively in the nontrauma cases (P=.008).
Renal Function at Presentation
Assessments of renal function and blood pressure were performed at presentation (Table I). Trauma patients had higher MDRD GFRs than nontrauma patients (P=.027), but lower systolic blood pressures (P=.011).
Table I.
Demographic Data, Renal Function, and Blood Pressure at Presentation by Etiology
| Parameter | Overall (n=26) | Trauma (n=9) | Nontrauma (n=17) | P Value |
|---|---|---|---|---|
| Mean age (±SD), y | 36.7 (±20.4) | 18.1 (±12.0) | 46.5 (±16.8) | <.001a |
| Male sex, % | 53.8 (n=14) | 66.7 (n=6) | 47.1 (n=8) | .296 |
| Mean MDRD GFR, mL/min/1.73m2 | 60.72 (44.38) | 94.28 (56.30) | 42.95 (22.87) | .027a |
| Mean SBP, mm Hg | 176.73 (25.60) | 162.33 (13.42) | 184.35 (27.49) | .011a |
| Mean DBP, mm Hg | 104.88 (17.43) | 102.89 (7.83) | 105.94 (20.99) | .680 |
| Mean MAP, mm Hg | 128.78 (16.67) | 122.59 (9.02) | 132.06 (18.99) | .173 |
Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; MDRD GFR, Modification of Diet in Renal Disease glomerular filtration rate; SBP, systolic blood pressure; SD, standard deviation. aStatistically significant.
Presenting Features
Flank pain was noted in 57.4% (n=15) and ecchymoses in 50.0% (n=13) of patients, with no differences by etiology (P=.497 and P=.213, respectively). Proteinuria occurred in 57.7% (n=15) and hematuria in 50.0% (n=13) of patients, similarly with no difference by etiology (P=.598 and P=.500, respectively) (Table II).
Table II.
Presenting Features, Investigations, and Pathological Findings by Etiology
| Overall (n=26), % | Trauma (n=9), % | Nontrauma (n=17), % | P Value | |
|---|---|---|---|---|
| Presenting features | ||||
| Flank pain | 57.4 (n=15) | 66.7 (n=6) | 52.9 (n=9) | .497 |
| Ecchymosis | 50.0 (n=13) | 33.3 (n=3) | 58.8 (n=10) | .213 |
| Imaging modalities | ||||
| Excretory urogram | 57.7 (n=15) | 55.6 (n=5) | 58.8 (n=10) | .873 |
| Kidneys ureters bladder x‐ray | 38.5 (n=10) | 33.3 (n=3) | 41.2 (n=7) | .694 |
| Angiography | 38.5 (n=10) | 33.3 (n=3) | 41.2 (n=7) | .694 |
| Computerized tomography | 46.2 (n=12) | 44.4 (n=4) | 47.1 (n=8) | .899 |
| Ultrasound | 38.5 (n=10) | 33.3 (n=3) | 41.2 (n=7) | .694 |
| Intravenous pyelogram | 11.5 (n=3) | 0 (n=0) | 17.6 (n=3) | .097 |
| Magnetic resonance | 3.8 (n=1) | 0 (n=0) | 5.9 (n=1) | .351 |
| Urinalysis | ||||
| Proteinuria | 57.7 (n=15) | 55.6 (n=5) | 58.8 (n=10) | .598 |
| Hematuria | 50.0 (n=13) | 44.4 (n=4) | 52.9 (n=9) | .500 |
| Pathological features | ||||
| Perinephric hematoma | 34.6 (n=9) | 33.3 (n=3) | 35.3 (n=6) | .635 |
| Subcapsular hematoma | 42.3 (n=11) | 44.4 (n=4) | 41.2 (n=7) | .873 |
Imaging and Pathological Findings
Multiple imaging modalities were employed throughout the study period. Excretory urogram was the most common modality, used in 57.7% (n=15), with magnetic resonance angiography being the least commonly employed, used in 3.8% (n=1). There were no significant differences in the rate of usage of each imaging modality by etiological group. Perinephric hematoma (Figure 2) was noted in 34.6% (n=9) and subcapsular hematoma in 42.3% (n=11) (Table II), with no differences between trauma and nontrauma patients.
Figure 2.
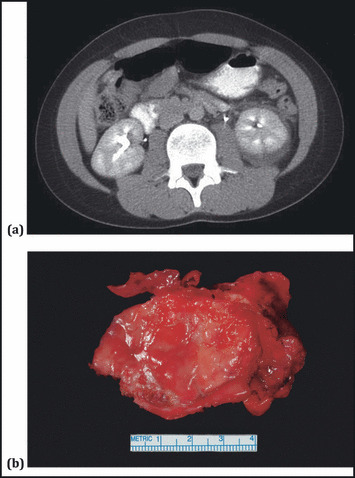
A case of an 11‐year‐old boy who developed Page kidney, following a fall from a horse. (A) A contrast‐enhanced abdominal computed tomographic scan shows a left renal hematoma. (B) A gross pathological specimen from laparoscopic evacuation and decortication of this hematoma. Pathology showed a fibrous wall with focal chronic inflammation and organizing hematoma. Five years after the original injury blood pressure normalized and angiotensin‐converting enzyme inhibitor therapy was discontinued.
Management
Surgical treatment was employed in 65.4% of patients (n=17), with no difference between trauma and nontrauma patients (P=.105). Nephrectomy was required in 9 cases, all of which were nontraumatic in nature (P=.002). Other procedures included evacuation of a hematoma in 23.1% (n=6), and capsule removal in 15.4% (n=4) of patients, with no differences between trauma and nontrauma cases. Medical treatment was employed in 73.1% of patients (n=19), often with surgical treatment, and 1 patient (3.8%) was observed.
Antihypertensive Therapy
At presentation with hypertension, a mean of 1.96±1.16 antihypertensive medications were prescribed in the study cohort. Significantly, trauma patients were prescribed fewer antihypertensive medications (mean 1.13±0.35) than nontrauma patients (2.38±1.20) on presentation (P=.001). Treatment with any antihypertensive agent was required in 66.7% (n=6) of trauma cases compared with 64.7% (n=11) of nontrauma cases at presentation (P=.635). Of note, trauma patients required fewer antihypertensive medications (0.44±0.53) than nontrauma patients (1.47±1.28) after intervention (P=.009). Numbers and/or doses of medications were decreased in 77.8% (n=7) of trauma cases compared with 41.2% (n=7) of nontrauma cases (P=.012). The use of any antihypertensive after intervention was required in 15 of 26 cases (58%), including 7 cases (4 trauma and 3 nontrauma) without a history of hypertension before the diagnosis of Page kidney.
β‐Blockers were used predominantly in nontrauma patients, particularly after intervention (P=.027). On presentation, diuretics were prescribed exclusively in the nontrauma group (P=.014) and, after intervention, calcium channel blockers were prescribed for this group only (P=.027). α‐Blockers were exclusively prescribed in the nontrauma group (P=.027). This pattern of medication use likely reflects the comorbidities associated with the advanced age of the nontrauma group.
Renal Function Postintervention
Assessments of renal function and blood pressure were also performed after intervention and/or medical treatment. Trauma patients had higher postintervention MDRD GFRs than nontrauma patients (P=.050) (Table III).
Table III.
Assessments of Renal Function and Blood Pressure Following Intervention by Etiology, Including Change in Parameter
| Parameter | Overall (n=26) | Trauma (n=9) | Nontrauma (n=17) | P Value |
|---|---|---|---|---|
| Mean MDRD GFR, mL/min/1.73m2 | 71.93 (50.67) | 104.13 (65.92) | 52.61 (25.85) | .050a |
| Mean change in creatinine, mg/dL | 0.29 (1.55) | 0.45 (1.16) | 0.19 (1.80) | .702 |
| Mean change in MDRD GFR, mL/min/1.73m2 | 10.45 (17.97) | 9.86 (17.35) | 10.81 (18.92) | .903 |
| Mean SBP, mm Hg | 142.69 (25.78) | 131.00 (18.15) | 148.88 (27.50) | .093 |
| Mean DBP, mm Hg | 85.23 (15.70) | 80.56 (15.65) | 87.71 (15.62) | .278 |
| Mean MAP, mm Hg | 104.33 (17.61) | 97.30 (16.27) | 108.06 (17.60) | .142 |
| Mean change in SBP, mm Hg | 34.04 (23.96) | 31.33 (11.95) | 35.47 (28.62) | .610 |
| Mean change in DBP, mm Hg | 20.04 (16.43) | 23.44 (16.43) | 18.24 (16.63) | .453 |
| Mean change in MAP, mm Hg | 24.38 (16.20) | 25.22 (13.26) | 23.94 (17.93) | .852 |
Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; MDRD GFR, Modification of Diet in Renal Disease Glomerular Filtration Rate; SBP, systolic blood pressure. aStatistically significant.
Successful clinical response based on assessments of blood pressures were noted to occur in 92.3% (n=24) of patients, with no differences between trauma and nontrauma patients (P=.587). MDRD GFR >60 mL/min/1.73m2 at the last follow‐up was seen in 60% (n=15) of patients, with a predominance in trauma patients (88.9% [n=8] vs 43.8% [n=7] of nontrauma patients, P=.020).
The Changing Face of Page Kidney Over Time
Imaging modalities predominantly employed in the 1960s and 1970s were the excretory urogram (EXU) (P=.001) and plain radiography (KUB) (P=.022). There were no differences in the use of renal angiography (P=.100), intravenous pyelogram (P=.298), or magnetic resonance imaging (P=.436) over time. Computed tomography was increasingly performed during the 1980s and 1990s compared with previous decades, with borderline statistical significance (P=.057). Similarly, ultrasonography was performed in every patient who presented with Page kidney during the 1980s and 1990s (P=.003).
There were no differences in the rates of surgical treatment (P=.361), medical treatment (P=.179), MDRD GFR >60 mL/min/1.73m2 at follow‐up (P=.650) and response to treatment throughout the study period (P=.829). There were no differences in the rates of response in MDRD GFR by year of presentation with Page kidney (P=.774).
Medication use changed during the study period, consistent with the introduction and increased availability of multiple antihypertensive medications. ACE inhibitors were not used before the 1980s and their use increased each decade thereafter (P=.001); there was a trend towards the increased use of β‐blockers in the 1980s more than any other decade (P=.078); diuretics and calcium channel blockers were used equally throughout the study period (P=.122 and P=.177, respectively); methyldopa was used predominantly in the 1960s and 1970s (P=.024); and α‐blockers were similarly used throughout the study period (P=.190).
Discussion
Our case series of 26 patients reports a comparative analysis of clinical parameters and outcomes between patients who developed a renal hemorrhage and resultant hypertension (ie, Page kidney) due to either trauma (classical Page kidney) or nontrauma causes, such as tumor, arteriovenous malformation, cyst rupture, glomerulonephritis, vasculitis, and native kidney biopsy. We excluded cases associated with renal allograft biopsy. As classically described, the majority of our trauma cases were young and male, without prior histories of hypertension. In contrast, the nontrauma cases were older, with approximately half of them having a prior history of hypertension.
Despite these differences, these two groups were similar with respect to presenting features, imaging modalities, urinalysis results, and pathological features. Both groups demonstrated improved GFR after treatment. One explanation is that unilateral kidney injury may result in an immediate GFR drop, with subsequent treatment and/or hypertrophy of the contralateral kidney leading to renal function improvement. In addition, in patients with parenchymal disease, other treatments based on biopsy findings may have contributed to the improvement in GFR.
With respect to hypertension, the majority who were hypertensive prior to the insult were from the nontrauma group. All of our trauma patients commenced antihypertensive medications for the first time at presentation with Page kidney. However, hypertension due to Page kidney was not reversible in all trauma cases, as 44.4% (n=4) of this group continued to require treatment after intervention. In the nontrauma group, 3 patients without prior histories of hypertension required antihypertensive therapy postintervention. Taken together, our data suggest that, regardless of etiology, one third of the patients (7 of 18) without a previous history of hypertension are at risk for chronic hypertension after developing Page kidney. This suggests that, after the initial insult and treatment, close follow‐up of these patients’ blood pressure is indicated. This is in contrast to previous reports, which suggest that patients with Page kidney have an excellent chance of “relieving the hypertension.” 24
Consistent with previous reports, our study shows that most patients with Page kidney are young and male. Trauma patients were significantly younger and had higher baseline MDRD GFR and lower systolic blood pressures than nontrauma cases at presentation. This suggests a different pathophysiology in trauma cases, but may also reflect age bias in MDRD GFR calculation. However, as this study is observational, it is not designed to demonstrate a causal relationship between GFR and blood pressure. Nontrauma patients were older and more likely to have underlying chronic kidney disease (as evidenced by the higher number of these patients with MDRD GFR <60 mL/min/1.73m2).
Although presenting features and treatments (both medical and surgical) were similar in both groups, other siginificant differences were noted. The use of imaging modalities changed significantly during the 50‐year study period, with EXU and KUB being most frequently performed in the 1960s and 1970s and ultrasonogaphy and computed tomography most frequently being used in the 1980s and 1990s. This likely reflects the introduction of newer and more refined technologies over the study period.
The most striking difference was in the rate of nephrectomy, which was performed only in nontrauma cases. Indications included renal hemorrhage in association with arteriovenous malformation, aneurysm, cyst rupture, and hypernephroma. Individual medical therapies were also different. Trauma cases required fewer antihypertensive medications, likely related to lower SBP at presentation, and were also more likely to have had antihypertensive medications decreased after intervention. Although postintervention serum creatinine measurements were similar in both groups, trauma cases had higher MDRD GFR, again potentially due to age bias.
Similarly, antihypertensive agents used for medical treatment changed significantly, consistent with changes in the guidelines for antihypertensvie therapies and the availability of agents during the study period. In addition, the number of patients included in this series makes it difficult to determine the reasons for the differing treatment strategies for trauma and nontrauma patients, and likely reflects interphysician variability. No cases were prescribed direct renin inhibitors, such as aliskerin, which may potentially be a useful treatment option, as hypertension in Page kidney is renin‐mediated. These agents were not available for prescription during the study period, and perhaps could be examined in future studies.
Limitations
The main weakness of our study is its retrospective design, during a time when significant changes in diagnostic imaging and treatment options, both surgical and medical, occurred. The numbers in this study were small, reflecting the rarity of this diagnosis. Strict methodology for chart review and recording of data ensured that we excluded cases without a true diagnosis of Page kidney and included those with accepted diagnostic clinical criteria. A systematic prospective comparison of clinical outcomes would have been ideal, which, given the rarity of this clinical entity and the variability of its presentation, would be possible only through a multicenter study.
Conclusions
Despite these limitations, our study clearly identifies distinct etiological subgroups that merit different approaches to investigation and management. Compared with nontrauma patients, trauma patients tend to be young and male, without a history of hypertension, and have less marked renal impairment. In addition, this series highlights Page kidney to be a risk factor for chronic hypertension, regardless of etiology, thus calling for close surveillance of blood pressures following initial work‐up and intervention.
Disclosure: The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
References
- 1. Page I. The production of persistent arterial hypertension by cellophane perinephritis. JAMA. 1939;113:\2046–2048. [Google Scholar]
- 2. Engel WJ, Page IH. Hypertension due to renal compression resulting from subcapsular hematoma. J Urol. 1955;73:735–739. [DOI] [PubMed] [Google Scholar]
- 3. Massumi RA, Andrade A, Kramer N. Arterial hypertension in traumatic subcapsular perirenal hematoma (Page kidney). Evidence for renal ischemia. Am J Med. 1969;46:635–639. [DOI] [PubMed] [Google Scholar]
- 4. Elias AN, Anderson GH Jr, Dalakos TG, Streeten DH. Renin angiotensin involvement in transient hypertension after renal injury. J Urol. 1978;119:561–562. [DOI] [PubMed] [Google Scholar]
- 5. von Knorring J, Fyhrquist F, Ahonen J. Varying course of hypertension following renal trauma. J Urol. 1981;126:798–801. [DOI] [PubMed] [Google Scholar]
- 6. Spark RF, Berg S. Renal trauma and hypertension: the role of renin. Arch Intern Med. 1976;136:1097–1100. [PubMed] [Google Scholar]
- 7. Vo NJ, Hanevold CD, Edwards R, et al. Recurrent Page kidney in a child with a congenital solitary kidney requiring capsular artery embolization. Pediatr Radiol. 2010;40:1837–1840. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mooppan MM, Kim H. Subcapsular urinoma: unusual form of “Page kidney” in newborn. Urology. 1984;23:585–587. [DOI] [PubMed] [Google Scholar]
- 9. Moriarty KP, Lipkowitz GS, Germain MJ. Capsulectomy: a cure for the Page kidney. J Pediatr Surg. 1997;32:831–833. [DOI] [PubMed] [Google Scholar]
- 10. John J, Allen S, Perry M, et al. Page kidney phenomenon presenting as acute renal failure after partial nephrectomy: a case report and review of the literature. Urol Int. 2008;80:440–443. [DOI] [PubMed] [Google Scholar]
- 11. Sasaguri M, Noda K, Matsumoto T, et al. A case of hyperreninemic hypertension after extracorporeal shock‐wave lithotripsy. Hypertens Res. 2000;23:709–712. [DOI] [PubMed] [Google Scholar]
- 12. Mufarrij P, Sandhu JS, Coll DM, Vaughan ED Jr. Page kidney as a complication of percutaneous antegrade endopyelotomy. Urology. 2005;65:592. [DOI] [PubMed] [Google Scholar]
- 13. Kamar N, Sallusto F, Rostaing L. Acute Page kidney after a kidney allograft biopsy: successful outcome from observation and medical treatment. Transplantation. 2009;87:453–454. [DOI] [PubMed] [Google Scholar]
- 14. Heffernan E, Zwirewich C, Harris A, Nguan C. Page kidney after renal allograft biopsy: sonographic findings. J Clin Ultrasound. 2009;37:226–229. [DOI] [PubMed] [Google Scholar]
- 15. Dempsey J, Gavant ML, Cowles SJ, Gaber AO. Acute Page kidney phenomenon: a cause of reversible renal allograft failure. South Med J. 1993;86:574–577. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen BD, Nghiem DD, Adatepe MH. Page kidney phenomenon in allograft transplant. Clin Nucl Med. 1994;19:361–363. [DOI] [PubMed] [Google Scholar]
- 17. Gibney EM, Edelstein CL, Wiseman AC, Bak T. Page kidney causing reversible acute renal failure: an unusual complication of transplant biopsy. Transplantation. 2005;80:285–286. [DOI] [PubMed] [Google Scholar]
- 18. Chung J, Caumartin Y, Warren J, Luke PP. Acute Page kidney following renal allograft biopsy: a complication requiring early recognition and treatment. Am J Transplant. 2008;8:1323–1328. [DOI] [PubMed] [Google Scholar]
- 19. McCune TR, Stone WJ, Breyer JA. Page kidney: case report and review of the literature. Am J Kidney Dis. 1991;18:593–599. [DOI] [PubMed] [Google Scholar]
- 20. Hoshiyama F, Nakanou I, Toyoshima Y, et al. A case of domestic violence‐related Page kidney. Hinyokika Kiyo. 2009;55:331–333. [PubMed] [Google Scholar]
- 21. Pintar TJ, Zimmerman S. Hyperreninemic hypertension secondary to a subcapsular perinephric hematoma in a patient with polyarteritis nodosa. Am J Kidney Dis. 1998;32:503–507. [DOI] [PubMed] [Google Scholar]
- 22. Nakano S, Kigoshi T, Uchida K, et al. Hypertension and unilateral renal ischemia (Page kidney) due to compression of a retroperitoneal paraganglioma. Am J Nephrol. 1996;16:91–94. [DOI] [PubMed] [Google Scholar]
- 23. Dopson SJ, Jayakumar S, Velez JC. Page kidney as a rare cause of hypertension: case report and review of the literature. Am J Kidney Dis. 2009;54:334–339. [DOI] [PubMed] [Google Scholar]
- 24. Sufrin G. The Page kidney: a correctable form of arterial hypertension. J Urol. 1975;113:450–454. [DOI] [PubMed] [Google Scholar]


