Abstract
J Clin Hypertens (Greenwich). 2012; 14:455–460. ©2012 Wiley Periodicals, Inc.
Microparticles (MPs) are associated with several cardiovascular complications. As multifunction biomarkers, they may contribute to the pathogenesis of diabetes‐associated vascular diseases. A total of 39 patients with diabetes and hypertension, 24 patients with diabetes without hypertension, and 20 healthy controls were enrolled. Flow cytometry was applied to detect plasma MPs, including endothelial MPs (EMPs), annexin V+MPs, platelet‐derived MPs (PMPs), and leukocyte‐derived MPs (LMPs). Brachial ankle pulse wave velocity (baPWV) was also performed in admission. Plasma EMPs, annexin V+MPs, PMPs, and LMPs in diabetics with or without hypertension were higher than those in control patients. Among diabetics, only EMPs in patients with hypertension was higher than in those without hypertension. Correlation analysis showed that systolic blood pressure and mean blood pressure were positively correlated with EMPs. Multivariant analysis demonstrated that EMP was an independent risk factor for the presence of hypertension in diabetics (odds ratio, 2.822; 95% confidence interval, 1.265–6.296; P=.011). Furthermore, baPWV in diabetics with hypertension (1910±355 cm/s) was higher than that in control patients (1441±198 cm/s) and diabetics without hypertension (1727±2 cm/s). Multivariant analysis identified EMP as a potent contributor to the development of impaired artery elasticity in diabetics (odds ratio, 4.401; 95% confidence interval, 1.529–12.673; P=.006). Plasma EMP was associated with the presence of hypertension and impaired arterial stiffness in type 2 diabetes.
The prevalence of diabetes is gradually increasing, and it is well known that diabetes and hypertension go hand in hand, with a 75% prevalence of hypertension among patients with diabetes. 1 Studies have found that arterial stiffness is increased in diabetes, which is considered an independent predictor of all‐cause and cardiac mortality in diabetes. 2 , 3 Although traditional risk factors such as age, sex, glycemic control, and insulin resistance are associated with the incidence of hypertension and changes in arterial stiffness in diabetes, these risk factors could not completely account for those modifications. 2 , 4 , 5
Microparticles (MPs) are small membrane vesicles shed from the plasma membrane surface and express a panel of phospholipids and proteins specific to the cells from which they are derived. Currently, research shows that MPs are associated with several cardiovascular complications and diseases, such as diabetes, hypertension, and the metabolic syndrome. 6 , 7 , 8 More importantly, most of the experimental evidence available so far indicates that MPs influence diverse biological function. MPs isolated from blood of patients with clinical cardiovascular disease could initiate thrombosis and cause endothelial dysfunction in vitro and in experimental vascular models. 8 , 9 , 10 In the present study we investigated whether the levels of MPs were associated with the presence of hypertension and arterial stiffness in patients with type 2 diabetes.
Patients and Methods
In this study, 39 patients with diabetes and hypertension, 24 patients with diabetes without hypertension, and 20 healthy controls were enrolled. Patients with diabetes were selected from August 2007 to November 2007 in the department of endocrinology in our hospital, and patients with ketoacidosis, hyperosmolar nonketotic diabetic coma, acute infection, or autoimmune disease were excluded. Diagnosis of diabetes was based on 1999 World Health Organization diagnostic criteria for diabetes mellitus. Hypertensive patients were characterized by a supine arterial blood pressure (BP) (after 10 minutes of rest) either systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg as measured by mercury sphygmomanometer twice in the morning. Clinical characteristics of patients are shown in Table I. The study was approved by the local ethics committee and informed consent was obtained from each patient.
Table I.
Clinical and Biological Characteristics of the Study Population
| Controls | DM‐HP(−) | DM‐HP(+) | |
|---|---|---|---|
| Patients, No. | 20 | 24 | 39 |
| Sex, male/female | 7/13 | 16/8a | 14/25b |
| Age, y | 57.9±6.1 | 56.4±6.7 | 64.9±8.0a,b |
| BMI, kg/m2 | 22.7±3.1 | 23.5±4.3 | 24.3±3.2 |
| SBP, mm Hg | 119±13 | 124±11 | 153±19a,b |
| DBP, mm Hg | 73±8.9 | 76±8.2 | 82±9.5a,b |
| FBG, mmol/L | 4.7±0.4 | 9.7±3.1a | 9.9±3.4a |
| PBG, mmol/L | 12.3±3.4 | 14.8±4.3b | |
| Glycated hemoglobin, % | 5.0±0.9 | 9.3±2.1a | 9.4±2.5a |
| Duration of DM | 1.8±2.7 | 7.2±6.9# | |
| Total cholesterol, mmol/L | 3.6±0.6 | 4.9±1.1a | 5.4±1.2a |
| Triglycerides, mmol/L | 0.9±0.2 | 2.7±4.3a | 2.9±3.2a |
| LDL cholesterol, mmol/L | 1.9±0.5 | 2.9±1.1a | 3.1±1.1a |
| HDL cholesterol, mmol/L | 1.3±0.3 | 1.0±0.3a | 1.1±0.3a |
| BUN, mmol/L | 6.4±2.7 | 6.0±1.8 | |
| Cr, μmol/L | 70±25 | 80±37 | |
| UA, mmol/L | 297±94 | 292±100 | |
| UMA, mg/L | 102±175 | 308±1194 | |
| Medication, No. | |||
| Insulin | – | 9 | 18 |
| Metformin | – | 9 | 18 |
| Sulfonylurea | – | 9 | 11 |
| Acarbose | – | 6 | 13 |
| Statin | – | 10 | 13 |
| ACE inhibitor/ARB | – | 4 | 30 |
| CCB | – | 1 | 10 |
| β‐Blocker | – | 0 | 5 |
| Diuretic | – | 0 | 6 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, mean body mass index; BUN, serum urea nitrogen; CCB, calcium channel blocker; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HP, hypertension; PBG, postprandial blood glucose; SBP, systolic blood pressure; UA, uric acid; UMA, urine microalbuminuria. a P<.05 compared with healthy patients. b P<.05 compared with diabetic patients without hypertension.
Detection of MP
Detection of plasma MP was performed by flow cytometry as previously described. 11 , 12 In brief, 50 μL of platelet‐poor plasma, separated from a 2‐mL blood sample by centrifugation at 160 × g (10 minutes) and then 1000 × g (6 minutes) was incubated (20 minutes, room temperature) with either 4 μL of antiCD‐31‐FITC plus 4 μL of anti‐CD42‐PE or anti‐CD45‐PE (BD Biosciences, Franklin Lakes, NJ), then diluted with 1 mL of PBS. While detecting annexin V+MP, platelet‐poor plasma was incubated with 4 μL of annexin V‐FITC solution (BD Biosciences) in the presence of CaCl2, while control experiments were performed in the presence of annexin V‐FITC without calcium. For flow cytometry assay (FACSCalibur; BD Biosciences), events calibrated by an internal standard beads (0.8 μm; Sigma, St. Louis, MO) were identified in forward‐scatter and side‐scatter intensity dot representation, gated as MP, and plotted on one‐ or two‐color fluorescence histograms. For MP numeration, calibrator beads (3 μm; Sigma) were added to the sample (Figure 1). EMP was defined as CD31‐positive and CD42‐negative particles. PMP was defined as CD31‐positive and CD42‐positive particles. LMP was defined as CD45‐positive particles.
Figure 1.
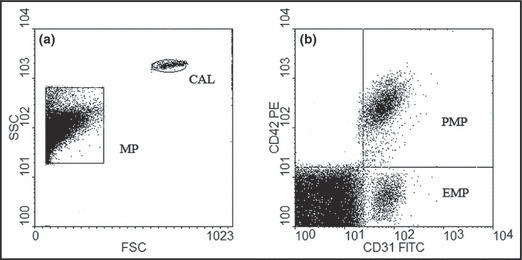
Cytofluorometry analysis of circulating microparticles (MPs). (a) Circulating MP and calibrator beats (CAL) are represented on a forward‐scatter/side‐scatter dot plot histogram. (b) Size selected (MP) are analyzed for fluorescence associated with specific antibodies. PMP indicates platelet‐derived microparticle.
Assessment of Brachial Ankle Pulse Wave Velocity
Brachial ankle pulse wave velocity (baPWV) was measured using a waveform analyzer (VP‐1000; Colin Company, Komaki, Japan) after at least 5 minutes of rest. baPWV was obtained using a volume‐plethysmographic apparatus by simultaneous BP and waveform measurements on all four limbs along with electrocardiographic and phonographic tracings. Pulse wave velocity was automatically calculated by time‐phasic analysis using the following formula: distance between two sites divided by pulse wave transit time. baPWV was measured from the ascending point of right brachial pulse volume recorder to the ascending point of each ankle pulse volume recorder. The distance between the right brachium and ankle was estimated based on body height and derived from statistical studies. All procedures took about 5 minutes. Mean baPWV value was calculated with (right baPWV+left baPWV)/2.
Statistical Analysis
All data were analyzed using SPSS 11.5 software (IBM, Armonk, NY). Quantitative data were presented as mean±standard deviation or median (minimum–maximum) and compared by one‐way analysis of variance or Kruskal‐Wallis test between groups. Categoric data were expressed as rate and compared by chi‐square test. Pearson or Spearman correlation analysis was conducted for the variables with the trend of correlation. Univariate and multivariate logistic regression was adopted for multiple factor analysis. P<.05 was considered statistically significant.
Results
Plasma Levels of Circulating MPs in Patients
The levels of EMPs, annexin V+MPs, PMPs, and LMPs in patients with diabetes with or without hypertension were significantly higher than those in healthy patients (P<.05). When comparing the different MPs among diabetes, only the level of EMP in diabetics with hypertension was significantly higher than those without hypertension (P<.05). Although the levels of PMPs, LMPs, and annexin V+MPs in diabetics with hypertension were higher than in those without hypertension, they did not reach statistical significance (Figure 2).
Figure 2.
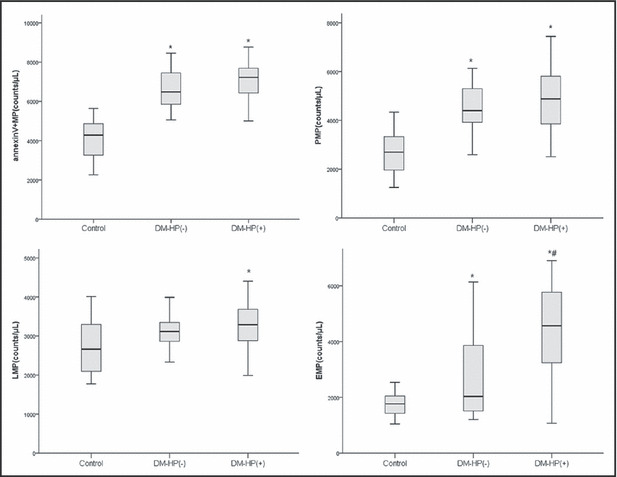
Levels of plasma microparticles in diabetes with hypertension [DM‐HP(+)], diabetes without hypertension [DM‐HP(−)] and control group (Control). Results were expressed as box plots. Line within boxes represent median values, the upper and lower lines of the boxes represent the 25th and 75th percentiles, and the upper and lower bar outside the boxes represent the 90th and 10th percentiles. *P<.05 compared with healthy patients. #P<.05 compared with diabetes without hypertension.
Correlations Between EMP and BP in All Patients
Correlation analysis demonstrated that SBP was positively correlated with EMP, age, glycated hemoglobin (HbAlc), total cholesterol, low‐density lipoprotein (LDL) cholesterol, fasting blood glucose, and postprandial blood glucose. DBP was correlated with LDL cholesterol and fasting blood glucose and EMP. Mean BP (MBP) was positively correlated with EMP, age, HbAlc, total cholesterol, LDL cholesterol, and fasting blood glucose (Table II). After adjustment by the factors of lipid levels and blood glucose, EMP was still positively related to SBP (r s=0.360, P=.009) and MBP (r s=0.408, P=.003).
Table II.
Univariate Correlations Between EMP and Biological Parameters
| EMP | Systolic Blood Pressure | Diastolic Blood Pressure | Mean Blood Pressure | Age | |
|---|---|---|---|---|---|
| EMP | |||||
| Systolic blood pressure | 0.439a | ||||
| Diastolic blood pressure | 0.229a | 0.640a | |||
| Mean blood pressure | 0.414a | 0.905a | 0.253a | ||
| Age | 0.329a | 0.446a | 0.159 | 0.474a | |
| Glycated hemoglobin | 0.512a | 0.318a | 0.173 | 0.309a | 0.230a |
| Total cholesterol | 0.376a | 0.316a | 0.134 | 0.324a | 0.217 |
| LDL cholesterol | 0.240a | 0.332a | 0.306a | 0.254a | 0.202 |
| HDL cholesterol | −0.063 | 0.063 | 0.034 | 0.061 | 0.154 |
| Triglycerides | 0.145 | 0.092 | 0.186 | 0.013 | −0.047 |
| Fasting blood glucose | 0.505a | 0.313a | 0.309a | 0.223a | 0.220a |
| Postprandial blood glucose | 0.155 | 0.263a | 0.173 | 0.224 | 0.046 |
Abbreviations: EMP, endothelial microparticle; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. a P<.05.
Level of EMPs and the Presence of Hypertension in Patients With Diabetes
In multivariate logistic regression analysis, hypertension was used as a dependent variable, while age, body mass index, blood glucose, lipid levels, sex, duration of diabetes, HbAlc, and plasma EMPs were used as independent variables. We found that age, duration of diabetes, and plasma EMPs were significantly independent risk factors for the presence of hypertension in patients with diabetes (Table III).
Table III.
Multivariate Logistic Regression Analysis of Risk Factors for Hypertension in Patients With Diabetes Mellitus
| OR (95% CI) | P Value | |
|---|---|---|
| Age | 1.135 (1.010–1.276) | .033 |
| EMP | 2.822 (1.265–6.296) | .011 |
| Duration of diabetes | 2.590 (1.118–5.999) | .026 |
Abbreviations: CI, confidence interval; EMP, endothelial microparticle; OR, odds ratio.
baPWV and MPs
The value of baPWV in diabetics was significantly greater than in the control patients (1839±332 cm/s vs 1441±198 cm/s, t=5.075, P<.001). Furthermore, baPWV in diabetics with hypertension was significantly higher than in those without hypertension (1910±355 cm/s vs 1727±2 cm/s; t=2.18, P=.033). These results indicated that arterial stiffness was increased in diabetics, especially in those with hypertension. We defined patients as having severe arterial stiffness when their baPWV values were higher than the 75th percentile of the distribution of baPWV in diabetes, and applied logistic regression analysis to investigate the risk factors of severe arterial stiffness. The univariate logistic regression analysis showed that age, HbAlc, total cholesterol, BP, annexin V+MP, LMP, and EMP were significant risk factors of severe arterial stiffness in diabetes (Table IV). Multivariate analysis indicated that EMP was the most significant and independent risk factor for severe arterial stiffness in diabetes (Table V).
Table IV.
Univariate Logistic Regression Analysis of Risk Factors for Severe Arterial Stiffness in Diabetes
| OR (95% CI) | P Value | |
|---|---|---|
| Age | 1.114 (1.031–1.204) | .006 |
| Sex | 1.498 (0.487–4.606) | .482 |
| Glycated hemoglobin | 1.410 (1.121–1.775) | .003 |
| Fasting blood glucose | 1.173 (0.990–1.389) | .065 |
| Smoking | 0.877 (0.171–4.491) | .875 |
| Duration of diabetes | 1.449 (0.882–2.380) | .144 |
| Total cholesterol | 1.647 (1.035–2.620) | .035 |
| LDL cholesterol | 1.060 (0.630–1.785) | .826 |
| HDL cholesterol | 1.360 (0.279–6.624) | .704 |
| Triglycerides | 0.967 (0.79–1.183) | .742 |
| Systolic blood pressure | 1.060 (1.026–1.095) | <.001 |
| Diastolic blood pressure | 1.030 (0.972–1.092) | .314 |
| Mean blood pressure | 1.084 (1.040–1.130) | <.001 |
| Annexin V+MP | 2.257 (1.324–3.848) | .003 |
| PMP | 1.436 (0.886–2.326) | .142 |
| LMP | 1.671 (1.004–2.781) | .048 |
| EMP | 6.125 (2.297–16.332) | <.001 |
Abbreviations: CI, confidence interval; EMP, endothelial microparticle; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LMP, leukocyte‐derived microparticle; OR, odds ratio; PMP, platelet‐derived microparticle; MP, microparticle. Bold values indicate significance.
Table V.
Multivariate Logistic Regression Analysis of Risk Factors for Severe Arterial Stiffness in Diabetes
| OR (95% CI) | P Value | |
|---|---|---|
| MAP | 1.065 (1.015–1.117) | .010 |
| EMP | 4.401 (1.529–12.673) | .006 |
Abbreviations: CI, confidence interval; EMP, endothelial microparticles; MAP, mean arterial pressure; OR, odds ratio.
Discussion
This study demonstrated that both plasma EMPs and baPWV were elevated in diabetics with hypertension. Level of EMPs was an independent risk factor for the presence of hypertension and a potent contributor to the development of severe arterial stiffness in diabetes.
Currently the number of patients with type 2 diabetes is increasing in the population, and it is well‐known that diabetes and hypertension go hand in hand, with a 75% prevalence rate of hypertension among patients with diabetes. 1 Previous studies have reported the association between traditional risk factors (such as age, sex, and treatment regimen) and the incidence of hypertension in diabetes. However, these traditional factors just partly explain the incidence of hypertension in patients with diabetes. Therefore, it is necessary to explore other factors that are involved in the presence of hypertension in diabetes. 4 , 5
As a marker of endothelial injury, EMP is detectable in the plasma of healthy patients and its amount is increased under pathological conditions. Elevated plasma EMP is associated with most of the cardiovascular complications and diseases. 6 , 7 , 8 Accumulating evidence indicates that EMP contributes to the pathogenesis of cardiovascular disease. Nozaki and colleagues 13 showed that plasma EMPs in patients with a high risk for coronary heart disease were independent predictors of long‐term cardiovascular events. Koga and associates 14 found that level of plasma EMP was an independent predictor for the presence of coronary artery disease in diabetes. Our present study found that levels of EMP, annexin V+MP, PMP, and LMP were elevated in diabetics. Among diabetic patients, only the level of plasma EMP in diabetics with hypertension was significantly higher than in those without hypertension. Multivariate logistic regression analysis showed that age, duration of diabetes, and level of endothelial MP were independent risk factors for the presence of hypertension in diabetes. Therefore, we presumed that elevated EMP was one of risk factors for the incidence of hypertension in type 2 diabetes. Indeed, it was demonstrated that EMP was not only a bystander but also a critical inducer of endothelial dysfunction. Elevated level of plasma EMP in diabetes could impair the integrity and vasorelaxation of endothelium and initiate the progression of hypertension. 8 , 10 , 15 , 16 Higher level of plasma EMP was associated with higher prevalence of hypertension in diabetes.
Previous studies indicated that arterial stiffness was increased in diabetes, which was considered an independent predictor of all‐cause and cardiac mortality. 2 , 3 As one of noninvasive standard measurement of large artery stiffness, the measurement of baPWV has recently been developed. 17 Our study showed that the value of baPWV was higher in diabetics than that in healthy patients and increased significantly in diabetics with hypertension, which indicated that the vascular elasticity was impaired in patients with diabetes, especially in diabetics with hypertension. Furthermore, we defined patients as having severe arterial stiffness when their baPWV value was higher than the 75th percentile of the distribution of baPWV in diabetes, and found that EMP and mean MAP were independent risk factors for severe arterial stiffness. It suggested that in diabetics with hypertension, not only elevated BP was involved in the impairment of arterial stiffness, but also that elevated EMP might play an important role in the progression of arterial stiffness. The association between circulating EMP and arterial stiffness in the present study was in accordance with previous studies. 7 , 18 They demonstrated that plasma EMP was positively related to baPWV in hypertension or in healthy patients. Although our study was unable to infer the causal relationship between the two parameters, a handful of evidence has shown that EMP may participate in the process of arterial stiffness. Previous studies found that EMP could impair the release of nitric oxide from vascular endothelial cells and the formation of the vascular network of human umbilical vein endothelial cells. They could also reduce the proliferation of endothelial cells and increase occurrence of apoptosis. 8 , 10 , 16 Meanwhile, MPs significantly increased endothelial progenitor cells apoptosis and reduced their colony‐forming capacity. 15 Loss of endothelial progenitor cells might represent impaired vascular reparation, which contributed to the increase of vascular tone and arterial stiffness. In addition, EMP might participate in the process of inflammation by increasing the inflammation factors, such as interleukin 1, tumor necrosis factor α, and neutrophil recruitment. 19 Bearing multiple biological functions make endothelial MPs possible to participate in the progression of arterial stiffness, through different mechanisms, including impact on endothelial cells, endothelial progenitor cells, and inflammation.
Conclusions
Increased levels of plasma endothelial MPs were associated with the presence of hypertension and arterial stiffness in patients with type 2 diabetes.
Conflict of interest: The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
Acknowledgments and disclosures: All authors participated in the development and writing of the paper and approved the final manuscript for publication. The authors take full responsibility for the content of the paper and are grateful to the staff of Department of Endocrinology, East Hospital, for their facilitation of the patient sample collection.
References
- 1. Cheung BM, Ong KL, Cherny SS, et al. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443–453. [DOI] [PubMed] [Google Scholar]
- 2. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 4. Janghorbani M, Amini M. Hypertension in type 2 diabetes mellitus in Isfahan, Iran: incidence and risk factors. Diabetes Res Clin Pract. 2005;70:71–80. [DOI] [PubMed] [Google Scholar]
- 5. Sahakyan K, Klein BE, Myers CE, et al. Novel risk factors in long‐term hypertension incidence in type 1 diabetes mellitus. Am Heart J. 2010;159:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diabetes Metab. 2008;1:S27–S32. [DOI] [PubMed] [Google Scholar]
- 7. Wang JM, Su C, Wang Y, et al. Elevated circulating endothelial microparticles and brachial–ankle pulse wave velocity in well‐controlled hypertensive patients. J Hum Hypertens. 2009;23:307–315. [DOI] [PubMed] [Google Scholar]
- 8. Agouni A, Lagrue‐Lak‐Hal AH, Ducluzeau PH, et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leroyer AS, Anfosso F, Lacroix R, et al. Endothelial‐derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–463. [DOI] [PubMed] [Google Scholar]
- 10. Densmore JC, Signorino PR, Ou J, et al. Endothelium‐derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006;26:464–471. [DOI] [PubMed] [Google Scholar]
- 11. Jimenez JJ, Jy W, Mauro LM, et al. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. [DOI] [PubMed] [Google Scholar]
- 12. Feng B, Chen Y, Luo Y, et al. Circulating level of microparticles and their correlation with arterial elasticity and endothelium‐dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–269. [DOI] [PubMed] [Google Scholar]
- 13. Nozaki T, Sugiyama S, Koga H, et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–608. [DOI] [PubMed] [Google Scholar]
- 14. Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of VE‐cadherin‐positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;17:1622–1630. [DOI] [PubMed] [Google Scholar]
- 15. Pirro M, Schillaci G, Paltriccia R, et al. Increased ratio of CD31+/CD42‐ microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–2535. [DOI] [PubMed] [Google Scholar]
- 16. Mezentsev A, Merks RM, O’Riordan E, et al. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–H1114. [DOI] [PubMed] [Google Scholar]
- 17. Munakata M, Ito N, Nunokawa T, et al. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003;16:653–657. [DOI] [PubMed] [Google Scholar]
- 18. Wang JM, Huang YJ, Wang Y, et al. Increased circulating CD31+/CD42‐microparticles are associated with impaired systemic artery elasticity in healthy subjects. Am J Hypertens. 2007;20:957–964. [DOI] [PubMed] [Google Scholar]
- 19. Buesing KL, Densmore JC, Kaul S, et al. Endothelial microparticles induce inflammation in acute lung injury. J Surg Res. 2011;166:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


