Abstract
J Clin Hypertens (Greenwich). 2012; 14:330–335. ©2012 Wiley Periodicals, Inc.
The activation of innate immune receptors, such as Toll‐like receptors (TLRs), participates in the pathogenesis of cardiovascular diseases. The authors evaluated TLR2 and TLR4 gene expression in the peripheral monocytes of nondiabetic hypertensive patients compared with normotensive individuals and investigated the effect of intensive systolic blood pressure (SBP)–lowering. Included were 43 nondiabetic hypertensive patients with essential hypertension who were randomly assigned to an intensive treatment arm, with an SBP target of <130 mm Hg, or a standard arm, with an SBP target of <140 mm Hg. TLR2 and TLR4 messenger RNA (mRNA) levels in monocytes were estimated before and 12 weeks after therapy initiation. Sixteen healthy individuals were included for comparison. Hypertensives revealed significantly higher TLR4 mRNA levels compared with normotensives (985±885 vs 554±234, P=.005). In contrast, no statistically significant difference was found in TLR2. Compared with standard treatment, intensive treatment significantly downregulated TLR2 and TLR4 mRNAs, expressed as fold induction (0.66±0.49 vs 1.38±1.65 and 0.62±0.3 vs 1.9±1.2, respectively; P<.001 for both). In conclusion, TLR4 mRNA levels in peripheral monocytes are significantly elevated in nondiabetic hypertensive patients. Intensive control of SBP results in attenuation of TLR2 and TLR4 gene expression in those patients. Our findings suggest that a strict SBP target in nondiabetic hypertensive patients may offer additional benefits.
Hypertension is recognized as an important risk factor for cardiovascular disease and organ damage. Chronic inflammatory processes represent a hallmark of essential hypertension and contribute to the development of target organ damage and atherosclerosis. 1 Atherosclerosis is a process mediated by circulating immune cells, such as monocytes. Circulating peripheral monocytes and their activation play an important part in the early stages of atherosclerotic lesion formation and play a crucial role in the cardiovascular complications of hypertensives. 2 It has been postulated that the activation of innate immune receptors, such as Toll‐like receptors (TLRs), participates in the pathogenesis of cardiovascular diseases. TLRs may be activated following tissue injury and have been linked to atherosclerosis. An increase in circulating TLR2‐ or TLR4‐positive monocytes has been observed in unstable angina, acute myocardial infarction, and chronic heart failure. 3 , 4 The renin‐angiotensin‐aldosterone system modulates the expression of TLRs, which are key elements of the innate immune system. More specifically, angiotensin II exerts proinflammatory effects and leads to activation of the innate immune system by a novel mechanism involving the upregulation of TLR4. 5 However, there are a lack of data regarding the role of TLRs in essential hypertension.
It is well established that antihypertensive therapy effectively reduces cardiovascular morbidity and mortality in proportion to the degree to which blood pressure (BP) is reduced; it is thus vital to achieve strict BP control in order to benefit maximally from the lowering of BP. 6 , 7 Optimal pressure control is crucial for the prevention of cardiovascular events in hypertensive patients. Most guidelines for the treatment of hypertension recommend a BP goal of <140/90 mm Hg and a more aggressive goal of <130/80 mm Hg for patients who have diabetes mellitus and are at high risk. However, some previous studies have indicated that a more intensive antihypertensive treatment with a systolic BP (SBP) target of <130 mm Hg is also safe and beneficial in nondiabetic patients. 8
In the present study, we evaluated TLR2 and TLR4 gene expression levels in the peripheral monocytes of untreated nondiabetic patients with essential hypertension compared with normotensive individuals. TLR receptors are expressed abundantly on monocytes, which play a crucial role in the cardiovascular complications of hypertensives. We also compared the effect of more intensive vs standard BP control on TLR2 and TLR4 gene expression levels in the monocytes of those patients.
Methods
Study Population
We prospectively enrolled patients with untreated grade 1 or grade 2 essential hypertension and with no indications of other organic heart disease. The diagnosis of hypertension was based on 3 outpatient measurements of BP >140/90 mm Hg at intervals of no longer than 2 weeks, according to the recommendations of the European Society of Hypertension/European Society of Cardiology. 9 Participants with BP >140/90 mm Hg on the final visit underwent 24‐hour ambulatory BP monitoring. To be eligible for inclusion in the study, a mean 24‐hour BP >130/80 mm Hg was required.
The patients had not previously taken any hypertensive medication and did not take any other drugs for 3 weeks before the studies. The following were criteria for exclusion: smokers; pregnant, lactating, or potentially childbearing women; previous history or medication for hypertension; patients with grade 3 hypertension or secondary hypertension; tachyarrythmias or bradyarrythmias; coronary artery disease; symptoms of heart failure and/or ejection fraction of left ventricle <55%; diabetes mellitus; cerebrovascular, liver, renal, or neoplastic disease; albumin excretion rate >200 μg/min; history of drug or alcohol abuse; any chronic inflammatory or other infectious disease during the past 6 months; and thyroid gland disease.
All patients gave written informed consent. The institutional ethics committee approved the study. Eligible patients were randomly assigned to one of two treatment targets: an intensive arm with an SBP target of <130 mm Hg or a standard arm with an SBP target of <140 mm Hg. The medications administered were open‐labeled and the choice of drugs in individual patients was left to the physicians’ discretion. Antihypertensive drug treatment included combinations of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium antagonists, and diuretics. If a dose was missed, the patient was instructed to take the next dose as scheduled. A full clinical examination and BP measurement were performed before and 4, 8, and 12 weeks after the initiation of treatment. On each visit, at least two measurements were taken, with a 5‐minute interval between them, and the second BP reading was used in the data analysis. In the intensively treated group, an SBP reading >130 mm Hg at week 4 led to exclusion from the study. Conversely, investigators were instructed that achievement of SBP <130 mm Hg in the standard group should lead to down‐titration of treatment.
A standard echocardiography study was also performed and blood samples were taken for analysis of full clinical chemistry and hematology markers. Blood samples for the evaluation of TLR messenger RNA (mRNA) levels in peripheral monocytes were drawn at baseline and at 12 weeks. The control group consisted of 16 normotensive individuals whose clinical and laboratory examinations were normal.
Blood samples were collected into ethylenediaminetetraacetic acid collection tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by Histopaque‐ficoll (SIGMA) centrifugation, and CD14+ monocytes were purified from PBMCs by positive selection using MACS high‐gradient magnetic separation columns type MS and negative magnetic bead selection. Purity assessed by FACS (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ) analysis was >95%.
RNA Isolation and Quantitative RT‐PCR
Total RNA was isolated from monocytes using the TRI Reagent (Ambion, Austin, TX) and 1‐μg RNA was reverse‐transcribed with oligo‐(dT) using the Reverse Transcription System (Promega, Madison, WI) in 20‐μL reactions. Measurements of mRNA levels were performed by real‐time reverse‐transcription polymerase chain reaction using the Stratagene Mx3000P Detection System (Santa Clara, CA). Polymerase chain reaction assays were performed in cDNA template 1 μL using the SYBR Green PCR Master Mix (Bio Rad, Hercules, CA). All measurements were performed in triplicate. The standard curve method was used for absolute quantification of the amplification products and specificity was determined by performing a melting curve analysis. Standard curves for expression of each gene were generated by serial dilution of known quantities of cDNA template. The housekeeping gene glyceraldehyde‐3‐phosphate‐dehydrogenase (GAPDH) was used as an endogenous reference gene, and relative quantification was done by normalizing the signals of the different genes with the GAPDH signal. Primers used were 5‐GGG AGA CAC AGA TGG CTG GGA‐3′ (sense) and 5′‐CAA GGA GCA TTG CCC AAC AGG A‐3′ (antisense) for TLR4, 5′‐CCT CTC GGT GTC GGA ATG T‐3′ (sense) and 5′‐CAT CCC GCT CAC TGT AAG AAA‐3′ (antisense) for TLR2, 5′‐CCA TCT TCC AGG AGC GAG‐3′ (sense) and 5′‐GCA GGA GGC ATT GCT GAT‐3′ (antisense) for GAPDH. For each patient, normalized values at follow‐up were expressed as fold differences from values before treatment.
Statistical Analysis
Descriptive statistics are presented as mean±standard deviation or frequencies, as appropriate. Continuous parameters were compared between groups using independent sample t tests, while categoric variables were compared using Fisher exact or chi‐square tests. The association between continuous variants was assessed using Pearson’s correlation coefficient. All tests were performed at the two‐sided 5% level of significance with the IBM‐SPSS 19 software package (SPSS, IBM, Armonk, NY).
Results
A total of 52 hypertensive patients were enrolled in the study. Sixteen healthy individuals were used as a control group. Adverse events were experienced by 10 patients. Peripheral edema was noticed in 6 patients in the intensive group (leading to exclusion from the study in 2) and in 3 in the standard group (leading to exclusion from the study in 1) and fatigue occurred in 1 patient in the standard treatment group. In the intensive treatment group, 2 patients were lost to 8‐week follow‐up and 4 patients were excluded because they did not achieve the target BP levels; thus, 43 patients were eligible for statistical analyses.
Hypertensive patients had significant baseline differences in SBP, diastolic blood pressure (DBP), left atrial dimension, and left ventricular mass index compared with controls (Table I). No changes in the patients’ physical characteristics—body weight or body mass index—were observed at the end of the treatment period (data not shown). At baseline, mean office sitting BP was comparable in the intensive and standard treatment groups (Table II). The average BP levels achieved in the intensive‐target subgroup and the standard‐target subgroup were 125±3/79±4 mm Hg and 136±3/85±5 mm Hg, respectively (P<.001 for both SBP and DBP). Hypertensive patients revealed significantly higher TLR4 mRNA levels compared with normotensive individuals, as expressed in arbitrary units (985±885 in hypertensives vs 554±234, P=.005). In contrast, we found no significant difference in TLR2 mRNA levels between hypertensives and controls (459±1219 vs 638±311, respectively, P=not significant [NS]).
Table I.
Clinical and Laboratory Data of the Participants
| Variable | Hypertensives (n=43) | Controls (n=16) | P Value |
|---|---|---|---|
| Age, y | 56±8 | 62±7 | NS |
| Male/female | 29/14 | 10/6 | NS |
| Smokers | 29 | 11 | NS |
| Body mass index, kg/m2 | 28.9±4.5 | 29.2±3.5 | NS |
| Fasting glucose, mg/dL | 93±15 | 97±10 | NS |
| Creatinine, mg/dL | 0.9±0.13 | 1.0±0.14 | NS |
| Haemoglobin, g/dL | 14.6±3.1 | 14.2±1.6 | NS |
| Total cholesterol, mg/dL | 226±47 | 244±38 | NS |
| Triglycerides, mg/dL | 199±89 | 227±102 | NS |
| LDL cholesterol, mg/dL | 149±50 | 164±63 | NS |
| HDL cholesterol, mg/dL | 38±65 | 37±15 | NS |
| Uric acid, mg/dL | 5.7±1.3 | 5.9±1.1 | NS |
| Systolic BP, mm Hg | 158±3 | 126±4 | <.001 |
| Diastolic BP, mm Hg | 94±6 | 78±4 | <.001 |
| Heart rate, beats per min | 72±13 | 70±4 | NS |
| Left atrium, mm | 40±6 | 31±1 | <.001 |
| LVMI, g/m2 | 170±44 | 101±9 | <.001 |
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVMI, left ventricular mass index; NS, not significant. Values are expressed as number or mean±standard deviation.
Table II.
Clinical and Laboratory Data of Patients in the Intensive and Standard Treatment Groups
| Variable | Intensive (n=18) | Standard (n=25) | P Value |
|---|---|---|---|
| Age, y | 51±7 | 53±9 | NS |
| Male/female | 12/6 | 17/8 | NS |
| Smokers | 11 | 18 | NS |
| Body mass index, kg/m2 | 28.2±4.4 | 27.2±4.5 | NS |
| Fasting glucose, mg/dL | 95±12 | 93±10 | NS |
| Hemoglobin, g/dL | 14.9±3.1 | 14.2±3.6 | NS |
| Total cholesterol, mg/dL | 217±50 | 232±46 | NS |
| Triglycerides, mg/dL | 190±95 | 197±108 | NS |
| LDL cholesterol, mg/dL | 146±65 | 148±69 | NS |
| HDL cholesterol, mg/dL | 37±65 | 38±55 | NS |
| Uric acid, mg/dL | 6.2±1.4 | 5.7±1.2 | NS |
| Systolic BP, mm Hg | 158±3 | 156±3 | NS |
| Diastolic BP, mm Hg | 94±5 | 91±4 | NS |
| Heart rate, beats per min | 74±12 | 71±13 | NS |
| Left atrium, mm | 41±7 | 39±6 | NS |
| LVMI, g/m2 | 172±48 | 168±40 | NS |
| Medications, No. | |||
| Angiotensin‐converting enzyme inhibitor | 10 | 14 | NS |
| Angiotensin receptor blockers | 8 | 10 | NS |
| Calcium antagonists | 6 | 9 | NS |
| Diuretics | 8 | 10 | NS |
| Statins | 10 | 12 | NS |
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVMI, left ventricular mass index; NS, not significant. Values are expressed as number or mean±standard deviation.
Antihypertensive treatment reduced TLR4 gene expression levels significantly only in the intensive treatment group (fold induction 0.62±0.3 vs 1.9±1.2 in the standard treatment group, P<.001; Figure 1). Although not so profound, a similar significant effect of treatment was revealed in TLR2 gene expression only in the intensively treated hypertensives (fold induction 0.66±0.49 vs 1.38±1.65 in the standard treatment group, P=.048).
Figure 1.

Intensive and standard blood pressure treatments revealed a significantly different effect on Toll‐like receptor (TLR)4 and TLR2 fold induction in peripheral monocytes from nondiabetic hypertensives (P<.001).
TLR4 fold induction was significantly associated with the changes in SBP (dSBP) and DBP (dDBP) before and after treatment (r=−0.53 and r=−0.51, respectively, P<.001; Figure 2), while no significant associations with the response to treatment were found for TLR2 (r=−0.2, P=NS for both). In addition, modest negative correlations with borderline significance was found between the degree of TLR4 elevation and the baseline level of SBP (r=−0.301, P=.05) and DBP (r=−0.26, P=.09).
Figure 2.
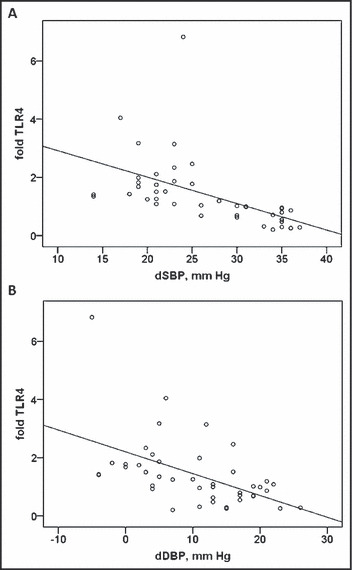
Toll‐like receptor (TLR)4 fold induction was significantly associated with changes in systolic blood pressure (dSBP) and diastolic blood pressure (dDBP) before and after treatment (r=−0.53 and r=−0.51, respectively, P<.001).
Discussion
Our findings demonstrate for the first time that TLR4 mRNA levels in peripheral monocytes are significantly elevated in nondiabetic hypertensive patients. In addition, intensive BP‐lowering results in a significantly lower gene expression of both TLR2 and TLR4 in the monocytes of those patients, compared with the standard treatment.
Activation of innate immune receptors, such as TLRs, participates in the pathogenesis of cardiovascular diseases. TLRs have been identified as central innate immune receptors, are highly expressed in the cardiovascular system, and could thus be a key link between cardiovascular diseases and the activation of the immune system.
The expression of TLR4 and TLR2 in atherosclerotic plaques is well documented; they are localized mainly to macrophages and endothelial cells, 10 , 11 while activation of TLR signalling can promote atherosclerosis by multiple mechanisms. 12 Increased TLR2 and TLR4 expression in monocytes has also been observed in unstable angina, acute myocardial infarction, and chronic heart failure. 3 , 4 The renin‐angiotensin‐aldosterone system modulates the expression of TLRs, which are key elements of the innate immune system. More specifically, angiotensin II exerts proinflammatory effects and leads to an activation of the innate immune system by a novel mechanism involving the upregulation of TLR4. 5
Our aim was to investigate the gene expression of receptors that reflect activation of the innate immune system that has not been studied in hypertensive patients so far. For this purpose, we chose TLR2 and TLR4, which are implicated in monocyte activation 13 and thus may play an important role in the hypertension‐associated end‐organ damage and the initiation and progression of atherosclerosis. Activation of the TLR4‐mediated signalling pathway leads to macrophage activation and migration, 14 and TLR4 stimulation in monocytes induces the production of matrix metalloproteinase 9, which has been suggested as a marker for extracellular matrix degradation. 15 In addition, according to animal studies, TLR4 is involved in vascular remodeling, probably via activation by endogenous ligands, and affecting collagen accumulation in the artery. 16 , 17 Recently, enhanced cardiac gene expression of TLR4 was reported in hypertensive rats. 18 However, the experimental data regarding the role of TLRs in cardiac hypertrophy and the prevention and treatment of hypertension‐induced myocardial hypertrophy and cardiac fibrosis are controversial. 19 , 20 , 21
In the literature, there is a lack of human studies examining the role and gene expression of TLR2 and TLR4 in essential hypertension. We found that TLR4 mRNA levels were significantly elevated in the monocytes of nondiabetic individuals compared with normotensives. We did not find any statistical difference in TLR2 gene expression. This may have been due to the fact that TLR2, similar to other inflammatory biomarkers, 22 may not be significantly elevated in hypertensive patients who have low cardiovascular risk. However, we cannot exclude the possibility that it might be the result of the small number and the clinical heterogeneity of the participants in this study. We found a large variability in TLR2 mRNA levels among hypertensive patients and this might have masked any differences. TLR2 plays a key role in macrophage recruitment, endothelial cell activation, and proinflammatory cytokine production; however, the broad spectrum of factors that contribute to this response remains unclear and needs further elucidation. 23
In addition, we provide novel data that a strict SBP target <130 mm Hg in nondiabetic hypertensives significantly decreased both TLR2 and TLR4 gene expression in peripheral monocytes compared with the standard treatment, which had a target of <140 mm Hg but >130 mm Hg.
Hypertension guidelines recommend that SBP and DBP be reduced to values <140/85 mm Hg, with a more aggressive BP target in certain high‐risk subgroups of patients, such as those with diabetes mellitus, chronic kidney disease, and coronary artery disease. Achieving the maximum reduction in cardiovascular morbidity and mortality is the primary goal of BP control; however, there are some findings that argue for a lower BP goal than this.
Few studies have investigated the efficacy of antihypertensive treatment targeted at lower BP levels than these in nondiabetic patients. In the Italian Study on the Cardiovascular Effects of Systolic Blood Pressure Control (Cardio‐Sis) trial, 7 a systolic BP goal <130 mm Hg was more effective in reducing left ventricular hypertrophy than was a treatment goal <140 mm Hg. Solomon and colleagues 24 reported that aggressive BP‐lowering was associated with improved annular relaxation velocity—a measure of diastolic function—in nondiabetic patients with hypertension and diastolic dysfunction, while the patients who achieved the greatest BP reduction had the best improvement in diastolic function.
Discussion
This is the first demonstration to our knowledge of increased TLR4 expression and activity in monocytes from nondiabetic hypertensive patients. Intensive SBP treatment results in a significant decrease of TLR2 and TLR4 gene expression in those patients. Although we did not find any statistically significant difference in TLR2 mRNA levels between hypertensives and normotensives, their reduction with intensive antihypertensive treatment might be of similar importance as for TLR4, since it has been demonstrated that the TLR2 level in monocytes, in parallel with conventional risk factors, is an independent risk factor and might play a critical role in atherosclerotic plaque formation. 25
Our findings support previous data that a strict SBP target <130 mm Hg in nondiabetic hypertensives may provide additional benefits and stresses the need for an evaluation of a BP treatment goal <130 mm Hg in further randomized clinical trials.
Study Limitations
As mentioned above, one limitation of this study is the relatively small sample size. However, it still produced interesting results. Some participants were taking statin treatment that might have an action on TLR gene expression levels. Since this percentage was similar in the two groups, it is unlikely to have affected the difference in our results. Also, we cannot exclude the possibility that the higher doses of antihypertensive medications used for achieving the SBP targets in the intensive group might be associated with an increased anti‐inflammatory effect and could have affected the response in terms of TLR gene expression. However, this does not diminish the value of our findings in supporting the benefits of strict BP control in spite of differences in medication.
Conclusions
Innate immune regulation is capable of dramatically influencing chronic inflammation and signaling cascade involving atherosclerotic evolution. Future studies will determine the contribution of our findings to their clinical outcome and the incidence of cardiovascular events. However, the measurement and modification of these innate immune pathways provides new opportunities for screening patients at risk for organ damage and tailoring therapy toward the organ systems specifically at risk.
Disclosure: The authors declare no conflict of interest.
References
- 1. Ghanem FA, Movahed A. Inflammation in high blood pressure: a clinician perspective. J Am Soc Hypertens. 2007;1:113–119. [DOI] [PubMed] [Google Scholar]
- 2. Tian N, Penman AD, Mawson AR, et al. Association between circulating specific leukocyte types and blood pressure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Hypertens. 2010;4:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Methe H, Kim JO, Kofler S, et al. Expansion of circulating Toll‐like receptor 4‐positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. [DOI] [PubMed] [Google Scholar]
- 4. Foldes G, von Haehling S, Okonko DO, et al. Fluvastatin reduces increased blood monocyte Toll‐like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol. 2008;124:80–85. [DOI] [PubMed] [Google Scholar]
- 5. Wolf G, Bohlender J, Bondeva T, et al. Angiotensin II upregulates Toll‐like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17:1585–1593. [DOI] [PubMed] [Google Scholar]
- 6. Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens. 2003;21:1055–1076. [DOI] [PubMed] [Google Scholar]
- 7. Turnbull F, Neal B, Algert C, et al.; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–1419. [DOI] [PubMed] [Google Scholar]
- 8. Verdecchia P, Staessen JA, Angeli F, et al.; On behalf of the Cardio‐Sis investigators . Usual versus tight control of systolic blood pressure in non‐diabetic patients with hypertension (Cardio‐Sis): an open‐label randomised trial. Lancet. 2009;374:525–533. [DOI] [PubMed] [Google Scholar]
- 9. Mancia G, de Backer G, Dominiczak A, et al.; Guidelines for the Management of Arterial Hypertension . The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1751–1762. [DOI] [PubMed] [Google Scholar]
- 10. Xu XH, Shah PK, Faure E, et al. Toll‐like receptor‐4 is expressed by macrophages in murine and human lipid‐rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. [DOI] [PubMed] [Google Scholar]
- 11. Edfeldt K, Swedenborg J, Hansson GK, et al. Expression of Toll‐like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 12. Björkbacka H. Multiple roles of Toll‐like receptor signaling in atherosclerosis. Curr Opin Lipidol. 2006;17:527–533. [DOI] [PubMed] [Google Scholar]
- 13. Farina C, Theil D, Semlinger B, et al. Distinct responses of monocytes to Toll‐like receptor ligands and inflammatory cytokines. Int Immunol. 2004;16:799–809. [DOI] [PubMed] [Google Scholar]
- 14. Lemieux I, Poirier P, Bergeron J, et al. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23:23B–31B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundstrom J, Evans JC, Benjamin EJ, et al. Relations of plasma matrix metalloproteinase‐9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. [DOI] [PubMed] [Google Scholar]
- 16. Hollestelle SC, De Vries MR, Van Keulen JK, et al. Toll‐like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–398. [DOI] [PubMed] [Google Scholar]
- 17. Pasterkamp G, Galis ZS, de Kleijn DP. Expansive arterial remodeling: location, location, location. Arterioscler Thromb Vasc Biol. 2004;24:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eissler R, Schmaderer C, Rusai K, et al. Hypertension augments cardiac Toll‐like receptor 4 expression and activity. Hypertens Res. 2011;34:551–558. [DOI] [PubMed] [Google Scholar]
- 19. Ehrentraut H, Weber C, Ehrentraut S, et al. The Toll‐like receptor 4‐antagonist eritoran reduces murine cardiac hypertrophy. Eur J Heart Fail. 2011;13:602–610. [DOI] [PubMed] [Google Scholar]
- 20. Liu YY, Cai WF, Yang HZ, et al. Bacillus Calmette‐Guerin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol. 2008;180:7349–7357. [DOI] [PubMed] [Google Scholar]
- 21. Ha T, Li Y, Hua F, et al. Reduced cardiac hypertrophy in Toll‐like receptor 4‐deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–234. [DOI] [PubMed] [Google Scholar]
- 22. Jastrzebski M, Czarnecka D, Rajzer M, et al. Increased levels of inflammatory markers in hypertensives with target organ damage. Kardiol Pol. 2006;64:802–809. [PubMed] [Google Scholar]
- 23. Hayashi C, Madrigal AG, Liu X, et al. Pathogen‐mediated inflammatory atherosclerosis is mediated in part via Toll‐like receptor 2‐induced inflammatory responses. J Innate Immun. 2010;2:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solomon SD, Verma A, Desai A, et al.; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction Investigators . Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55:241–248. [DOI] [PubMed] [Google Scholar]
- 25. Kuwahata S, Fujita S, Orihara K, et al. High expression level of Toll‐like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis. 2010;209:248–254. [DOI] [PubMed] [Google Scholar]


