Abstract
J Clin Hypertens (Greenwich). 2012;14:149–157. ©2012 Wiley Periodicals, Inc.
Most patients with hypertension require combination therapy in order to achieve blood pressure (BP) goals. This 40‐week open‐label extension of the 12‐week double‐blind Triple Therapy With Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients Study (TRINITY) evaluated the efficacy and safety of triple‐combination treatments with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide (OM/AML/HCTZ) in 2112 participants with moderate to severe hypertension. Following 2 weeks of initial treatment with OM 40/AML 5/HCTZ 12.5 mg, participants not achieving BP goal were titrated to OM 40/AML 5/HCTZ 25 mg or OM 40/AML 10/HCTZ 12.5 mg on a randomized basis. At week 16, participants who did not achieve BP goal were further titrated to OM 40/AML 10/HCTZ 25 mg. At the end of the study, 44.5% to 79.8% of participants reached BP goal and the mean BP decreased from 168.6/100.7 mm Hg (baseline BP at randomization) to 125.0 to 136.8 mm Hg/77.8 to 82.5 mm Hg, depending on treatment. Long‐term treatment with OM/AML/HCTZ was well tolerated and effective with no new safety concerns.
Recent data from the National Health and Nutrition Examination Survey (NHANES) indicate that 76.4 million adults in the United States have high blood pressure (BP), defined as systolic BP (SBP) ≥140 mm Hg, diastolic BP (DBP) ≥90 mm Hg, or use of prescription medication to lower BP. 1 , 2 Furthermore, the prevalence of this condition, 33.5%, is more than twice the national Healthy People 2010 goal of 16% and has not decreased significantly in the past 10 years. 1 , 2 , 3 With the aging population, the prevalence of hypertension (not age‐adjusted) in 2030 is projected to be 37.3%. 4
Hypertension is a risk factor for cardiovascular disease, including myocardial infarction, heart failure, stroke, and kidney failure, leading to increased morbidity and mortality. 2 , 5 Clinical trial data demonstrate that effective BP lowering reduces the risk of cardiovascular and cerebrovascular events. 5 , 6
Hypertension control has improved from 27.3% of treated patients whose BP was <140/90 mm Hg in 1988–1994 to 50.1% in 2007–2008, thus achieving the Healthy People 2010 national objective of controlling BP in 50% of individuals with hypertension. 1 , 7 One factor that contributes to hypertension control is the increasing use of combination therapy secondary to the fact that most patients with hypertension require multiple therapeutic agents to reach BP goal. 5 , 8 , 9
Successful management of hypertension requires both health‐promoting lifestyle changes and long‐term pharmacotherapy. 5 Adherence to treatment requires medications that are effective, well tolerated, and convenient, as only approximately 40% of treated patients continue treatment for 10 years. 10
Although clinical trial data demonstrate that most patients with hypertension can achieve BP goal, 11 , 12 , 13 most will require >1 antihypertensive agent and many will require ≥3 agents to achieve BP goal. 11 , 12 , 13 The requirement for combination therapy is not unexpected, as physiologic compensatory mechanisms, such as diuretic‐induced activation of the renin‐angiotensin‐aldosterone system, may lead to tolerance of single‐agent therapy, limiting long‐term response. 14
The Triple Therapy With Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients Study (TRINITY) showed that triple‐combination treatment with OM 40 mg, AML 10 mg, and HCTZ 25 mg was effective in controlling BP compared with the component dual‐combination treatments and was well tolerated. 15 The purpose of this open‐label extension was to assess the long‐term efficacy and safety (using a sequential algorithm that models real‐world clinical practice) of the triple‐combination treatment OM/AML/HCTZ to treat study participants to BP goal.
Methods
The TRINITY study was a phase 3, randomized, parallel‐group evaluation of participants 18 years and older with mean seated BP (SeBP) ≥140/100 mm Hg or ≥160/90 mm Hg (off antihypertensive medication) conducted at 317 clinical sites in the United States and Puerto Rico. The trial consisted of a 3‐week washout (period I); a 12‐week, double‐blind, randomized treatment (period II); a 40‐week open‐label treatment (period III); and a 2‐week post‐treatment follow‐up. Details of the study design and results of the 12‐week randomized treatment period have previously been reported. 15 In the first 2 weeks of the double‐blind treatment period, participants were randomized to receive dual‐combination treatment and a subset of participants received placebo. Thereafter, all participants taking placebo were switched to dual‐combination treatment, which was continued from weeks 2 to 4. Triple‐combination treatment with OM 40/AML 10/HCTZ 25 mg was initiated in a subset of participants from each of the 3 dual‐combination treatment groups at week 4 and continued until week 12. 15 The change in SeBP for triple‐combination treatment was compared with the component dual‐combination treatments at week 12. 15
On completion of the 12‐week randomized period, study participants were enrolled in the open‐label extension and all participants were switched to OM 40/AML 5/HCTZ 12.5 mg (administered as OM 40/AML 5 mg fixed‐dose combination plus HCTZ 12.5 mg). Participants not achieving BP goal (<140/90 mm Hg or <130/80 mm Hg for participants with diabetes, chronic renal disease, or chronic cardiovascular disease) after 2 weeks (week 14) were randomly titrated to 1 of 2 treatments (OM 40/AML 10/HCTZ 12.5 mg [administered as OM 40/AML 10 mg fixed‐dose combination plus HCTZ 12.5 mg] or OM 40/AML 5/HCTZ 25 mg [administered as OM 40/AML 5 mg fixed‐dose combination plus HCTZ 25 mg]) using an interactive voice response system. Participants not achieving BP goal 2 weeks after this titration (week 16) were further titrated to OM 40/AML 10/HCTZ 25 mg (administered as OM 40/AML 10 mg fixed‐dose combination plus HCTZ 25 mg). Participants achieving BP goal generally remained on the same treatment throughout the open‐label treatment period but could be uptitrated at any time as per the investigator’s discretion. If participants experienced symptoms of hypotension or intolerance to study medications, back‐titration to a lower dose of triple‐combination treatment (but not to dual‐combination treatment) was at the investigator’s discretion. All participants were treated per the investigator’s discretion at the conclusion of the open‐label extension.
Study visits were scheduled at weeks 12 (start of the open‐label extension), 14, 16, 18, 20, 28, 36, 44, and 52 to assess BP response and safety, and a follow‐up visit was scheduled at week 54 to evaluate safety issues. Three BP assessments were made at each visit (trough BP measurement) using a validated cuff oscillometric monitor (Omron HEM‐705CP, Omron Healthcare, Inc, Bannockburn, IL) and the mean of these 3 measurements was used as the BP for that visit.
The primary efficacy variable for this evaluation was seated DBP (SeDBP) and seated SBP (SeSBP) at each scheduled visit during the open‐label extension. Secondary efficacy variables included the titration effect corresponding to changes in dosing regimen on SeDBP and SeSBP, the proportion of study participants reaching BP goal at each visit, and the proportion of participants achieving prespecified BP targets (SeBP <140/90 mm Hg, SeBP <130/80 mm Hg, SeBP <120/80 mm Hg, SeSBP <140 mm Hg, and SeDBP <90 mm Hg) at any time during the open‐label treatment period.
Safety assessments included adverse events (AEs), physical examinations, 12‐lead electrocardiography (ECG), and clinical laboratory tests. All AEs occurring during and up to 14 days following the open‐label treatment period were recorded and categorized by the treatment regimen in which the AE started. AEs developing prior to the open‐label treatment period (ie, during the 12‐week randomized treatment period) were counted as AEs for the open‐label extension only if they worsened during the open‐label extension.
Statistical Analysis
The primary efficacy population included all study participants who entered the open‐label period, received at least 1 dose of open‐label study medication, and provided at least one post‐dose assessment of BP. SeDBP and SeSBP at each open‐label visit, titration effect, proportion of participants reaching BP goal, proportion of participants achieving BP targets, and change in SeDBP and SeSBP from baseline (week 0) to week 52/early termination (ET) were assessed using summary statistics by treatment group. The comparative efficacy of the 2 randomized titration regimens (OM 40/AML 10/HCTZ 12.5 mg and OM 40/AML 5/HCTZ 25 mg) in study participants not achieving BP goal at week 14 was assessed using an analysis of covariance model with titrated treatment as a fixed effect and BP value at week 14 as a covariate.
The primary safety population included all study participants who entered the open‐label period and received at least 1 dose of open‐label study medication. AEs were assessed using summary statistics by onset dosing regimen and laboratory and ECG assessments were assessed using summary statistics by final dosing regimen.
Results
Demographic and Baseline Characteristics
Overall, 2112 study participants entered the open‐label treatment period and received at least 1 dose of study medication (safety population), 2098 participants provided at least 1 post‐dose BP assessment (efficacy population), and 1796 participants completed the 40‐week open‐label extension (Figure 1). Reasons for premature discontinuation in 316 (15%) participants were AEs (127 [6.0%]), withdrawn consent (85 [4.0%]), lost to follow‐up (55 [2.6%]), protocol violations (24 [1.1%]), pregnancy (2 [0.1%]), and other (23 [1.1%]).
Figure 1.
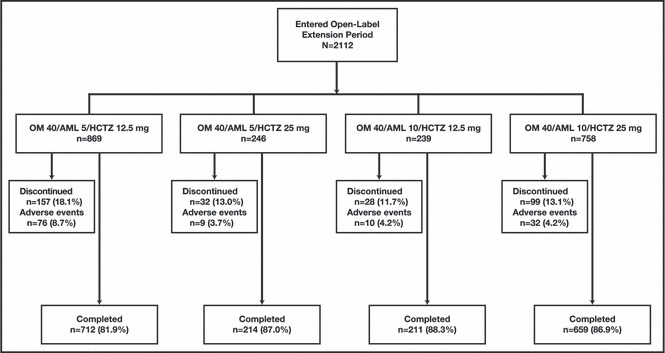
Study participant disposition by final dosing regimen for the open‐label treatment period. AML indicates amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil.
Table I summarizes demographic and clinical characteristics of study participants entering the open‐label extension by dosing regimen. Baseline demographics and characteristics of participants who entered the open‐label extension were comparable to those of participants who entered the 12‐week double‐blind period of the study. 15 In the open‐label extension compared with the 12‐week study, participant mean age was 55.4 and 55.1 years, mean duration of hypertension was 10.1 and 9.9 years, and mean baseline SeBP was 168.6/100.7 mm Hg and 168.5/100.9 mm Hg, respectively. Overall, 53.3% of the open‐label extension and 52.9% of the 12‐week study participants were men and 67.7% of the open‐label extension and 66.8% of the 12‐week study participants were Caucasian.
Table I.
Demographic and Baseline Characteristics of Study Participants Entering the Open‐Label Study by Final Dosing Regimena
| OM 40/AML 5/HCTZ 12.5 mg (n=869) | OM 40/AML 5/HCTZ 25 mg (n=246) | OM 40/AML 10/HCTZ 12.5 mg (n=239) | OM 40/AML 10/HCTZ 25 mg (n=758) | |
|---|---|---|---|---|
| Age, mean (SD), y | 54.6 (10.8) | 57.0 (10.5) | 55.6 (11.4) | 55.8 (10.3) |
| Age group, No. (%) | ||||
| <65 y | 722 (83.1) | 188 (76.4) | 179 (74.9) | 609 (80.3) |
| ≥65 y | 147 (16.9) | 58 (23.6) | 60 (25.1) | 149 (19.7) |
| ≥75 y | 25 (2.9) | 10 (4.1) | 8 (3.3) | 23 (3.0) |
| Sex, No. (%) | ||||
| Male | 429 (49.4) | 114 (46.3) | 135 (56.5) | 448 (59.1) |
| Ethnicity | ||||
| Hispanic or Latino | 125 (14.4) | 47 (19.1) | 37 (15.5) | 100 (13.2) |
| Race, No. (%)b | ||||
| White | 629 (72.4) | 175 (71.1) | 149 (62.3) | 477 (62.9) |
| Black | 211 (24.3) | 63 (25.6) | 84 (35.1) | 264 (34.8) |
| Asian | 21 (2.4) | 4 (1.6) | 4 (1.7) | 10 (1.3) |
| Other | 10 (1.1) | 5 (2.0) | 3 (1.2) | 9 (1.1) |
| BMI, mean (SD), kg/m2 | 32.0 (6.7) | 33.8 (7.3) | 32.5 (7.1) | 34.6 (7.4) |
| BMI category, No. (%) | ||||
| BMI <30 kg/m2 | 370 (42.6) | 87 (35.4) | 105 (43.9) | 218 (28.8) |
| BMI ≥30 kg/m2 | 499 (57.4) | 159 (64.6) | 134 (56.1) | 540 (71.2) |
| Diabetes, No. (%) | 63 (7.2) | 34 (13.8) | 40 (16.7) | 197 (26.0) |
| Chronic kidney disease, No. (%)c | 30 (3.5) | 13 (5.3) | 14 (5.9) | 36 (4.7) |
| Chronic cardiovascular disease, No. (%) | 55 (6.3) | 30 (12.2) | 20 (8.4) | 86 (11.3) |
| Duration of hypertension, mean (SD), y | 8.6 (8.6) | 9.5 (9.3) | 11.3 (9.7) | 11.7 (10.4) |
| Baseline SeSBP, mean (SD), mm Hgd | 163.8 (11.8) | 169.6 (13.0) | 167.8 (12.6) | 174.1 (14.7) |
| Baseline SeDBP, mean (SD), mm Hgd | 99.8 (6.9) | 100.5 (7.4) | 100.5 (7.2) | 102.0 (8.5) |
Abbreviations: AML, amlodipine besylate; BMI, body mass index; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. aBaseline data were collected prior to randomization into the double‐blind period of the study. bStudy participants could select more than one race. cDefined as screening creatinine clearance ≥30 mL/min and ≤60 mL/min. dBaseline BP was defined as the mean of the randomization visit and the visit immediately preceding randomization.
Efficacy
Mean BP by treatment group at weeks 12 (start of the open‐label extension), 14, 16, 28, and 52/ET are summarized in Table II. At week 12, mean SeBP of the open‐label cohort was 134.8/82.3 mm Hg (mean SeBP was measured prior to the first dose of open‐label study medication but while receiving blinded antihypertensive study medication). At week 52/ET, the mean SeBP by final dosing regimen was 125.0/77.8 mm Hg for OM 40/AML 5/HCTZ 12.5 mg, 130.7/80.2 mm Hg for OM 40/AML 5/HCTZ 25 mg, 129.4/78.8 mm Hg for OM 40/AML 10/HCTZ 12.5 mg, and 136.8/82.5 mm Hg for OM 40/AML 10/HCTZ 25 mg.
Table II.
Mean Seated Blood Pressure and Blood Pressure Goal by Treatment Group From Week 12 to End of Study
| Time Point | OM 40/AML 5/HCTZ 12.5 mg | OM 40/AML 5/HCTZ 25 mg | OM 40/AML 10/HCTZ 12.5 mg | OM 40/AML 10/HCTZ 25 mg |
|---|---|---|---|---|
| Week 12a | ||||
| No. | 2098 | 0 | 0 | 0 |
| Mean (SD) | 134.8/82.3 (16.2/10.1) | ― | ― | ― |
| No. (%) to goal | 1076 (51.3) | ― | ― | ― |
| Week 14 | ||||
| No. | 2060 | 0 | 0 | 0 |
| Mean (SD) | 134.2/81.8 (16.2/9.9) | ― | ― | ― |
| No. (%) to goal | 1130 (54.9) | ― | ― | ― |
| Week 16 | ||||
| No. | 1111 | 449 | 463 | 7 |
| Mean (SD) | 127.2/78.5 (12.6/8.5) | 139.2/83.2 (14.8/9.1) | 138.9/83.4 (14.2/9.7) | 144.7/86.7 (10.3/13.4) |
| No. (%) to goal | 868 (78.1) | 167 (37.2) | 171 (36.9) | 1 (14.3) |
| Week 28 | ||||
| No. | 844 | 237 | 259 | 590 |
| Mean (SD) | 125.1/77.4 (12.2/8.0) | 131.7/80.2 (12.4/8.6) | 131.3/79.6 (12.7/9.4) | 137.7/83.0 (13.5/9.8) |
| No. (%) to goal | 701 (83.1) | 154 (65.0) | 161 (62.2) | 241 (40.8) |
| Week 52/early termination | ||||
| No. | 857 | 247 | 240 | 751 |
| Mean (SD) | 125.0/77.8 (14.2/9.1) | 130.7/80.2 (13.7/9.2) | 129.4/78.8 (12.6/9.2) | 136.8/82.5 (15.5/10.0) |
| No. (%) to goal | 684 (79.8) | 159 (64.4) | 165 (68.8) | 334 (44.5) |
Abbreviations: AML, amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation. aData summarized at week 12 were measured before the first dose of the open‐label study medication.
Overall, approximately 45% of study participants did not achieve BP goal within 2 weeks of starting open‐label treatment with OM 40/AML 5/HCTZ 12.5 (week 14) and 912 participants were subsequently randomized to OM 40/AML 10/HCTZ 12.5 mg or OM 40/AML 5/HCTZ 25 mg. Both treatment regimens resulted in statistically significant reductions in SeDBP and SeSBP (P<.0001) at week 16 compared with week 14, as expected. The difference between the 2 groups was not statistically significant (SeDBP, P=.2889; SeSBP, P=.8588) (Table S1). As a result, an additional 37% of participants titrated to each of these triple‐combination treatments at week 14 achieved BP goal by week 16.
When data from all dose titrations that occurred throughout the open‐label extension were analyzed, each titration resulted in mean reductions in SeDBP and SeSBP that ranged from −2.9 to −5.7 mm Hg and −5.9 to −10.2 mm Hg, respectively. Titration from OM 40/AML 5/HCTZ 25 mg to OM 40/AML 10/HCTZ 25 mg was associated with the greatest mean reduction in SeDBP (−5.7 mm Hg) and titration from OM 40/AML 10/HCTZ 12.5 mg to OM 40/AML 10/HCTZ 25 mg was associated with the greatest mean reduction in SeSBP (−10.2 mm Hg) (Table III).
Table III.
Titration Effect: Change in Blood Pressure—All Participants Entering the Open‐Label Extension Period With Dosage Change in Study Medication
| OM 40/AML 5/HCTZ 12.5 mg to OM 40/AML 5/HCTZ 25 mg | OM 40/AML 5/HCTZ 12.5 mg to OM 40/AML 10/HCTZ 12.5 mg | OM 40/AML 5/HCTZ 25 mg to OM 40/AML 10/HCTZ 25 mg | OM 40/AML 10/HCTZ 12.5 mg to OM 40/AML 10/HCTZ 25 mg | |
|---|---|---|---|---|
| No.a | 604 | 639 | 360 | 383 |
| Change in SeDBP, mean (SD) | −2.9 (8.3) | −4.4 (8.7) | −5.7 (9.4) | −5.0 (9.1) |
| Change in SeSBP, mean (SD) | −5.9 (13.6) | −6.8 (13.3) | −9.9 (15.0) | −10.2 (13.8) |
Abbreviations: AML, amlodipine besylate; BP, blood pressure; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure. Titration effect was calculated as BP measurement at last visit on the new dose regimen minus BP measurement at last visit of the previous dose regimen. aNumber of titration events. Six participants had duplicate titration events from OM 40/AML 5/HCTZ 12.5 mg to OM 40/AML 10/HCTZ 12.5 mg. Two participants had duplicate titration events from OM 40/AML 10/HCTZ 12.5 mg to OM 40/AML 10/HCTZ 25 mg.
Mean changes in BP from baseline (start of the 12‐week randomized period) to week 52/ET were comparable across all 4 treatment groups (SeDBP: −19.4 mm Hg to −22.1 mm Hg; SeSBP: −37.2 mm Hg to −39.1 mm Hg; Figure 2). Final dosing correlated with study participant’s baseline BP: participants with the lowest baseline BP tended to receive OM 40/AML 5/HCTZ 12.5 mg, participants with intermediate baseline BP tended to receive OM 40/AML 10/HCTZ 12.5 mg or OM 40/AML 5/HCTZ 25 mg, and participants with the highest baseline BP tended to receive OM 40/AML 10/HCTZ 25 mg.
Figure 2.
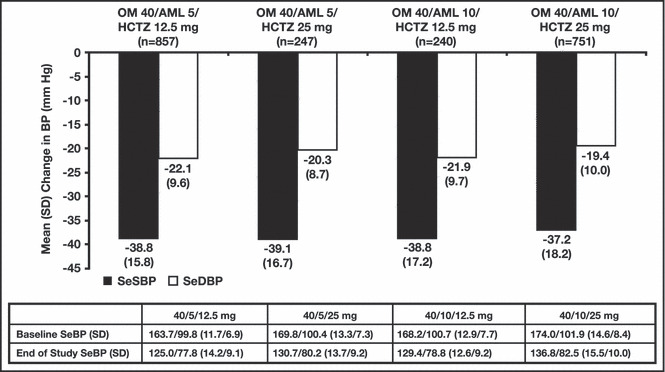
Change in blood pressure (BP) from baseline (week 0) to end of study by final dosing regimen. Baseline BP was defined as the mean of the randomization visit and the visit immediately preceding randomization; n is the number of study participants with values at both time points. AML indicates amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation; SeBP, seated BP; SeDBP, seated diastolic BP; SeSBP, seated systolic BP.
The percentage of study participants who reached BP goal at week 52/ET was 79.8% in the OM 40/AML 5/HCTZ 12.5 mg group, 68.8% in the OM 40/AML 10/HCTZ 12.5 mg group, 64.4% in the OM 40/AML 5/HCTZ 25 mg group, and 44.5% in the OM 40/AML 10/HCTZ 25 mg group (Table II). The lower percentage of goal attainment in participants who received OM 40/AML 10/HCTZ 25 mg may be explained by the combination of higher BP at baseline and a greater prevalence of diabetes, which required the lower BP goal of <130/80 mm Hg. Most BP targets (with the exception of <120/80 mm Hg) were achieved by a greater percentage of participants who received OM 40/AML 10/HCTZ 25 mg compared with participants receiving any of the other 3 triple‐combination treatments (Figure 3).
Figure 3.
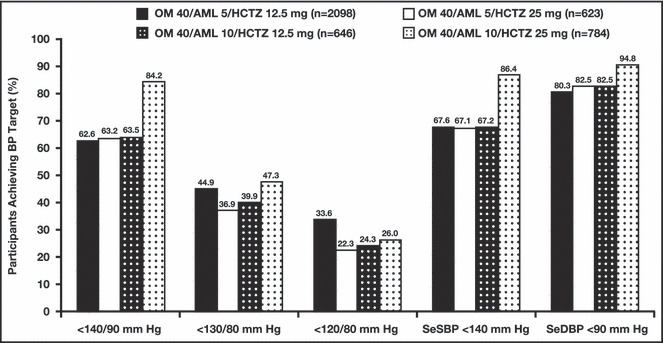
Proportion of study participants achieving blood pressure (BP) targets at any time during the open‐label treatment period by final dosing regimen. AML indicates amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SeDBP, seated diastolic BP; SeSBP, seated systolic BP.
Safety and Tolerability
During the open‐label extension, mean exposure to any triple‐combination treatment was 256 days. Exposure to the individual triple‐combination treatments was 117.3 days for OM 40/AML 5/HCTZ 12.5 mg, 104.0 days for OM 40/AML 5/HCTZ 25 mg, 105.9 days for OM 40/AML 10/HCTZ 12.5 mg, and 200.8 days for OM 40/AML 10/HCTZ 25 mg. No new safety concerns were identified for any of the triple‐combination treatments that were not known to occur with the individual component treatments.
In total, 1515 (71.7%) study participants experienced an AE and 536 (25.4%) experienced a drug‐related AE during the open‐label extension. Most AEs and drug‐related AEs were considered to be of mild or moderate severity (Table IV). Overall, 106 (5.0%) study participants had a serious AE (SAE) and 5 (0.2%) had a drug‐related SAE. These included acute renal insufficiency, presyncope, and hypotension in 3 study participants who received OM 40/AML 5/HCTZ 12.5 mg, acute renal insufficiency with hyperkalemia in 1 participant, and syncope in 1 participant who receieved OM 40/AML 5/HCTZ 25 mg. Although a greater percentage of participants treated with OM 40/AML 10/HCTZ 25 mg experienced an AE, drug‐related AE, or SAE, the extent of exposure to this triple‐combination treatment was approximately twice as long compared with the other 3 treatment groups.
Table IV.
Study Participants With Adverse Events by Onset Dosing Regimen
| OM 40/AML 5/HCTZ 12.5 mg (n=2112) | OM 40/AML 5/HCTZ 25 mg (n=627) | OM 40/AML 10/HCTZ 12.5 mg (n=652) | OM 40/AML 10/HCTZ 25 mg (n=790) | |
|---|---|---|---|---|
| All AEs | 989 (46.8) | 228 (36.4) | 244 (37.4) | 467 (59.1) |
| Mild | 531 (25.1) | 121 (19.3) | 120 (18.4) | 231 (29.2) |
| Moderate | 380 (18.0) | 86 (13.7) | 99 (15.2) | 189 (23.9) |
| Severe | 78 (3.7) | 21 (3.3) | 25 (3.8) | 47 (5.9) |
| Drug‐related AEsa | 311 (14.7) | 73 (11.6) | 69 (10.6) | 156 (19.7) |
| Serious AEs (SAEs) | ||||
| All SAEs | 40 (1.9) | 11 (1.8) | 17 (2.6) | 38 (4.8) |
| Drug‐related SAEs | 3 (0.1) | 2 (0.3) | 0 | 0 |
| Deaths | 1 (0.0) | 0 | 1 (0.2) | 1 (0.1) |
| Discontinuationsb | ||||
| All AEs | 74 (3.5) | 10 (1.6) | 12 (1.8) | 31 (3.9) |
| AEs starting in the open‐label treatment period | 67 (3.2) | 10 (1.6) | 11 (1.7) | 28 (3.5) |
| Drug‐related AEs starting in the open‐label treatment period | 45 (2.1) | 7 (1.1) | 7 (1.1) | 13 (1.6) |
| AEs occurring in ≥2% on any dosing regimen | ||||
| Dizziness | 91 (4.3) | 22 (3.5) | 22 (3.4) | 38 (4.8) |
| Headache | 47 (2.2) | 16 (2.6) | 17 (2.6) | 26 (3.3) |
| Peripheral edema | 42 (2.0) | 8 (1.3) | 26 (4.0) | 48 (6.1) |
| Cough | 44 (2.1) | 16 (2.6) | 8 (1.2) | 18 (2.3) |
| Upper respiratory tract infection | 72 (3.4) | 11 (1.8) | 16 (2.5) | 33 (4.2) |
| Nasopharyngitis | 55 (2.6) | 15 (2.4) | 14 (2.1) | 31 (3.9) |
| Urinary tract infection | 59 (2.8) | 15 (2.4) | 7 (1.1) | 23 (2.9) |
| Back pain | 31 (1.5) | 8 (1.3) | 15 (2.3) | 16 (2.0) |
| Arthralgia | 27 (1.3) | 7 (1.1) | 8 (1.2) | 27 (3.4) |
| Muscle spasms | 22 (1.0) | 10 (1.6) | 6 (0.9) | 18 (2.3) |
Abbreviations: AE, adverse event; AML, amlodipine besylate; OM, olmesartan medoxomil; HCTZ, hydrochlorothiazide. aDrug‐related was defined as definitely, probably, or possibly related to randomized study medication. bTwo participants discontinued the study due to SAEs that were considered possibly related to study medication (one receiving OM 40/AML 5/HCTZ 12.5 mg had acute renal insufficiency and another receiving OM 40/AML 5/HCTZ 25 mg had syncope). Data are presented as number (percentage) of study participants.
The percentage of participants who discontinued the study during the open‐label extension was low and similar across treatment groups (Table IV). Overall, 127 (6.0%) participants discontinued due to an AE, 72 (3.4%) discontinued due to a drug‐related AE, and 23 (1.1%) discontinued due to an SAE (2 SAEs were considered possibly related to study medication). Three deaths occurred during the open‐label extension; however, none were considered to be related to study drug. One participant died from complications of a pharyngeal abscess while receiving OM 40/AML 5/HCTZ 12.5 mg, another died from cardiac arrest during an acute coronary syndrome while receiving OM 40/AML 10/HCTZ 25 mg, and the cause of death in the third participant (who received OM 40/AML 10/HCTZ 12.5 mg) was unknown.
The most frequently reported AEs (>3%) by the onset of treatment regimen were dizziness (4.3%) and upper respiratory tract infection (URTI, 3.4%) in study participants who received OM 40/AML 5/HCTZ 12.5 mg; dizziness (3.5%) in participants who received OM 40/AML 5/HCTZ 25 mg; peripheral edema (4.0%) and dizziness (3.4%) in participants who received OM 40/AML 10/HCTZ 12.5 mg; and peripheral edema (6.1%), dizziness (4.8%), URTI (4.2%), nasopharyngitis (3.9%), arthralgia (3.4%), and headache (3.3%) in participants who received OM 40/AML 10/HCTZ 25 mg (Table IV). With the exceptions of peripheral edema (which was more common in participants who received AML 10 mg than AML 5 mg as part of the triple‐combination treatment) and arthralgia (which was more common in participants receiving OM 40/AML 10/HCTZ 25 mg than other treatments), there were no clinically meaningful differences in AEs between treatment groups.
Discussion
Many patients with hypertension will require ≥2 antihypertensive agents in order to achieve BP goal. 11 , 12 , 13 Consequently, using a fixed‐dose combination may lead to more rapid goal attainment, enhanced adherence due to regimen simplification, and better overall BP control. 8 , 9 , 16 , 17 , 18 , 19 , 20 The requirement for ≥2 antihypertensive agents is not unexpected due to the multifactorial pathogenesis of the condition. 21 In a meta‐analysis of data from 42 randomized trials, combining agents from different antihypertensive drug classes was shown to produce a BP reduction 5‐fold greater than that produced by doubling the dose of a single agent. 22
OM, AML, and HCTZ each target a different pathway in the control of hypertension, and these complementary mechanisms of action make these 3 agents a rational choice for combination therapy. 14 , 20 , 22 Previous clinical trials have evaluated the efficacy and safety of combining ≥2 of these agents and have clearly demonstrated an additive BP‐lowering effect. 23 , 24 , 25 , 26 , 27 , 28 Moreover, a fixed‐dose combination of these 3 agents, by facilitating adherence, may improve the treatment of patients with resistant hypertension, defined as BP that remains above goal despite concurrent use of 3 antihypertensive agents of different classes, including a diuretic. 14 , 29 , 30 , 31 , 32
The current open‐label extension of the 12‐week TRINITY study demonstrates that long‐term administration of the combination of OM/AML/HCTZ is both effective and well tolerated. Mean baseline reductions in SeDBP and SeSBP at week 52/ET ranged from −19.4 to −22.1 mm Hg and −37.2 to −39.1 mm Hg, respectively. As a result, baseline mean SeBP decreased from 168.6/100.7 mm Hg to 125.0–136.8/77.8–82.5 mm Hg at the conclusion of the study. These BP reductions were similar to those seen in study participants who received the triple‐combination treatment (OM 40/AML 10/HCTZ 25 mg) during the double‐blind portion of the study (−37.1/21.8 mm Hg), 15 demonstrating the durable antihypertensive efficacy of the triple combination.
The open‐label extension algorithm is modeled after real‐world clinical practice for titrating patients to goal. 5 Although there was a defined, randomized dose titration for study participants not achieving BP goal, dose adjustments, either up or down, were at the discretion of the investigator who managed the participant. Using this algorithm, 55% of participants reached BP goal within 2 weeks of initiating open‐label treatment with OM 40/AML 5/HCTZ 12.5 mg, and, across treatment groups, 44.5% to 79.8% were at BP goal at week 52/ET. Although BP goal attainment appeared to be inversely related to the strength of the triple‐combination treatment, this observation may reflect specific subgroup variables and not treatment‐related factors. This premise is supported by the observations that participants who were titrated to the highest dose of triple‐combination treatment also had the highest BP at baseline and the highest prevalence of diabetes, which required a more rigorous BP goal of <130/80 mm Hg.
Physician therapeutic inertia (the failure to initiate or intensify therapy when indicated) may also have played a role in some study participants not achieving BP goal. 33 , 34 , 35 As previously mentioned, titration during the open‐label extension was at the discretion of the investigator. As data indicate that physicians are less likely to intensify treatment when patients are close to BP goal, 34 , 36 the investigator may have chosen not to uptitrate participants’ treatment. For example, a survey of primary care physicians found that 52% would not initiate treatment for hypertension in a middle‐aged adult with SBP between 140 mm Hg and 159 mm Hg; furthermore, 33% would not intensify treatment for persistent SBP between 140 mm Hg and 158 mm Hg, and 25% would not intensify treatment for persistent DBP between 90 mm Hg and 94 mm Hg. 36 The availability of fixed‐dose combinations may help overcome physicians’ therapeutic inertia and improve BP control rates.
Finally, triple‐combination treatment with OM/AML/HCTZ was safe and well tolerated during this 40‐week open‐label extension. Discontinuation due to AEs was infrequent and similar across treatment groups.
Conclusions
This open‐label extension of the TRINITY study demonstrated the long‐term efficacy and safety of OM/AML/HCTZ triple‐combination treatment. This triple‐combination therapy provides a safe and effective option for patients with hypertension who are not adequately controlled on dual‐combination treatments.
Acknowledgments and disclosures: Research funds for this study and preparation of the manuscript were provided by Daiichi Sankyo, Inc, Parsippany, New Jersey. Editorial support for this article was provided by Vrinda Mahajan, PharmD, of Peloton Advantage, LLC, Parsippany, New Jersey. The opinions expressed in the current article are those of the authors. The authors received no honoraria/fee for service or other form of financial support related to the development of this article. Dean J. Kereiakes, MD, reports no disclosure information. Steven G. Chrysant, MD, has served as a consultant and speakers bureau member for and received grant/research support and honoraria from Daiichi Sankyo, Inc. Joseph Izzo, MD, has served as a consultant or investigator for Daiichi Sankyo, Inc, Boehringer‐Ingelheim, Novartis, GlaxoSmithKline, Takeda Pharmaceuticals, and Forest Laboratories. Suzanne Oparil, MD, has received grant/research support from Amgen Inc and Merck & Co., Inc; is a member of the speakers bureau for Daiichi Sankyo, Inc and Novartis; has served as a consultant for Boehringer‐Ingelheim, Daiichi Sankyo, Inc, Eli Lilly, Forest Laboratories, NicOx, Novartis, and Omron Healthcare; and has received honorarium from Daiichi Sankyo, Inc, Novartis, and Pfizer Inc. Michael Melino, PhD, James Lee, PhD, Victor Fernandez, BS, and Reinilde Heyrman, MD, are employees of Daiichi Sankyo, Inc.
Supporting information
Table S1. Titration Effect: Change in Blood Pressure From Week 14 to Week 16.
Supporting info item
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoon SS, Ostchega Y, Louis T. Recent Trends in the Prevalence of High Blood Pressure and Its Treatment and Control, 1999–2008 [NCHS data brief, no. 48]. National Center for Health Statistics. http://www.cdc.gov/nchs/data/databriefs/db48.pdf. Accessed April 25, 2011. [PubMed] [Google Scholar]
- 4. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:1–13. [DOI] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 6. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 7. Egan BM, Basile JN. Controlling blood pressure in 50% of all hypertensive patients: an achievable goal in the healthy people 2010 report? J Investig Med. 2003;51:373–385. [DOI] [PubMed] [Google Scholar]
- 8. Calhoun DA. Use of single‐pill combination therapy in the evolving paradigm of hypertension management. Expert Opin Pharmacother. 2009;10:1869–1874. [DOI] [PubMed] [Google Scholar]
- 9. Chrysant SG. Using fixed‐dose combination therapies to achieve blood pressure goals. Clin Drug Investig. 2008;28:713–734. [DOI] [PubMed] [Google Scholar]
- 10. van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10‐year persistence with antihypertensive drugs. J Hypertens. 2005;23:2101–2107. [DOI] [PubMed] [Google Scholar]
- 11. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 12. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 13. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 14. Elijovich F, Laffer C. A role for single‐pill triple therapy in hypertension. Ther Adv Cardiovasc Dis. 2009;3:231–240. [DOI] [PubMed] [Google Scholar]
- 15. Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double‐blind, 12‐week, parallel‐group study. Clin Ther. 2010;32:1252–1269. [DOI] [PubMed] [Google Scholar]
- 16. Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 17. Brixner DI, Jackson KC, Sheng X, et al. Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free‐ and fixed‐dose combinations. Curr Med Res Opin. 2008;24:2597–2607. [DOI] [PubMed] [Google Scholar]
- 18. Jackson KC, Sheng X, Nelson RE, et al. Adherence with multiple‐combination antihypertensive pharmacotherapies in a US managed care database. Clin Ther. 2008;30:1558–1563. [DOI] [PubMed] [Google Scholar]
- 19. Feldman RD, Zou GY, Vandervoort MK, et al. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. [DOI] [PubMed] [Google Scholar]
- 20. Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:42–50. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Donaire JA, Ruilope LM. Multiple action fixed combination. Present or future? Fundam Clin Pharmacol. 2010;24:37–42. [DOI] [PubMed] [Google Scholar]
- 22. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. [DOI] [PubMed] [Google Scholar]
- 23. Chrysant SG, Weber MA, Wang AC, Hinman DJ. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17:252–259. [DOI] [PubMed] [Google Scholar]
- 24. Chrysant SG, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double‐blind, placebo‐controlled, 8‐week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. [DOI] [PubMed] [Google Scholar]
- 25. Volpe M, Miele C, Haag U. Efficacy and safety of a stepped‐care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate‐to‐severe hypertension: an open‐label, long‐term study. Clin Drug Investig. 2009;29:381–391. [DOI] [PubMed] [Google Scholar]
- 26. Kereiakes DJ, Neutel J. Efficacy of an olmesartan medoxomil‐based treatment algorithm in patients with hypertension and type 2 diabetes: analysis of diurnal blood pressure control as assessed by 24‐hour ambulatory blood pressure monitoring. Ther Adv Cardiovasc Dis. 2010;4:285–293. [DOI] [PubMed] [Google Scholar]
- 27. Weir MR, Hsueh WA, Nesbitt SD, et al. A titrate‐to‐goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed‐dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide. J Clin Hypertens (Greenwich). 2011;13:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chrysant SG, Oparil S, Melino M, et al. Efficacy and safety of long‐term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens (Greenwich). 2009;11:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263–1269. [DOI] [PubMed] [Google Scholar]
- 30. Hermida RC, Ayala DE, Calvo C, et al. Effects of time of day of treatment on ambulatory blood pressure pattern of patients with resistant hypertension. Hypertension. 2005;46:1053–1059. [DOI] [PubMed] [Google Scholar]
- 31. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 32. Viera AJ, Hinderliter AL. Evaluation and management of the patient with difficult‐to‐control or resistant hypertension. Am Fam Physician. 2009;79:863–869. [PubMed] [Google Scholar]
- 33. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–834. [DOI] [PubMed] [Google Scholar]
- 34. Basile J. Clinical inertia and blood pressure goal attainment. J Clin Hypertens. 2009;12(Suppl 1):S5–S12. [Google Scholar]
- 35. Basile J, Neutel J. Overcoming clinical inertia to achieve blood pressure goals: the role of fixed‐dose combination therapy. Ther Adv Cardiovasc Dis. 2010;4:119–127. [DOI] [PubMed] [Google Scholar]
- 36. Hyman DJ, Pavlik VN. Self‐reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence‐based medicine. Arch Intern Med. 2000;160:2281–2286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Titration Effect: Change in Blood Pressure From Week 14 to Week 16.
Supporting info item


