Abstract
J Clin Hypertens (Greenwich). 2011;13:598–604.©2011 Wiley Periodicals, Inc.
Hypertension is prevalent in the United States and remains uncontrolled. The primary objective of the study was to determine the effect of once‐daily dosing of a combination therapy for blood pressure (BP) and dyslipidemia using home BP monitoring on reaching clinical BP and the effect of daily dosing of combination therapy on reaching lipid goals. The study was conducted in middle‐aged, indigent, African Americans who had high‐risk, resistant hypertension and dyslipidemia. Patients were randomly assigned to either the home and clinic BP group or usual care group and were followed for 6 months. The average BPs for each group were compared and used to titrate the study drug appropriately. Both groups achieved significant declines in BP, total cholesterol, and low‐density lipoprotein (LDL) (P<.0001). These findings demonstrate that BP control could be achieved at a rate of 43.5% compared with the 2004 national control rate of 35%. The LDL control rate was also improved. Cardiovascular risk reduction has been proven to be achieved through managing lipids and BP. This trial demonstrates that these goals can be achieved similar to other groups in indigent African Americans with high‐risk hypertension and dyslipidemia.
One in three adults in the United States has hypertension. 1 African Americans are among the population with the highest prevalence of hypertension, currently at 41.4% and 44% in black men and women, respectively. 2 Although awareness and treatment have improved significantly to 72% and 63%, respectively, control rates remain at 37% in African Americans. 2
Often multiple risk factors for cardiovascular disease occur concurrently. 3 Among nondiabetic veterans, the prevalence of concomitant hypertension (HTN) and dyslipidemia (DYS) was 23.8%. 4 According to the heart disease and stroke statistics of 2008, the prevalence of DYS among US adults was 35.6% and only one third of those treated reached the National Cholesterol Education Program (NCEP)–defined goal for low‐density lipoprotein (LDL) cholesterol. 2 Although control rates for DYS improved from 1999 to 2004 from 4% to 25.1%, there is still need for improvement. 2 The control rates of combined HTN/DYS are even lower, at 9%. Combined HTN and DYS lead to an increased risk of cardiovascular (CV) events, and this risk is greater than the sum of CV event risks of HTN and DYS alone. 5 , 6 Therefore, developing better approaches to managing both HTN and DYS can improve control rates and decrease CV morbidity and mortality. 7
Many tools have been used to improve control of HTN and DYS. One tool is the use of combination therapy, which can improve adherence to medication. 8 Another tool specifically for HTN is home blood pressure (BP) monitoring (HBPM), which has many potential advantages, including improvement of patient adherence to medication. 9 However, the reports in the literature regarding improvements in BP using HBPM have been inconsistent. 10 Yet, there is sufficient evidence that HBPM is a useful strategy to improve adherence to medication and BP control to investigate the most effective use for this tool in clinical practice. 10 , 11
While several studies have compared HBPM vs clinic BP monitoring, we have found none that have assessed the effect of clinic and HBPM with combination therapy to improve BP control and DYS. The primary objective of the study is to determine the effect of once‐daily dosing of a combination therapy for BP and DYS using HBPM on reaching clinical BP and the effect of combination therapy on reaching lipid goals.
Methods
The IMPACT trial was conducted at UT‐Southwestern Medical Center in Dallas, TX, in patients from the Parkland Hospital System hypertension clinic from 2005 to 2006. At an initial screening, patients signed written informed consent. Criteria for inclusion included being diagnosed with HTN but with untreated systolic BP of 159 mm Hg to 200 mm Hg and/or an untreated diastolic BP of 99 mm Hg to 120 mm Hg. Patients being treated were required to have a systolic BP of 140 mm Hg to 200 mm Hg and/or a treated diastolic BP of 90 mm Hg to 120 mm Hg. Patients were currently being treated with a statin drug for LDL cholesterol >100 mg/dL. Exclusion criteria consisted of evidence of unstable angina, CV event (myocardial infarction, angina, revascularization, stent replacement, arrhythmia) in the past 6 months, evidence of cerebrovascular event (transient ischemic attack/stroke) in the past 6 months, severe renal insufficiency/failure with serum creatinine >2.0 or calculated glomerular filtration rate <40 mL/min, presence of a life‐threatening disease, substance abuse, and the inability to reside in Dallas County, TX, for 6 months.
Patients were randomly assigned to either the home and clinic BP group (HOME/CLINIC) or usual care group with only clinic BP readings (USUAL). Patients were seen at baseline, 3 months, and 5 months for laboratory testing and were also seen monthly for BP checks. At baseline and the end of the study, all participants underwent 24‐hour ambulatory BP monitoring (ABPM). All currently prescribed medications were continued with the exceptions of calcium channel blockers (CCBs), 3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase inhibitors (statins), and nonsteroidal anti‐inflammatory drugs, which were discontinued for a 2‐week wash‐out period. During the washout period, all patients were placed on a diuretic (hydrochlorothiazide 12.5–25 mg) daily or furosemide (twice‐daily dosing of appropriate dose) then discontinued at the initiation of the study drug. Loop diuretics were used in patients with chronic kidney disease. BP was monitored for safety to remain below 200/110 mm Hg.
At the end of the 2‐week washout, patients started an open‐label combination tablet of amlodipine 5 mg daily, with the corresponding atorvastatin dosage based on a conversion table. At later visits, treatment would be stepwise intensified by increasing the amlodipine component to 10 mg and at visit 3 (usually the 3‐month mark), the atorvastatin dose was adjusted according to their LDL level to achieve NCEP guideline goals for cholesterol control. If BP control was not achieved at the maximum study drug dose, antihypertensives were added in the following stepwise manner: step 1=add angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; step 2=add β‐blocker; step 3=add diuretic; step 4=add direct vasodilator (minoxidil/hydralazine); and step 5=add aldosterone blockade. All patients had an evaluation of BP every 4 weeks in the clinic, and if BP was >140/90 mm Hg (<130/80 mm Hg for diabetes, renal insufficiency, or congestive heart failure [CHF]) if the goal had not been reached, then amlodipine was increased to the maximum of 10 mg daily with no change in the atorvastatin dose. All of the study participants were considered “hard to treat” by their primary care physician. At baseline, these patients had uncontrolled hypertension while taking 3 medications.
In the USUAL group, clinic BPs were used to determine the need for titrating doses of medications. In the HOME/CLINIC group, the higher of the clinic or HBPMs were used to determine the need for titrating doses of medication. The average of the clinic BPs was compared with the average of the home BPs taken by the patient. The home‐measurement patients were instructed to measure their BP daily for 7 days before the clinic visits with the calibrated OMRON CP 773 (OMRON, Kyoto, Japan) automated device provided to them. The self‐measured BP was the average of all readings collected during the 7 days prior to each follow‐up visit. Patients were instructed to rest for 5 minutes in the seated position and then to perform 3 consecutive self‐measurements of their BP 3 times a day. The patients kept a log of their BP readings and the readings were also stored in the memory of the device. When the patient returned for their follow‐up visit, the average of the prior 7 days was taken and compared with the average BP in the clinic that day. In the clinic during their follow‐up visit, the clinic BP was taken with an automated device and the average of 3 consecutive readings was taken by the physician after patients rested for 5 minutes in the sitting position. The need for titrating medication to reach BP goals was based on the highest of the home average or the clinic average for that visit.
In addition, at randomization and the final visit, patients underwent 24‐hour ambulatory monitoring with Spacelabs 90207 (Spacelabs Healthcare, Issaquah, WA) recorders programmed to obtain BP readings at 15‐minute intervals from 8 am to 10 pm and at 30‐minute intervals from 10 pm to 8 am. At randomization, 3 months, and the final follow‐up visit, laboratory testing was performed including total cholesterol, high‐density lipoprotein (HDL) cholesterol, LDL cholesterol, electrolytes, creatinine, and urinary albumin/creatinine ratio. If needed, patients could receive an optional visit to assess the titration of the medication further if they were not at goal. The target for both HOME/CLINIC and USUAL group BP measurements was SBP <140 mm Hg (<130 mm Hg if diabetic) and DBP <90 mm Hg (<80 mm Hg if diabetic). At enrollment and at the final visit, patients completed a short‐form 12 Quality of Life Questionnaire (Figure 1).
Figure 1.
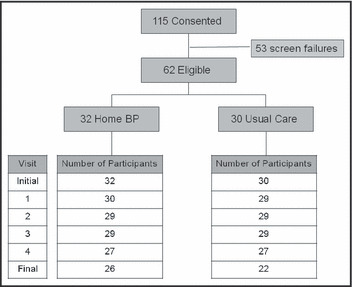
Study design flowchart of patient visits and screening. *Six patients who returned for a final visit did not complete an ambulatory blood pressure measurement and they were equally distributed between the study groups.
Statistical Methods
Randomization to study arm was stratified by sex, age, and initial number of BP medications and balanced in blocks of size 10 within strata. Age was dichotomized at 55 years and older, and initial number of BP medications was stratified at ≤1. Baseline characteristics were compared using Fisher exact test for categoric characteristics and the Wilcoxon rank‐sum test for continuous characteristics. The intention‐to‐treat analysis included all 62 randomized patients. Patients with missing data were assumed not at goal. Fisher exact test was used to compare study arms on the primary outcome of at‐goal status at the end of the study. Sensitivity analyses included (1) only the 42 patients with complete at‐goal data at the end of the study, and (2) all randomized patients but with missing data assumed at goal. Mixed linear regression models were used to compare changes in the secondary outcomes of clinic SBP, clinic DBP, 24‐hour SBP, 24‐hour DBP, total cholesterol, LDL cholesterol, and triglycerides. These models included fixed effects of visit, study arm, and visit×study arm and accounted for first‐order autocorrelation of repeated measurements within patients. SAS/STAT, version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
Patient Demographics
A total of 115 patients consented to participate in the study and were randomly assigned to the HOME/CLINIC or USUAL care group. Of these, 62 (53.9%) patients were included in the intention‐to‐treat analysis and 53 were screen failures (Figure 1). At the end of the study, only 42 patients had all information needed for the study. At baseline, the USUAL group (n=30) and the HOME/CLINIC group (n=32) had similar characteristics (Table I) and BP values (Figure 2). The two groups were stratified by age, sex, office BP, previous CCB therapy, and presence of diabetes, renal failure, or CHF. As shown in Table I, the average age was 55 years, and the group was comprised primarily of women (63% in the USUAL group and 72% in the HOME/CLINIC groups) and African Americans (73% in the USUAL and 78% in the HOME/CLINIC groups). Most patients were taking several BP medications, with the average number of BP medications being 3.4 (±0.2). A total of 97% of patients were taking statins at entry into the study.
Table I.
Baseline Demographics of the IMPACT Study Participants (N=62)
| Parameter | Usual Care Group (Clinic Only), n=30 | Home+Clinic Group, n=32 | P Value |
|---|---|---|---|
| Age, y | 55.3 | 55.4 | .90 |
| African American, % | 73 | 78 | .42 |
| Caucasian, % | 20 | 9 | |
| Hispanic, % | 3 | 3 | |
| Women, % | 63 | 72 | .59 |
| Diabetic, % | 50 | 38 | .44 |
| Body mass index, kg/m2 | 34.0 | 35.6 | .24 |
| Weight, kg | 95.0 | 97.9 | .59 |
| Waist circumference, cm | 108 | 116 | .11 |
| Hip circumference, cm | 115 | 123 | .01 |
| Alcohol use, % | 50 | 38 | .44 |
| Tobacco use, % | 17 | 26 | .53 |
| Caffeine use, % | 87 | 71 | .21 |
| Exercise, % | 57 | 58 | 1.0 |
Figure 2.
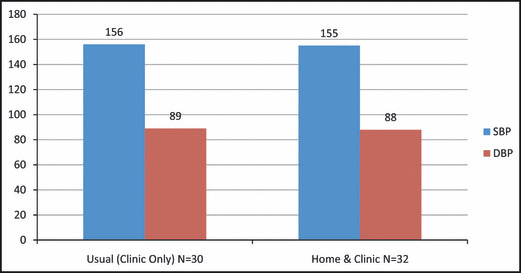
Baseline blood pressure measurements for usual care (n=30) and home and clinic care (n=32) groups. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
BP Control
Both groups achieved significant decline in BP (Figure 4). The mean baseline clinic BP for the USUAL group was 156/89 mm Hg. The mean baseline clinic BP for the HOME/CLINIC group was 155/88 mm Hg. These measurements were similar between the study groups, with P=.82 between groups for systolic BP (SBP) and P=.69 between groups for diastolic BP (DBP). The mean 24‐hour ABPM at baseline for the entire study population was 153/87 mm Hg. However, baseline 24‐hour ABPM was higher in the HOME/CLINIC group compared with the USUAL group (SBP, 155 mm Hg and 146 mm Hg, respectively, P<.01; DBP, 91 mm Hg and 83 mm Hg, respectively, P<.01).
Figure 4.
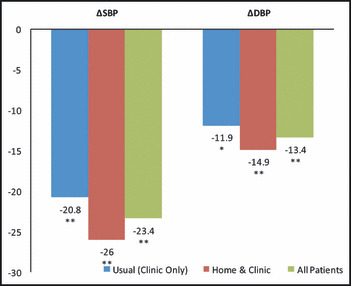
Ambulatory blood pressure monitoring showed significant declines in blood pressure for both treatment groups from baseline to final. There is a trend that diastolic blood pressure (DBP) declined greater in the home and clinic care group (P=.55) (n=42). *P<.01, **P<.0001.
There was a statistically significant decline in clinic BP for the entire study population, with −28.2 mm Hg and −11.8 mm Hg for SBP and DBP, respectively (P<.0001 and P<.0001, respectively) (Figure 3). The difference in clinic BP decrease between the two groups was similar, with no significant difference between SBP (USUAL −27.4 mm Hg and the HOME/CLINIC −29 mm Hg, P=.83) or DBP (USUAL −12.7 mm Hg and HOME/CLINIC −10.9 mm Hg, P=.69) (Figure 3).
Figure 3.
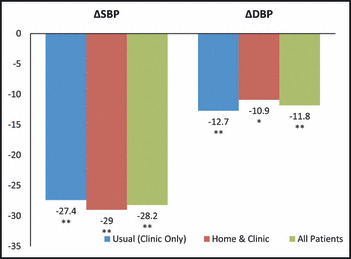
A statistically significant decline in clinic blood pressure for the entire study population from baseline to final with no group differences between systolic blood pressure (SBP) or diastolic blood pressure (DBP) decline from baseline to final (n=42). *P<.01, **P<.0001.
ABPM also showed significant declines in BP for both groups (SBP−23.4, P<.0001) and DBP −13.4, P<.0001) (Figure 4). In the USUAL group, the decline in SBP was −20.8 mm Hg and in the HOME/CLINIC the decline in SBP was −26 mm Hg, showing a trend of a greater decline in SBP in the HOME/CLINIC group, but this difference was not significant (P=.46, Figure 4). The USUAL group decline in DBP was −11.9 mm Hg and the HOME/CLINIC group decline in DBP was −14.9 mm Hg. Again, there is a trend that DBP declined greater in the HOME/CLINIC but was not statistically significant (P=.55) (Figure 4).
Lipid Level Control
The total cholesterol for both groups from baseline to the end of the study declined, with a change from baseline equaling −44.8 (P<.0001) (Table II). The LDL cholesterol levels for both groups also declined significantly, with a change from baseline of −41.1 (P≤.0001) (Table II). HDL cholesterol and triglyceride levels for both groups declined but not significantly (P=.08 and P=.42, respectively) (Table II). There were no significant differences between the treatment groups in the change from baseline to the end of the study in any of these parameters.
Table II.
Change in Cholesterol Profile From Baseline to Final in All IMPACT Study Participants
| Parameter | Baseline | Change From Baseline | P Value |
|---|---|---|---|
| Total cholesterol | 191.6 | −44.8 | <.0001 |
| LDL cholesterol | 116.7 | −41.1 | <.0001 |
| HDL cholesterol | 47.9 | −4.5 | .08 |
| Triglycerides | 153.7 | −16.2 | .42 |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Laboratory Results
The creatinine level for the entire population increased by +0.19, and all albumin:creatinine ratios decreased by −154.2. These changes were not statistically significant, and there was no statistical significance in the differences between treatment groups.
Adherence
The measures of adherence are shown in Table III. At each visit, the study medications were distributed and pill counts were conducted. Adherence rates were very high across the study population, with no significant difference between the adherence rates of each group. In the USUAL group, an average of 92% of the pills were taken and in the HOME/CLINIC group, an average of 93% of the pills were taken (P=.96). The average number of visit encounters were similar between the two groups (P=.54).
Table III.
Adherence Measures in IMPACT Study Participants
| Adherence Measure | Usual Care (Clinic BP Only) N=30 | Home+Clinic BP N=32 | P Value |
|---|---|---|---|
| Pills taken, % | 92 | 93 | .96 |
| Visit encounters, No. | 7.0 | 7.1 | .54 |
Abbreviation: BP, blood pressure.
Dual Control Rates
The control rate of at‐goal BP, defined as <140/90 mm Hg (<130/80 mm Hg if diabetic) in the USUAL group was 46.6% and in the HOME/CLINIC group was 40.6%. The control rate of at‐goal LDL cholesterol (defined by NCEP guidelines) in the USUAL group was 50% (n=15) and in the HOME/CLINIC group was 62.5%. The control rate of the entire study population of at‐goal BP (n=27) was 43.5% and the control rate of at‐goal LDL cholesterol (n=35) was 56.4%. The rate of combined control of BP and LDL cholesterol in the USUAL group was 36.6% and in the HOME/CLINIC group the rate was 28.1%. The control rate of at‐goal BP and at‐goal LDL cholesterol combined in the entire study population was 32.3%. Among the actual population that completed the study with all visits accounted for (n=42), the rate of at‐goal BP in the entire study population was 65.8% and the rate of at‐goal LDL cholesterol was 85.3%. The dual control rate of BP and LDL cholesterol was 48.7%.
Analyses of Add‐on Therapy
At the final visit, the mean number of BP medications in the entire study population was 3.4 (±0.2) (the final number does not include the study drug Caduet [Pfizer Labs, New York, NY]). Participants who reached goal BP took a mean of 3.2 (±0.3) BP medications. The participants who did not achieve BP goal were taking a mean of 3.6 (±0.2) BP medications at final visit. Of patients taking 1 BP medication at final visit (n=3), 100% were at goal, and of patients taking 2 BP medications at final visit (n=6), 50% were at goal. Of the patients taking 3 BP medications at final (n=23), there were 52.2% at goal. Of the patients taking 4 BP medications (n=16), 43.8% were at goal. Of the patients taking >5 medications (n=7), 28.5% reached BP goal. At the final visit, 49.1% (n=55) of all participants reached their BP goal.
Two patients decreased their number of BP medications by 3, and half were at goal. There were 11 patients who decreased their number of BP medications by 1 at final visit, with 45.5% being at goal. Twenty‐eight patients did not add or delete any BP medications at final visit and 57.1% were at goal. Perhaps the class of medication was more important in BP control than the number of medications. In the analysis of the quality‐of‐life data, there were no statistically significant differences between the study groups observed.
Discussion
Our findings demonstrate that in a hard‐to‐treat group of hypertensives, BP control could be established at a control rate of 43.5% compared with the 2004 national control rate of 35%, and the LDL control rate in our population also exceeded the 2004 national control rate (25.1%) at 56.4%. Furthermore, our dual control rate of HTN and DYS was 32.3% (compared with the national control rate at 9%). It is important to point out the significant BP and lipid control in the entire study group because this is an indigent population comprised of mostly middle‐aged African Americans who are among the most difficult populations to achieve BP and lipid goals. 12
One of the goals of this study was to investigate the effect of using home and clinic BP compared with the conventional use of clinic BP only in the management of HTN. There was no difference in the achievement of BP control between the USUAL and HOME/CLINIC groups. However, the baseline ABPM readings of the HOME/CLINIC group were significantly higher than in the USUAL group, a factor that may have affected the outcome. We hypothesized that the HOME/CLINIC group would have better BP control, particularly by 24‐hour ABPM measurement. Our results from the final 24‐hour ABPM show a trend for a greater decline in the HOME/CLINIC group compared with the USUAL group, yet it was not statistically significant. Our results are similar to a study by Niiranen and colleagues who looked at the comparison of home and ambulatory monitoring of BP. This study found that both ABPM and treatment based on HBPM led to good BP control. 13
The second goal was to observe the effects of combination therapy on lipid levels. There was no difference in the achievement of lipid control between the treatment groups. In the study population, total cholesterol decreased from baseline by −44.8 mg/dL (23% reduction), LDL cholesterol decreased by −41.1 mg/dL (17% reduction), and lipid control for the entire study population was 56.4%. In the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) study, which compared pravastatin treatment with usual care on reducing all‐cause 4‐year mortality, total cholesterol levels decreased by 17.2% in the pravastatin group and 7.6% in the usual care group. 14 ALLHAT was comprised of nearly half women and 38% African Americans and showed that African Americans were the only subgroup that demonstrated a benefit from lipid reduction in decreasing coronary heart disease event rates. 14 In our study, cholesterol levels were similarly reduced. ALLHAT–LLT and 8 other large long‐term statin trials demonstrate that an 18% reduction in mean total cholesterol is associated with a 27% reduction in congestive heart disease events and a 14% reduction in all‐cause mortality. 14 Although our study is not an event trial, the similarities in the population and achieved reductions compared with event trials suggests that there is likely CV protection and clinical benefit from the intervention in this trial.
There are several reasons for the success of this trial in treating BP and lipids. First, both groups were highly adherent to the medication and to the study visit schedule. Both groups were closely monitored and had monthly visits, pill counts, education, and one‐on‐one counseling with the physician and study coordinator in the trial. Most investigators report improvements in medication adherence with complex interventions such as these, especially HBPM. The effects were greatest when HBPM was combined with other adherence‐enhancing strategies. 8 , 15 The effects of HBPM alone could not be fully quantified in this trial because both groups did so well and showed dramatic improvement in BP control.
Second, the addition of a combination therapy such as amlodipine plus atorvastatin simplifies the medication regimen and assists participants with adherence to therapy. In addition, statins have been reported to have favorable effects on BP control. 5 In another study demonstrating the efficacy of amlodipine plus atorvastatin, investigators performed an 8‐week randomized, double‐blind placebo‐controlled trial that showed that dual‐goal attainment was significantly higher with amlodipine/atorvastatin plus therapeutic lifestyle changes compared with placebo plus therapeutic lifestyle changes (55.6% vs 5.0%, respectively; P<.001). 9 The Clinical Utility of Caduet in Simultaneously Achieving Blood Pressure and Lipid Endpoints in a Specific Patient Population (CAPABLE) trial also found that single‐pill amlodipine plus atorvastatin therapy was effective in targeting HTN and DYS in African Americans who were at risk for CV disease. 12 The CAPABLE participants’ baseline BP ranged from 146 mm Hg to 149 mm Hg systolic and 88 mm Hg to 93 mm Hg diastolic, and 48.3% of patients attained both BP and LDL cholesterol goals. 12 In our study, participants had higher baseline BP (156/89 mm Hg in the usual group and 155/88 mm Hg in the home/clinic group), were taking more antihypertensive drugs at baseline, and had higher LDL cholesterol levels at baseline. Both our study and the CAPABLE trial provide evidence that amlodipine plus atorvastatin can be helpful in controlling BP and reducing lipids, which can reduce CV events in African Americans.
Limitations
There were some limitations to our study. It is unclear why we found no significant difference between our groups in the primary hypothesis, but we have 3 possible explanations. First, it could be a result of our small sample size of 62 patients. Possibly, if our study population had been larger, the difference in the two groups would have been more evident. Further research with a larger group size should be performed to yield more comprehensive results.
Second, the patients in this trial were enrolled in the trial where they received very close follow‐up, interaction, and attention, which is different from the typical outpatient setting at this institution. Close follow‐up and frequent physician interaction was experienced by both groups, which could have confounded our results. Our study is composed of patients of a lower socioeconomic status and poor access to health care, therefore it was important in the design to have more comprehensive, focused, and patient‐centered interventions to improve adherence. 16 A study by Mohammadi and colleagues 17 looked at the partnership care model to improve the control of HTN. This model states that attention should be paid to the interactions between the patient and the nurse or the patient and physician, and that this partnership increases involvement, motivation, and responsibility of the people interacting. Their intervention of this model led to a significant decline in SBP and DBP in their intervention group. 17 Another study demonstrated an improvement in BP control through provider education, provider alerts, and patient education. 18 In our study population, enrollment into a clinical trial improved access to care, required patients to follow strict care protocol guidelines, provided more education, and increased the frequency of physician visits, all of which might have reduced the difference in the effect of the study intervention between the treatment groups.
A third factor to consider is the “trial effect,” also known as the “inclusion effect” or the Hawthorne effect, which has been described in other studies. The effect itself is a confounding factor that occurs when participants in clinical trials change their behavior because they know they are being observed. 19 Participants are constantly aware that they are involved in a trial, they expect improvement, and therefore work harder to achieve that improvement. 20 Trial participation may enhance the opportunity to receive more effective treatment, trial clinicians may be better informed or more attentive due to being observed, or trial participation may make the patient feel more involved in their care. 19 In our study population, all participants had increased provider interaction, with visits once a month and frequent phone calls. Therefore, there was no “control” group that did not receive this “enhanced” interaction, which may have affected the outcome between the study groups.
Conclusions
HTN and DYS commonly occur concurrently. Management of these CV risk factors is suboptimal particularly in indigent and minority populations, where the risk for disease is highest. Combined approaches to care that use home BP monitoring, combination therapy, and education will improve control rates of both cholesterol and HTN in high‐risk populations such as that seen in the IMPACT study.
References
- 1. Chun‐Ju H, Donald C, Beatty P, et al. National health statistics reports. National ambulatory medical care survey 2007. http://www.cdc.gov/nchs/data/nhsr/nhsr003.pdf. Number 27. Accessed November 3, 2010. [PubMed]
- 2. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen N, Magno C, Hinojosa M, et al. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928–934. [DOI] [PubMed] [Google Scholar]
- 4. Johnson M, Pietz D, Battleman D, et al. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10:926–932. [PubMed] [Google Scholar]
- 5. Borghi C. Interactions between hypercholesterolemia and hypertension: implications for therapy. Curr Opin Nephrol Hypertens. 2002;11:489–496. [DOI] [PubMed] [Google Scholar]
- 6. Kannel W. Risk stratification in hypertension: new insights from the Framingham study. Am J Hypertens. 2000;13:3S–10S. [DOI] [PubMed] [Google Scholar]
- 7. Neutel J, Bestermann W, Dyess E, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized placebo‐controlled, a multicenter study. J Clin Hypertens. 2009;11:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrodeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure‐lowering medication in ambulatory care? A systemic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732. [DOI] [PubMed] [Google Scholar]
- 9. Parati G, Omboni S, Albini F, et al. Home blood pressure telemonitoring improves hypertension control in general practice. The TeleBPCare study. J Hypertens. 2009;27:198–203. [DOI] [PubMed] [Google Scholar]
- 10. Yarows S, Julius S, Pickering T, et al. Home blood pressure monitoring. Arch Intern Med. 2000;160:1251–1256. [DOI] [PubMed] [Google Scholar]
- 11. Staessen J, Hond E, Celis H, et al. Antihypertensive treatment based on blood pressure measurements at home or in the physician’s office. A randomized controlled trial. JAMA. 2004;8:955–963. [DOI] [PubMed] [Google Scholar]
- 12. Flack J, Ronald V, Watson K, et al. Improved attainment of blood pressure and cholesterol goals using single‐pill amlodipine/atorvastatin in African Americans: the CAPABLE trial. Mayo Clin Proc. 2008;83(1):35–45. [DOI] [PubMed] [Google Scholar]
- 13. Niiranen T, Kantola I, Vesalainen R, et al. A comparison of home measurement and ambulatory monitoring of blood pressure in the adjustment of antihypertensive treatment. Am J Hypertens. 2006;19:468–474. [DOI] [PubMed] [Google Scholar]
- 14. Julius S, Nesbitt S, Egan B, et al. Trial of preventing hypertension. N Engl J Med. 2006;354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 15. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansoor G, White W. Self‐measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11):1017–1022. [DOI] [PubMed] [Google Scholar]
- 17. Mohammadi E, Abedi HA, Jalali F, et al. Evaluation of ‘partnership care model’ in the control of hypertension. Int J Nurs Pract. 2006;12:153–159. [DOI] [PubMed] [Google Scholar]
- 18. Roumie C, Elasy T, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med. 2006;145:165–175. [DOI] [PubMed] [Google Scholar]
- 19. Gale E. The Hawthorne studies‐a fable for our times. Q J Med. 2004;97:439–449. [DOI] [PubMed] [Google Scholar]
- 20. Kaptchuk T, Shaw J, Kerr C, et al. Maybe I made up the whole thing: placebos and patients experiences in a randomized controlled trial. Cult Med Psychiatry. 2009;33(3):382–411. [DOI] [PMC free article] [PubMed] [Google Scholar]


