Abstract
In the prospective, open‐label, titrate‐to‐goal Blood Pressure Control in All Subgroups With Hypertension (BP‐CRUSH) study, 999 patients with hypertension uncontrolled on monotherapy (mean age, 55.6±11.4 years; baseline blood pressure [BP], 153.7±9.2/91.9±8.6 mm Hg) were switched to fixed‐dose amlodipine/olmesartan medoxomil (AML/OM) 5/20 mg. Patients were uptitrated every 4 weeks to AML/OM 5/40 mg and 10/40 mg to achieve BP <120/70 mm Hg. Patients were subsequently uptitrated every 4 weeks to AML/OM+hydrochlorothiazide (HCTZ) 10/40+12.5 mg and 10/40+25 mg to achieve BP <125/75 mm Hg. The primary end point, the cumulative percentage of patients achieving seated systolic BP <140 mm Hg (<130 mm Hg for patients with diabetes) by week 12, was 75.8%. The mean (±standard error) BP changes from baseline during the titration periods ranged from −14.2±0.4 mm Hg/−7.7±0.3 mm Hg for AML/OM 5/20 mg to −25.1±0.7 mm Hg/−13.7±0.4 mm Hg for AML/OM+HCTZ 10/40+25 mg. By week 20, the cumulative BP threshold of <140/90 mm Hg was achieved by 90.3% of patients. An ambulatory BP monitoring substudy (n=243) showed that 24‐hour efficacy was maintained. Treatment‐emergent adverse events (TEAEs), mostly mild to moderate in severity, occurred in 529 patients (53.0%). Drug‐related TEAEs occurred in 255 patients (25.5%). This well‐tolerated, treat‐to‐goal algorithm enabled a large proportion of patients with uncontrolled hypertension on monotherapy to safely achieve BP control on single‐pill AML/OM combination therapy or triple therapy with the addition of HCTZ. J Clin Hypertens (Greenwich). 2011;13:404–412. ©2011 Wiley Periodicals, Inc.
An analysis of the 2007 to 2008 National Health and Nutrition Examination Survey (NHANES) showed that 29% of adults in the United States have hypertension. 1 Of the 73% of patients with hypertension who receive treatment, approximately 50% do not reach recommended blood pressure (BP) goals (<140/90 mm Hg for uncomplicated hypertension, <130/80 mm Hg for patients with diabetes). 1 Data suggest that although antihypertensive monotherapy allows only approximately one third of patients to reach recommended BP goals, 2 , 3 , 4 this type of therapy is prescribed to as many as 63% of patients. 2 , 4 , 5 , 6 , 7 Factors identified by physicians as reasons for not uptitrating therapy include evidence of a clear improvement in BP despite the lack of goal achievement, patient self‐measurements below BP goals, feeling that office BP measurements were not representative of true BP, low patient adherence, possible side effects, and significant comorbidities. 8 , 9 , 10
The Blood Pressure Control in All Subgroups With Hypertension (BP‐CRUSH) study (ClinicalTrials.gov identifier NCT00791258) evaluated improvement in BP goal achievement after patients who were previously uncontrolled on antihypertensive monotherapy per study protocol were switched to amlodipine/olmesartan medoxomil (AML/OM)±hydrochlorothiazide (HCTZ) combination therapy. The results of the BP‐CRUSH study, which included prespecified subgroups of patients with hypertension (elderly, black, Hispanic, Asian, obese, type 2 diabetics, and patients with the metabolic syndrome), are presented here. In this study, efficacy was assessed by seated cuff BP (SeBP) measurement. In addition, ambulatory BP monitoring (ABPM) was performed in a subpopulation of patients.
Methods
Study Design
The BP‐CRUSH study was a phase 4 (phase 3B in South Africa), prospective, open‐label, multicenter, single‐arm, dose‐titration study with a 20‐week active treatment period. The study was designed in order to have ≥700 patients complete the study. Men and women were eligible for enrollment if they were aged 18 to 80 years and had uncontrolled BP (mean systolic BP [SBP] ≥140 mm Hg [≥130 mm Hg for patients with diabetes] and ≤180 mm Hg and mean diastolic BP [DBP] ≤110 mm Hg on 2 consecutive visits during screening) after ≥1 month of antihypertensive monotherapy with an angiotensin‐converting enzyme inhibitor, angiotensin II receptor blocker (ARB), β‐blocker, calcium channel blocker (CCB), or diuretic. Patients uncontrolled on multiple antihypertensive therapies (including fixed‐dose combination therapy except for triamterene/HCTZ), with type 1 or 2 diabetes mellitus requiring insulin, type 2 diabetes mellitus and hemoglobin A1c≥9.0% at screening and serum creatinine levels >2.0 mg/dL or calculated glomerular filtration rate <40 mL/min at screening, significant cardiac disease, or serious systemic diseases or secondary hypertension, as well as women who were pregnant or lactating, were excluded. The BP‐CRUSH study aimed to enroll a population in which approximately 50% of patients were previously treated with ARB or dihydropyridine (DHP)‐CCB monotherapy for ≥1 month prior to screening. The study was designed to enroll ≥100 patients in each of the following subgroups: elderly (ie, 65 years or older), African American/black, Hispanic, Asian, type 2 diabetes, metabolic syndrome, and obese (ie, body mass index ≥30 kg/m2). African American/black, Hispanic, and Asian designations were based on patient self‐report. For this study, metabolic syndrome was defined as the presence of ≥3 of the following: high‐density lipoprotein cholesterol <50 mg/dL in women and <40 mg/dL in men; triglycerides ≥150 mg/dL; BP ≥130/85 mm Hg; or fasting glucose ≥100 mg/dL.
On day 1, patients who met the inclusion criteria were switched from their previous antihypertensive monotherapy to a fixed‐dose combination of AML/OM 5/20 mg. Active treatment was administered once daily at 8 am±120 minutes each morning. Uptitration was permitted every 4 weeks in accordance with the following schedule: uptitration to AML/OM 5/40 mg, AML/OM 10/40 mg, AML/OM 10/40 mg+HCTZ 12.5 mg, and AML/OM 10/40 mg+HCTZ 25 mg. Patients were eligible to uptitrate to dosages containing AML/OM if they had a mean SBP ≥120 mm Hg and <200 mm Hg or a mean DBP ≥70 mm Hg and <115 mm Hg. Patients were eligible to uptitrate to any dosage that included HCTZ if they had a mean SBP ≥125 mm Hg and <200 mm Hg or a mean DBP ≥75 mm Hg and <115 mm Hg. Patients taking any AML/OM‐only combination whose BP was <120/70 mm Hg, as well as patients taking any AML/OM+HCTZ combination whose BP was <125/75 mm Hg, who were eligible for uptitration but not symptomatic at any visit were not uptitrated to the next dosing level. These patients entered the maintenance phase and remained on their currently assigned study treatment. If a patient’s BP became uncontrolled during the maintenance phase, defined as SBP ≥130 mm Hg or DBP ≥80 mm Hg, the patient was uptitrated to the next higher dose and re‐entered the titration phase of the study. Patients with either a mean office SBP ≥200 mm Hg or a DBP ≥115 mm Hg at any visit exited the study, as did patients with either SBP <120 mm Hg or DBP <70 mm Hg with symptomatic hypotension.
At each visit of the active treatment phase, SeBP was measured; concomitant medication use, adherence, and adverse events (AEs) were assessed; and the study drug was dispensed. A physical examination was completed at baseline and at the end of the study (week 20), and clinical laboratory tests were assessed at weeks 12 and 20. SeBP was measured in triplicate using an automated BP device (Omron HEM‐705CP; Omron Corporation, Tokyo, Japan). The mean of the 3 measurements was recorded as SeBP for each visit and used to judge study and uptitration eligibility. A subset of patients enrolled at approximately 30 preselected sites also underwent ABPM at baseline, week 12, and end of study (week 20). ABPM was performed using a Spacelabs 90207 oscillometric device (Spacelabs Healthcare, Issaquah, WA). Patients reported to the study site at 8 am±90 minutes so the device could be applied and the patient dosed as close to 8 am as possible. After wearing the monitor for a full 24 hours, the patient returned to the study site for device removal. If the procedure was not successful, the procedure could be repeated once. If APBM failed a second time, it was not repeated.
Study Assessments
The primary efficacy end point of the study was the cumulative percentage of patients achieving the seated cuff SBP (SeSBP) goal of <140 mm Hg (<130 mm Hg in patients with diabetes) during the first 12 weeks of active treatment (ie, the percentage of patients who achieved the SeSBP goal at any time during the first 12 weeks of active treatment). Secondary SeBP end points included the noncumulative (last‐observation‐carried‐forward [LOCF]) percentage of patients achieving the SeSBP goal of <140 mm Hg (<130 mm Hg in patients with diabetes) at week 12 (ie, the percentage of patients who achieved the SeBP goal at the last post‐baseline visit at or prior to week 12); percentages of patients achieving the combined Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) SeBP goal of <140/90 mm Hg (<130/80 mm Hg for patients with diabetes) during 12 and 20 weeks of treatment; the change from baseline in mean SeSBP/seated cuff DBP (SeDBP) during each titration period (LOCF); and the percentage of patients achieving the SeBP threshold of <140/90 mm Hg during each titration period. In the ABPM subgroup, end points included the change from baseline in mean ambulatory SBP and DBP over 24 hours and during the daytime (8 am–4 pm), nighttime (10 pm–6 am), and final 2, 4, and 6 hours of the dosing interval after 12 and 20 weeks of treatment and the percentage of patients who achieved mean 24‐hour, daytime (8 am–4 pm), and nighttime (10 pm–6 am) BP targets of <130/80 mm Hg, <135/85 mm Hg, and <120/70 mm Hg, respectively, after 12 and 20 weeks of treatment. In addition, the number and percentage of patients who were classified as dippers at 12 and 20 weeks, defined as patients with any nocturnal decrease in ambulatory SBP and/or DBP ≥10% of mean daytime ambulatory SBP and/or DBP values, respectively, were assessed. Similarly, the number and percentage of nondippers at 12 and 20 weeks, defined as patients with maximum nocturnal decrease in ambulatory SBP and DBP <10% of mean daytime ambulatory SBP and DBP, respectively, were assessed.
Safety assessments included the evaluation of AEs, laboratory parameters and their changes from baseline, and physical examinations. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA, Chantilly, VA) version 12.0 and were summarized by primary system organ class and preferred term. Treatment adherence was assessed by the tablet count of the study medication returned at each required visit before in‐clinic dosing and defined as the ratio of the number of study drug tablets taken over the number of study drug tablets that should have been taken during the dosing period multiplied by 100.
Statistical Analysis
The study population included all patients who received ≥1 dose of active study medication. Demographic and baseline characteristics were summarized using descriptive statistics, as were laboratory data and their change from baseline. All analyses of BP data for a visit, or for a period defined by visits, used all available BP data, regardless of whether the patient was on treatment or the type of treatment. All analyses of BP data for a titration dose or for a period defined by titration doses used all on‐treatment data (ie, data that could be attributed to a study drug). If there was an interruption in dosing immediately before BP was taken, the BP data were not considered on‐treatment and were excluded. The cumulative BP goal achievement rate by visit was calculated as the ratio of the number of patients who achieved the goal at any time from the first dose date to the visit date over the number of patients who had any post‐baseline BP data by that visit. The cumulative BP goal achievement rate by titration dose was defined similarly, with “visit” replaced by “end of the titration dose period.” The noncumulative (LOCF) BP goal achievement rate by visit was calculated as the ratio of the number of patients who achieved the goal at the visit (when missing, at the last available visit, ie, LOCF) over the number of patients who had any post‐baseline BP data by that visit. The noncumulative BP goal achievement rate by titration dose was defined similarly, with “visit” replaced by “end of the titration dose period.”
Changes in seated and ambulatory BP from baseline were summarized by visit or titration dose. For the summaries by titration dose, the last available on‐treatment value within the dose was used. To test whether these changes were different from 0, the 1‐sample paired t test was used. All statistical analyses were performed at a 2‐sided significance level of 5%.
Results
Study Disposition, Demographics, and Baseline Characteristics
Of the 1406 patients screened, 999 entered the active treatment phase and represent the treatment population. Of these 999 patients, 243 underwent ABPM at baseline and week 12 or 20 and represent the ABPM population. The number of patients exposed to each titration dose was as follows: 999 to AML/OM 5/20 mg, 892 to AML/OM 5/40 mg, 795 to AML/OM 10/40 mg, 699 to AML/OM 10/40 mg+HCTZ 12.5 mg, and 497 to AML/OM 10/40 mg+HCTZ 25 mg. A total of 263 patients (26.3%) discontinued the study due to protocol violations (n=109), AEs (n=87), withdrawn consent (n=40), lost to follow‐up (n=18), or other reasons (n=9), leaving 736 patients who completed the study. At baseline, patients in the total population had a mean (standard deviation [SD]) age of 55.6 (11.4) years and a mean (SD) SeBP of 153.7 (9.2) mm Hg/91.9 (8.6) mm Hg (Table I). The corresponding data for the ABPM population were a mean (SD) age of 56.6 (10.1) years, mean (SD) SeBP of 153.5 (8.8) mm Hg/91.3 (7.7) mm Hg, and a mean (SD) 24‐hour ambulatory BP of 135.8 (11.7) mm Hg/81.3 (9.0) mm Hg.
Table I.
Demographics and Baseline Characteristics
| Characteristics | Total Cohort (N=999) | ABPM Cohorta (N=243) |
|---|---|---|
| Age, mean (SD), y | 55.6 (11.4) | 56.6 (10.1) |
| ≥65 y, No. (%) | 228 (22.8) | 54 (22.2) |
| Women, No. (%) | 491 (49.1) | 113 (46.5) |
| Weight, mean (SD), kg | 88.2 (21.5) | 89.7 (20.8) |
| BMI, mean (SD), kg/m2 | 31.0 (6.4) | 31.4 (6.5) |
| Race, No. (%) | ||
| Caucasian | 630 (63.1) | 169 (69.5) |
| Black | 234 (23.4) | 47 (19.3) |
| Asian | 129 (12.9) | 26 (10.7) |
| American Indian/Alaskan Native | 6 (0.6) | 1 (0.4) |
| Ethnicity, No. (%) | ||
| Hispanic or Latino | 105 (10.5) | 33 (13.6) |
| Type 2 diabetes, No. (%) | 192 (19.2) | 32 (13.2) |
| Metabolic syndrome, No. (%) | 462 (46.2) | 107 (44.0) |
| Prior monotherapy, No. (%) | ||
| ACE inhibitor | 289 (28.9) | – |
| ARB | 237 (23.8) | – |
| Diuretic | 167 (16.7) | – |
| DHP‐CCB | 118 (11.8) | – |
| β‐Blocker | 115 (11.5) | – |
| Non–DHP‐CCB | 20 (2.0) | – |
| Other | 20 (2.0) | – |
| Noneb | 33 (3.3) | – |
| SeSBP, mean (SD), mm Hg | 153.7 (9.2) | 153.5 (8.8) |
| SeDBP, mean (SD), mm Hg | 91.9 (8.6) | 91.3 (7.7) |
| Ambulatory SBP, mean (SD), mm Hg | NA | 135.8 (11.7) |
| Ambulatory DBP, mean (SD), mm Hg | NA | 81.3 (9.0) |
Abbreviations: ABPM, ambulatory blood pressure (BP) monitoring; ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; DBP, diastolic BP; DHP‐CCB, dihydropyridine–calcium channel blocker; NA, not available; SBP, systolic BP; SD, standard deviation; Se, seated. aABPM cohort was monitored for 24 hours at baseline, week 12, and week 20. bDid not take antihypertensive therapy within 1 day prior to the first amlodipine/olmesartan medoxomil dose. Note that 11 patients received triamterene/hydrochlorothiazide combination therapy prior to study entry. This was the only combination allowed per protocol due to triamterene’s weak activity and potassium‐sparing effects.
Efficacy
Seated Cuff BP At the end of 12 weeks of active treatment, the primary end point (the cumulative percentage of patients who achieved the SeSBP goal of <140 mm Hg [<130 mm Hg for patients with diabetes]) was 75.8% for the total population, including 80.1% for patients without diabetes and 57.9% for patients with diabetes (Figure 1). By the end of 12 and 20 weeks, the cumulative percentage of patients who achieved the SeBP goal of <140/90 mm Hg (<130/80 mm Hg for patients with diabetes) was 71.3% and 84.8%, respectively.
Figure 1.
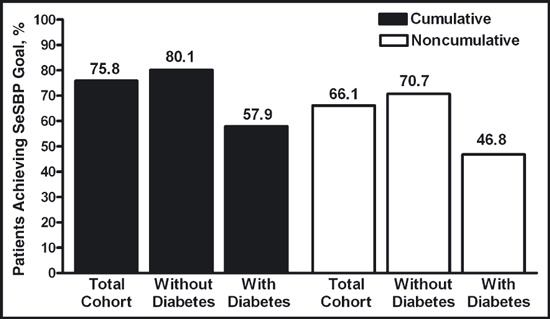
Proportions of patients achieving the seated cuff systolic blood pressure (SeSBP) goal (<140 mm Hg [<130 mm Hg for patients with diabetes]) by week 12. The cumulative blood pressure (BP) goal achievement rate at week 12 was calculated as the ratio of the number of patients who achieved the goal at any time from the first dose to week 12 over the number of patients who had any post‐baseline BP data. The noncumulative (last‐observation‐carried‐forward [LOCF]) BP goal achievement rate by visit was calculated as the ratio of the number of patients who achieved the goal at week 12 (when missing, at the last available visit, ie, LOCF) over the number of patients who had any post‐baseline BP data.
For each titration dose, the reductions from baseline in mean SeBP at week 12 (LOCF) were statistically significant (Figure 2A). Furthermore, there was a significant titration effect on mean SeBP at each titration dose. Titrating from AML/OM 5/20 mg to 5/40 mg and AML/OM 5/40 mg to 10/40 mg resulted in mean SeBP changes of −1.90/−0.83 mm Hg and −5.67/−3.26 mm Hg, respectively (P<.01 for each). Titrating from AML/OM 10/40 mg to AML/OM 10/40+HCTZ 12.5 mg and from AML/OM 10/40+HCTZ 12.5 mg to AML/OM 10/40+HCTZ 25 mg resulted in mean SeBP changes of −4.48/−2.60 mm Hg and −3.76/−1.65 mm Hg (P<.0001 for each). The cumulative achievement of the <140/90 mm Hg SeBP threshold (ie, the proportion of patients who attained target anytime from first dose to end of titration period) was 49.5% for patients receiving AML/OM 5/20 mg, 63.8% for AML/OM 5/40 mg, 77.1% for AML/OM 10/40 mg, 86.7% for AML/OM 10/40+HCTZ 12.5 mg, and 90.3% for AML/OM 10/40+HCTZ 25 mg (Figure 2B).
Figure 2.
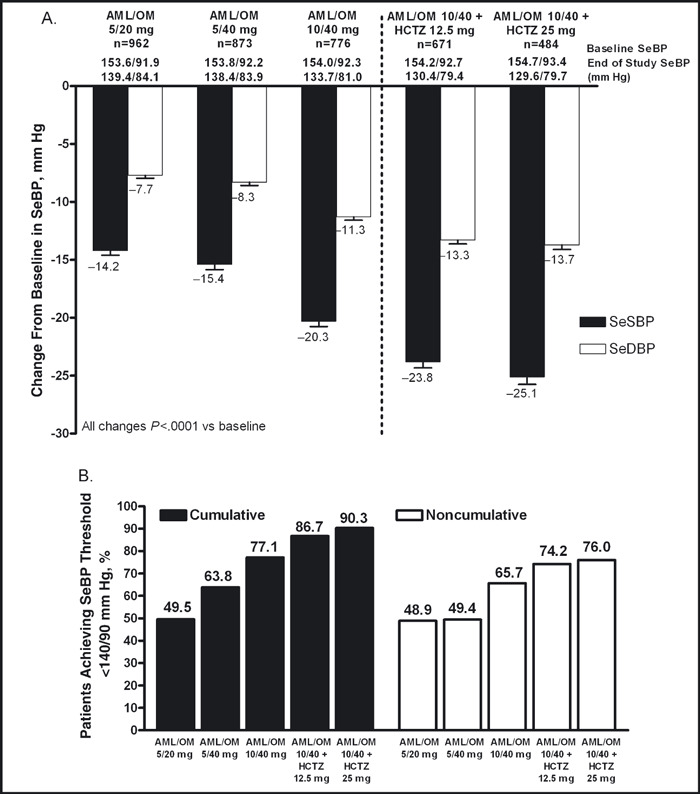
Change from baseline in seated cuff blood pressure (SeBP) (A) and proportions of patients achieving SeBP threshold of <140/90 mm Hg by titration dose (B). AML indicates amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SeDBP, seated cuff diastolic blood pressure; SeSBP, seated cuff systolic blood pressure. The cumulative blood pressure (BP) goal achievement rate at each titration period was calculated as the ratio of the number of patients who achieved the goal at any time from the first dose to the end of the titration dose period over the number of patients who had any post‐baseline BP data during the titration dose period. The noncumulative (last‐observation‐carried‐forward [LOCF]) BP goal achievement rate by visit was calculated as the ratio of the number of patients who achieved the goal at the end of the titration dose period (when missing, at the last available visit, ie, LOCF) over the number of patients who had any post‐baseline BP data during the titration dose period.
Of the patients who were uncontrolled on ARB monotherapy prior to study enrollment, 72.2% and 86.8% reached the SeBP goal of <140/90 mm Hg (<130/80 mm Hg for patients with diabetes) cumulatively by weeks 12 and 20, respectively. For patients previously treated with a DHP‐CCB combination, the cumulative SeBP goal achievement rate was 65.8% by week 12 and 83.8% by week 20.
24‐Hour ABPM The reductions from baseline in mean 24‐hour ambulatory BP were statistically significant at both weeks 12 and 20. From baseline to week 12 (Figure 3A) and baseline to week 20 (Figure 3B), the mean (standard error of the mean) changes from baseline in 24‐hour ambulatory BP were −14.8 (0.72) mm Hg/−9.4 (0.46) mm Hg and −21.0 (0.84) mm Hg/−13.3 (0.55) mm Hg, respectively (P<.0001 for all). There were also significant reductions from baseline in mean 24‐hour ambulatory BP during the daytime (8 am−4 pm), nighttime (10 pm–6 am), and last 2, 4, and 6 hours of the dosing interval at weeks 12 and 20 (Figures 3A and 3B). The ambulatory SBP during the entire 24‐hour dosing interval is shown in Figures 4A and 4B. At weeks 12 and 20, 73.4% and 90.5% of patients, respectively, achieved the mean 24‐hour ambulatory BP target of <130/80 mm Hg (Figure 5). The mean daytime (8 am–4 pm) ambulatory BP target of <135/85 mm Hg was achieved by 72.9% and 88.4% of patients at weeks 12 and 20, respectively, and the mean nighttime (10 pm–6 am) ambulatory BP target of <120/70 mm Hg was achieved by 62.0% of patients at week 12 and 78.9% of patients at week 20 (Figure 5).
Figure 3.
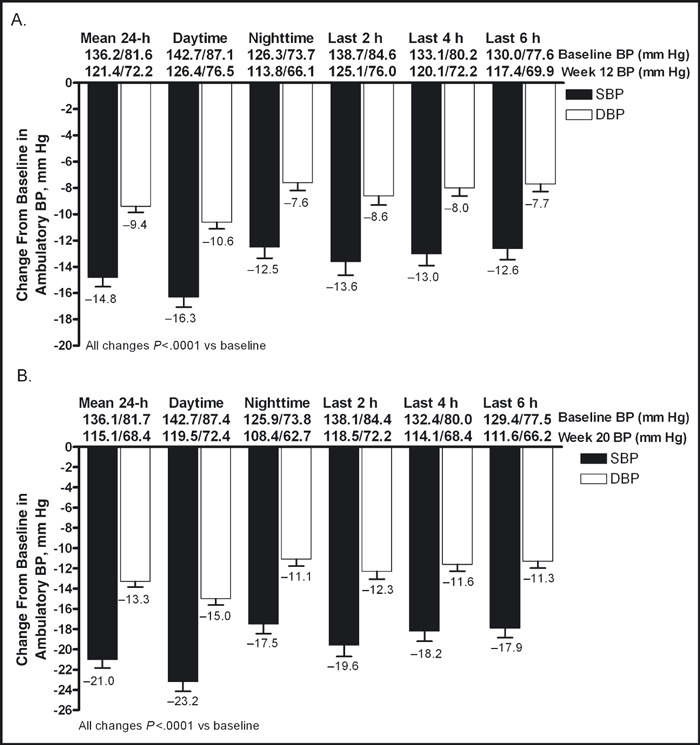
Change in mean ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) (±standard error of the mean) during the 24‐hour dosing interval and daytime, nighttime, and last 2, 4, and 6 hours of the dosing interval at week 12 (A) and week 20 (B).
Figure 4.
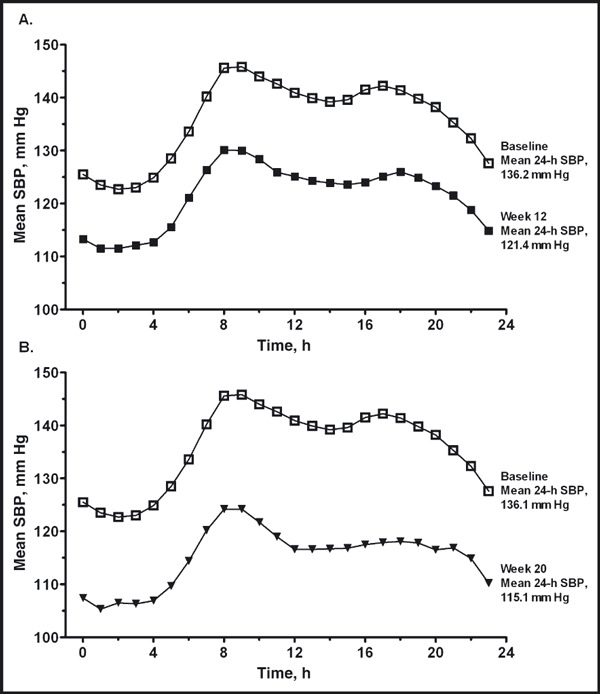
Ambulatory systolic blood pressure (SBP) over the 24‐hour dosing interval at week 12 (A) and week 20 (B). Time 0 represents 12 am. Dosing occurred at 8 am±120 minutes.
Figure 5.
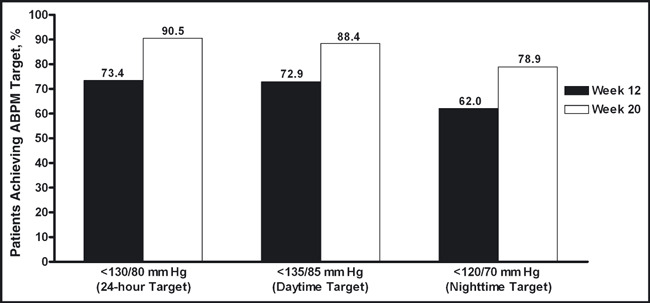
Proportion of patients achieving mean 24‐hour, daytime (8 am–4 pm), and nighttime (10 pm–6 am) ambulatory blood pressure targets at weeks 12 and 20. ABPM indicates ambulatory blood pressure monitoring.
At week 12, a total of 229 patients had valid ABPM data. Of the 169 patients who were ABPM dippers at baseline, 127 (75.2%) were dippers and 42 (24.9%) were nondippers at week 12. Of the 60 patients who were nondippers at baseline, 31 (51.7%) were dippers and 29 (48.3%) were nondippers at week 12. At week 20, 199 patients had valid ABPM data. Of the 150 patients who were ABPM dippers at baseline, 112 (74.7%) were dippers and 38 (25.3%) were nondippers at week 20. Of the 49 patients who were ABPM nondippers at baseline, 26 (53.1%) were dippers and 23 (46.9%) were nondippers at week 20.
Safety and Tolerability
Overall, the treatment regimen was well tolerated. In the total population, 529 patients (53.0%) experienced a treatment‐emergent AE (TEAE), including 255 patients (25.5%) whose TEAEs were deemed to be drug‐related (Table II). Thirteen patients experienced a serious TEAE (Table II). Although none of the serious TEAEs were recorded by the investigators as drug‐related on the clinical report form, information recorded in the ARISg risk management database (Aris Global LLC, Stamford, CT) by the investigator indicated that one of the serious TEAEs (abdominal pain) was considered to be possibly related to HCTZ 25 mg. A total of 86 patients (8.6%) withdrew from the study secondary to a TEAE, and 69 (6.9%) withdrew after a drug‐related TEAE. Drug‐related TEAEs occurring in >1% of the total cohort included peripheral edema (6.5%), nausea (1.4%), fatigue (2.0%), headache (2.0%), dizziness (7.6%), and hypotension (2.3%) (Table II). Aside from the incidence of peripheral edema, which was greater in recipients of AML/OM 10/40 mg compared with AML/OM 5/20 mg or 5/40 mg, none of the drug‐related TEAEs appeared to be dose‐related (Table II). Increased blood uric acid (1.2%) was the only drug‐related clinical laboratory AE that occurred in >1% of the total cohort. No other clinically meaningful changes in laboratory parameters, vital signs, or electrocardiography results were observed.
Table II.
Summary of AEs
| AE, No. (%) | AML/OM 5/20 mg (N=999) | AML/OM 5/40 mg (N=892) | AML/OM 10/40 mg (N=795) | AML/OM 10/40+HCTZ 12.5 mg (N=699) | AML/OM 10/40+HCTZ 25 mg (N=496) | Totala (N=999) |
|---|---|---|---|---|---|---|
| Patients with any TEAE | 214 (21.4) | 158 (17.7) | 188 (23.6) | 180 (25.8) | 99 (20.0) | 529 (53.0) |
| Patients with any drug‐related TEAE | 75 (7.5) | 39 (4.4) | 70 (8.8) | 74 (10.6) | 47 (9.5) | 255 (25.5) |
| Patients with any serious TEAE | 2 (0.2) | 3 (0.3) | 3 (0.4) | 3 (0.4) | 1 (0.2) | 12 (1.2)b |
| Patients with any drug‐related serious TEAE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0)c | 0 (0.0) |
| Drug‐related TEAEs: >1% of patients | ||||||
| Peripheral edema | 13 (1.3) | 8 (0.9) | 35 (4.4) | 7 (1.0) | 4 (0.8) | 65 (6.5) |
| Nausea | 6 (0.6) | 4 (0.4) | 1 (0.1) | 3 (0.4) | 1 (0.2) | 14 (1.4) |
| Fatigue | 9 (0.9) | 3 (0.3) | 2 (0.3) | 4 (0.6) | 2 (0.4) | 20 (2.0) |
| Dizziness | 20 (2.0) | 10 (1.1) | 11 (1.4) | 26 (3.7) | 13 (2.6) | 76 (7.6) |
| Dizziness, postural | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 2 (0.2) |
| Headache | 8 (0.8) | 5 (0.6) | 3 (0.4) | 3 (0.4) | 1 (0.2) | 20 (2.0) |
| Blood uric acid increase | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.3) | 6 (1.2) | 8 (0.8) |
| Hypotension | 3 (0.3) | 6 (0.7) | 5 (0.6) | 4 (0.6) | 5 (1.0) | 23 (2.3) |
| Orthostatic hypotensiond | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.2) | 2 (0.2) |
Abbreviations: AML, amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SAE, serious adverse event (AE); TEAE, treatment‐emergent AE. aThe total number of patients with an AE may not match the sum of the individual titration steps because a single patient could experience the same event at ≥1 titration step. bOne additional SAE (unstable angina and non–ST‐elevation myocardial infarction) occurred in 1 patient with a history of type 2 diabetes and hypertension while taking AML/OM 10/40+HCTZ 25 mg. This SAE, which was not considered by the investigator to be related to study drug, was not reported until 2 months after database lock and was not recorded in the clinical database. cOne serious TEAE (abdominal pain) was considered to be possibly related to HCTZ 25 mg as recorded in the ARISg risk management database (Aris Global LLC, Stamford, CT) by the investigator. This event was not recorded by the investigators as drug‐related on the clinical report form. dDrug‐related TEAE occurred at an incidence of <1%; however, it was considered to be a drug‐related TEAE of interest.
Discussion
The results of the BP‐CRUSH study demonstrate that a single‐pill AML/OM‐based titration regimen is a well‐tolerated means of effectively reducing BP and achieving BP goals in patients with hypertension who are uncontrolled on previous antihypertensive monotherapy. Following this regimen allowed a significant majority of patients uncontrolled on monotherapy to achieve BP goals. In this study, a majority of patients with and without diabetes achieved the SeSBP goal (<130 mm Hg and <140 mm Hg, respectively) on a single‐pill AML/OM‐based therapy regimen. At each titration step, the AML/OM‐based titration regimen significantly reduced the mean SeBP from baseline. Notably, switching patients with hypertension uncontrolled on an ARB or DHP‐CCB directly to fixed‐dose AML/OM allowed 72.2% of prior ARB and 65.8% of prior DHP‐CCB recipients cumulatively to achieve the SeBP goal of <140/90 mm Hg (<130/80 mm Hg for patients with diabetes) by week 12.
A unique aspect of the study was the prespecified enrollment of all major subpopulations with a high prevalence for hypertension, namely the elderly, blacks, and Hispanics, and patients with diabetes, obesity, and the metabolic syndrome. Patients were also switched directly from monotherapy to combination therapy, without a period of nontreatment, a scenario that closely mimics the real‐world experience of clinical medicine. Another important component of the study was that there was a large ABPM cohort of patients treated with both dual and triple combination therapy.
Although treating to goal is important for improving outcomes in patients with hypertension, 11 BP control rates remain suboptimal. 1 , 2 , 4 , 7 Treating to goal is particularly important for patients with diabetes, who tend to have a disproportionately higher prevalence of hypertension 12 and are at a greater risk of developing cardiovascular (CV) and renal disease. 13 , 14 Barriers to BP goal achievement include a lack of patient adherence to prescribed antihypertensive therapy regimens and clinical inertia, defined as the failure of clinicians to increase dosages or add new medications when BP treatment goals are unmet. 5 , 15 , 16 These factors are particularly relevant because for most patients, BP goal achievement necessitates the use of ≥2 antihypertensive therapies. 14
Clinical inertia is a multifactorial issue and physicians have a role to play in avoiding this problem by evaluating evidence that supports the use of aggressive treatment algorithms. The results of the BP‐CRUSH study may suggest that the tools to implement these strategies are available. The study demonstrated that in previously treated patient subpopulations that are often associated with clinical inertia and low rates of BP control, the use of a combination of antihypertensive drugs enabled a greater proportion to achieve their treatment goals. The development of single‐pill triple therapy formulations may also make these algorithms more acceptable by aiding patient adherence because intensifying therapy would not increase the pill burden, a factor associated with nonadherence. 17 Increased adherence has been previously demonstrated for fixed‐dose combinations of 2 antihypertensive agents. 18 , 19
An important measure of the efficacy of any antihypertensive therapeutic regimen is its ability to control BP throughout the 24‐hour dosing interval. The measurement of ambulatory BP during the 24‐hour period permits the assessment of BP control throughout the dosing interval. 14 Maintaining BP control during the last 6, 4, and 2 hours of the dosing interval is critical because this is the time when the morning surge in BP occurs, a factor known to be associated with an increased incidence of CV events. 20 , 21 In the present study, the AML/OM‐based titration regimen significantly reduced the mean 24‐hour, daytime (8 am–4 pm), and nighttime (10 pm–6 am) ambulatory BP, as well as the mean ambulatory BP during the last 2, 4, and 6 hours of the dosing interval, from baseline at both 12 and 20 weeks. Measurement of ambulatory BP also allows for the evaluation of whether nocturnal BP decreases by a prespecified percentage (ie, patient classified as a dipper) compared with daytime BP. Compared with patients who are classified as dippers, patients who are nondippers have an increased risk of CV events. 22 In the present study, 74.7% of patients who were dippers at baseline remained dippers at the end of the study. Furthermore, 53.1% of patients who were nondippers at baseline were dippers at the end of the study.
Currently, there are no guideline‐approved ambulatory BP goals, although the American Heart Association suggests that normal 24‐hour, daytime, and nighttime ambulatory BP targets should be <130/80 mm Hg, <135/85 mm Hg, and <120/70 mm Hg, respectively. 23 At the end of the study, the ABPM target of <130/80 mm Hg was reached by 90.5% of patients during the 24‐hour dosing period, the target of <135/85 mm Hg was reached by 88.4% of patients during the daytime (8 am–4 pm), and the target of <120/70 mm Hg was reached by 78.9% of patients during the nighttime (10 pm–6 am). Taken together, these ABPM data support the efficacy of an AML/OM‐based titration regimen in controlling BP throughout the 24‐hour dosing period.
In addition to being effective, the AML/OM‐based titration regimen explored in this study was safe and well tolerated, and no new safety issues were observed. The most common drug‐related TEAEs observed were dizziness, hypotension, fatigue, and nausea. As observed in previous studies, the addition of OM to AML appears to mitigate AML‐associated development of peripheral edema. 24 , 25 The addition of HCTZ to AML/OM did not affect the safety of the AML/OM‐based titration regimen. The incidence of most drug‐related TEAEs was similar among recipients of AML/OM+HCTZ and AML/OM‐based regimens. Although the incidence of dizziness was slightly higher with the addition of HCTZ to AML/OM, the incidence of hypotension was similar. The incidence of peripheral edema was slightly lower with triple therapy than previous titration steps.
Study Limitations and Strengths
A limitation of the BP‐CRUSH study is its open‐label, single‐arm design. This could introduce treatment bias. The strengths include the large study population, the use of ABPM to evaluate 24‐hour BP control, and the implementation of aggressive BP criteria for dose titration. The enrollment of a large study population that included a high proportion of patients with characteristics associated with difficult‐to‐treat hypertension demonstrates the efficacy of an AML/OM‐based titration regimen in achieving BP control in a diverse population of patients, thus increasing the applicability of the study to the general population.
Gaps remain between treatment guidelines and their implementation. In order to address the future needs of caring for patients with hypertension, physicians should be aware that patients can respond effectively and safely when switching from monotherapy to combination therapy. It is accepted that reducing BP improves patient outcomes. Evidence that olmesartan‐based therapies result in superior outcomes vs other regimens needs to be addressed in future studies. It also needs to be established that reducing clinical inertia and improving adherence to therapy results in improved patient outcomes. More studies are also needed to explore the benefits of the earlier introduction of 2‐ and even 3‐component single‐pill combination treatment in patients who are more than 20 mm Hg or 30 mm Hg from SBP control. The earlier attainment of BP control may result in reduced risk for CV events as a result of not only earlier control of BP, but also better long‐term control. 26 , 27
Conclusions
Switching to a single‐pill combination of AML/OM effectively controlled BP in a large proportion of patients with hypertension who were previously uncontrolled on antihypertensive monotherapy. Adding HCTZ to the titration regimen allowed more patients to achieve the SeBP goal of <140/90 mm Hg (<130/80 mm Hg for patients with diabetes). Titration to an AML/OM+HCTZ combination was well tolerated. Using a single‐pill combination of AML/OM, with the addition of HTCZ if required, and following a BP goal‐based treatment algorithm, the vast majority of patients previously uncontrolled on antihypertensive monotherapy can achieve BP goals without experiencing AEs.
Acknowledgments
Acknowledgements and disclosures: This study was supported by Daiichi Sankyo, Inc. Medical writing and editorial services were provided by Melanie Leiby, PhD, and Alan J. Klopp, PhD, of inScience Communications, a Wolters Kluwer business. Dr Weir is an ad‐hoc scientific consultant for Daiichi Sankyo, Inc, Novartis, Amgen, and NicOx. Dr Willa A. Hsueh has no financial relationships to disclose. Dr Shawna D. Nesbitt is on the speakers’ bureau for Novartis, Boehringer Ingelheim, and Forest Laboratories and serves on an advisory board for Daiichi Sankyo, Inc. Dr Thomas J. Littlejohn III has no financial relationships to disclose. Dr Alan Graff has no financial relationships to disclose. Ali Shojaee, PharmD, is employed by Daiichi Sankyo, Inc. William F. Waverczak, MS, is employed by Daiichi Sankyo, Inc. Chunlin Qian, PhD, is employed by Daiichi Sankyo, Inc. Christopher J. Jones, PhD, is employed by Wolters Kluwer. Dr Joel M. Neutel is on the speakers’ bureau for Novartis, Boehringer Ingelheim, Daiichi Sankyo, Inc, sanofi‐aventis, Bristol‐Myers Squibb, Forest, and Pfizer.
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Mori H, Ukai H, Yamamoto H, et al. Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res. 2006;29(3):143–151. [DOI] [PubMed] [Google Scholar]
- 3. Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289(16):2073–2082. [DOI] [PubMed] [Google Scholar]
- 4. Qvarnström M, Wettermark B, Ljungman C, et al. Antihypertensive treatment and control in a large primary care population of 21 167 patients. J Hum Hypertens. 2010;Aug 19. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5. Spranger CB, Ries AJ, Berge CA, et al. Identifying gaps between guidelines and clinical practice in the evaluation and treatment of patients with hypertension. Am J Med. 2004;117(1):14–18. [DOI] [PubMed] [Google Scholar]
- 6. Petrella RJ, Merikle EP, Jones J. Prevalence, treatment, and control of hypertension in primary care: gaps, trends, and opportunities. J Clin Hypertens (Greenwich). 2007;9(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filippi A, Paolini I, Innocenti F, et al. Blood pressure control and drug therapy in patients with diagnosed hypertension: a survey in Italian general practice. J Hum Hypertens. 2009;23(11):758–763. [DOI] [PubMed] [Google Scholar]
- 8. Ferrari P, Hess L, Pechere‐Bertschi A, et al. Reasons for not intensifying antihypertensive treatment (RIAT): a primary care antihypertensive intervention study. J Hypertens. 2004;22(6):1221–1229. [DOI] [PubMed] [Google Scholar]
- 9. Bramlage P, Thoenes M, Kirch W, et al. Clinical practice and recent recommendations in hypertension management–reporting a gap in a global survey of 1259 primary care physicians in 17 countries. Curr Med Res Opin. 2007;23(4):783–791. [DOI] [PubMed] [Google Scholar]
- 10. Nicodème R, Albessard A, Amar J, et al. Poor blood pressure control in general practice: in search of explanations. Arch Cardiovasc Dis. 2009;7:477–483. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Messerli F, Bakris G, et al. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR‐Trandolapril Study. Hypertension. 2007;50(2):299–305. [DOI] [PubMed] [Google Scholar]
- 12. Comaschi M, Coscelli C, Cucinotta D, et al. Cardiovascular risk factors and metabolic control in type 2 diabetic subjects attending outpatient clinics in Italy: the SFIDA (survey of risk factors in Italian diabetic subjects by AMD) study. Nutr Metab Cardiovasc Dis. 2005;15(3):204–211. [DOI] [PubMed] [Google Scholar]
- 13. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–444. [DOI] [PubMed] [Google Scholar]
- 14. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 15. Cushman WC, Basile J. Achieving blood pressure goals: why aren’t we? J Clin Hypertens (Greenwich). 2006;8(12):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834. [DOI] [PubMed] [Google Scholar]
- 17. Chapman RH, Petrilla AA, Benner JS, et al. Predictors of adherence to concomitant antihypertensive and lipid‐lowering medications in older adults: a retrospective, cohort study. Drugs Aging. 2008;25(10):885–892. [DOI] [PubMed] [Google Scholar]
- 18. Patel BV, Remigio‐Baker RA, Thiebaud P, et al. Improved persistence and adherence to diuretic fixed‐dose combination therapy compared to diuretic monotherapy. BMC Fam Pract. 2008;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaya FT, Du D, Gbarayor CM, et al. Predictors of compliance with antihypertensive therapy in a high‐risk Medicaid population. J Natl Med Assoc. 2009;101(1):34–39. [DOI] [PubMed] [Google Scholar]
- 20. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107(10):1401–1406. [DOI] [PubMed] [Google Scholar]
- 21. Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–743. [DOI] [PubMed] [Google Scholar]
- 22. Fagard RH, Thijs L, Staessen JA, et al. Night‐day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23(10):645–653. [DOI] [PubMed] [Google Scholar]
- 23. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. [DOI] [PubMed] [Google Scholar]
- 24. Chrysant SG, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double‐blind, placebo‐controlled, 8‐week factorial efficacy and safety study. Clin Ther. 2008;30(4):587–604. [DOI] [PubMed] [Google Scholar]
- 25. Volpe M, Brommer P, Haag U, et al. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double‐blind, parallel‐group, multicentre study. Clin Drug Investig. 2009;29(1):11–25. [DOI] [PubMed] [Google Scholar]
- 26. Bakris GL, Weir MR. Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed‐dose combination approaches. J Clin Hypertens (Greenwich). 2003;5(3):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown MJ, McInnes GT, Papst CC, et al. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel‐group trial. Lancet. 2011; 377(9762):312–320. [DOI] [PubMed] [Google Scholar]


