Abstract
J Clin Hypertens (Greenwich). 2012; 14:588–592. © 2012 Wiley Periodicals, Inc.
Blood pressure (BP) reductions when combining blockers of the renin‐angiotensin system (RAS) and β‐blockers have generally not been shown to be greater than for individual agents, possibly because of overlapping mechanisms of action. The authors tested the additivity of the β‐blocker nebivolol, which has vasodilating activity, with the angiotensin‐converting enzyme inhibitor lisinopril in patients with stage 2 diastolic hypertension. The BP effects of placebo (n=93), nebivolol 5 mg to 20 mg daily (n=185), lisinopril 10 mg to 40 mg daily (n=189), and nebivolol 5 mg to 20 mg + lisinopril 10 mg to 40 mg (n=189) during 6 weeks of treatment were compared. The primary end point was change in diastolic BP (DBP). For the full cohort, baseline BP was 163.8/104.4 mm Hg, mean age was 49.2 years, 58% were men, 62% were white, and 34% were black. DBP fell by 17.2±10.2 mm Hg with the combination, greater than placebo (8.0±9.2, P<.0001), nebivolol (13.3±8.9, P=.0010), and lisinopril (12.0±9.8, P<.0001). For systolic BP, corresponding reductions were 19.2±19.8 mm Hg, 9.9±16.4 (P<.0001 vs combination), 14.4±14.1 (P=.0470), and 16.1±17.2 (P=.0704). Adverse event rates were similar in all groups. This study demonstrated the potential antihypertensive benefits of combining nebivolol with a RAS blocker.
Combination treatment is required for effective blood pressure (BP) control in more than half of all patients with hypertension. 1 , 2 Several two‐drug combinations have been shown to be more effective than their single‐drug components in reducing BP. 3 , 4 , 5 , 6
A recent position paper that evaluated potential two‐drug combinations in hypertension treatment concluded, however, that the combined use of β‐blockers with blockers of the RAS is less effective for BP reduction. 7 Previous trials have demonstrated that such combinations were not meaningfully more effective than the individual drugs. 8 , 9 , 10 One explanation for this finding is that each of these drug types depends, at least in part, on a common mechanism of action—blockade of the RAS—so that their combination is less likely to provide useful additive antihypertensive effects. 10 , 11 , 12 , 13
An apparent exception to this finding was reported in the Glycemic Effects in Diabetes Mellitus: Carvedilol‐Metoprolol Comparison in Hypertensives (GEMINI) trial, in which addition of either of the β‐blockers, carvedilol or metoprolol, produced further BP reductions when added to ongoing treatment with RAS blockers in hypertensive patients with diabetes. 14 In a subsequent study, however, the BP effects of several combinations of carvedilol and the angiotensin‐converting enzyme (ACE) inhibitor lisinopril were rigorously compared with the individual drugs and failed to confirm additive effects. 15
Nebivolol is the most recently available β‐blocker in the United States. Beyond its β receptor–blocking effects, it has a vasodilatory action, presumably mediated by its ability to increase availability of vascular endothelial nitric oxide. 16 Because this mechanism of action appears to be independent of its renin inhibitory effects, an additional BP‐lowering effect could be anticipated when nebivolol is combined with a blocker of the RAS. In a previous study in which patients were already receiving RAS ACE inhibitors or angiotensin receptor blockers, nebivolol provided further efficacy. 17 In the present placebo‐controlled study we more carefully tested this hypothesis by comparing the antihypertensive effects of a free combination of nebivolol and lisinopril with each of these drugs given individually.
Methods
The objective of the study was to determine whether the diastolic BP (DBP)‐lowering effects of the combination of nebivolol and lisinopril (given as separate doses) were significantly greater than those of the individual drugs used in the same doses as in the combination.
Patients
The study was conducted in men and women (nonpregnant) aged 18 to 64 who had stage 2 diastolic hypertension while not receiving hypertension treatment. Patients could have been on no previous treatment or else withdrawn from treatment at the start of an initial 4‐week placebo phase. Eligibility was defined as DBP ≥100 mm Hg after 4 weeks of nontreatment (receiving placebo only), ≥100 mm Hg at two consecutive weekly visits after at least 2 weeks of the initial placebo treatment, or ≥110 mm Hg after at least 1 week of the initial placebo (and confirmed within 3 days).
Patients were excluded from entering the study if they had known secondary hypertension, systolic BP (SBP) ≥180 mm Hg or DBP ≥110 mm Hg during screening or at the start of the initial placebo period, evidence of chronic kidney disease (estimated glomerular filtration rate <60 mL/min), liver dysfunction, or a recent history (<6 months) of stroke, myocardial infarction, coronary revascularization, or other conditions that could interfere with the conduct of the trial. Patients who had contraindications to the use of β‐blockers or ACE inhibitors were also excluded.
Study Design
This was a double‐blind, placebo‐controlled parallel group trial that compared the effects of placebo, nebivolol monotherapy, lisinopril monotherapy, and the combination of nebivolol and lisinopril on DBP and SBP. Patients initially signed an informed consent approved by the appropriate institutional review board and then entered a 1‐week screening period to determine initial eligibility. The patients then entered a 4‐week single‐blind period during which they all received placebo. If patients were already receiving antihypertensive therapy it was discontinued (according to appropriate instructions for the particular agents involved) at the start of the washout period. As described in the previous section, patients became eligible for randomization into one of the study treatment groups if they satisfied the criteria for stage 2 diastolic hypertension.
The primary end point of the study was measured after 6 weeks of active treatment. At the beginning of this period, patients were randomized in a 1:2:2:2 ratio to placebo, nebivolol alone, lisinopril alone, or nebivolol + lisinopril. All drugs were administered in a double‐blind fashion. Initially, the patients assigned to nebivolol alone received 5 mg daily but were titrated to 20 mg daily after 2 weeks if they did not achieve target BP (<130/80 mm Hg if diabetic, otherwise <140/90 mm Hg) and stayed on this dose for the remaining 4 weeks of the study. For the lisinopril group, the respective doses were 10 mg and 40 mg daily, and for the combination group, the doses for each of the drugs were the same as in the monotherapy groups.
SBP and DBP were measured at randomization and at the end of the 6‐week treatment period. These were “trough” measurements taken in the morning within 2 hours of when the next drug dose was scheduled. The readings were taken after 5 minutes of rest in the seated position using an automated oscillometric device. The BP values were documented as the average of 3 readings on each occasion. Standard laboratory data were obtained at randomization and at the 6‐week treatment period. Any adverse effects were recorded at the 2‐week and 6‐week points of the 6‐week treatment period.
Data and Statistics
The primary end point of the study was the change in trough DBP from baseline (at randomization) to the end of the 6‐week treatment period. The last‐observation‐carried‐forward approach was used to impute missing on‐treatment values at week 6. All statistical tests were two‐sided hypothesis tests based on a 5% level of significance.
Comparisons of changes in DBP between the treatment groups were by an analysis of covariance model. Treatment group and study site were factors, and baseline DBP was a covariate. The initial comparison was between the combination group (nebivolol + lisinopril) and placebo; thereafter, the effects in the combination group were compared with nebivolol alone and lisinopril alone. To control the family‐wise error rate, hierarchical testing was employed such that the second set of comparisons (combination vs nebivolol and combination vs lisinopril) could be conducted only if the initial comparison, combination vs placebo, was significant. For this second step, a multiple testing procedure was employed 18 : first, if the combination effect on DBP was different (at the P=.05 level) from the average of the nebivolol and lisinopril effects, then the combination could be compared with nebivolol and with lisinopril. In addition, the effects in the nebivolol and lisinopril monotherapy groups were each compared with placebo.
Changes in SBP from baseline were the secondary end point in this study. These changes could be analyzed only if the results of analyzing the primary end point (DBP), as described above, were significant. If this condition was satisfied, then the analysis of the secondary end point could be carried out using the same step‐wise procedures as for the primary end point.
A responder analysis was conducted as an additional end point with responders defined as those achieving SBP <130 mm Hg and DBP <80 mm Hg for diabetic patients and SBP <140 mm Hg and DBP <90 mm Hg for all other patients.
Results
A total of 1126 patients were enrolled in the trial. The disposition of these study patients during the study is shown in Figure 1. After study entry, 462 patients were excluded from the study primarily due to failure to satisfy the study’s inclusion/exclusion criteria. As far as the intent‐to‐treat population was concerned, 93 patients were randomized to placebo, 185 to nebivolol monotherapy, 189 to lisinopril monotherapy, and 189 to combination nebivolol + lisinopril treatment.
Figure 1.

Disposition of patients who signed informed consents to enter the trial.
In the placebo group, 74 patients completed the double‐blind treatment (77.9%), as did 168 (88.9%) of nebivolol patients, 169 (88.9%) of lisinopril patients, and 173 (91.1%) of the combination patients. The main reasons for on‐treatment discontinuation were inadequate treatment response (mainly in the placebo group, 9.5% of patients), adverse events (2.1% in each of the active treatment groups), or withdrawal of consent, patients being lost to follow‐up, or technical violations of the protocol.
Baseline Patient Characteristics
The principal clinical and demographic patient characteristics in the safety population for the 4 treatment groups are summarized in Table I. There were no major differences in the compositions of these groups. The baseline DBPs, by definition, were all higher than 100 mm Hg, consistent with stage 2 diastolic hypertension. The mean body mass index (BMI) in all 4 groups was >32 kg/m2, indicating a high incidence of obesity (BMI ≥30) in stage 2 hypertension. There was a strong representation of black patients in all 4 treatment groups; for the study cohort as a whole, 33.6% of the patients (safety population) were black.
Table I.
Baseline Characteristics of Patients Randomized to Each of the 4 Treatment Groups
| Category | Placebo | Nebivolol | Lisinopril | Nebivolol + Lisinopril |
|---|---|---|---|---|
| Patients, No. | 95 | 188 | 189 | 189 |
| Age, y | 47.4±9.7 | 49.7±8.4 | 50.1±8.0 | 48.8±8.4 |
| Male, % | 50.5 | 56.4 | 60.8 | 60.3 |
| White, % | 68.4 | 64.9 | 61.4 | 56.1 |
| Black, % | 24.2 | 31.9 | 36.0 | 37.6 |
| Diabetes, % | 11.6 | 11.2 | 14.8 | 20.1 |
| Body mass index, kg/m2 | 32.9±7.9 | 32.7±6.8 | 32.5±7.4 | 32.1±6.7 |
| Heart rate, beats per min | 81.7±14.0 | 79.1±11.2 | 80.4±11.3 | 79.6±11.4 |
| Systolic BP, mm Hg | 164.7±13.5 | 160.3±11.3 | 163.5±13.2 | 164.3±12.9 |
| Diastolic BP, mm Hg | 104.3±3.6 | 104.1±3.7 | 104.2±3.8 | 105.0±4.4 |
Data are mean±standard deviation or percentage.
BP Effects
The primary end point for the study was treatment‐induced change in DBP. The baseline values and treatment effects on BP after 6 weeks of therapy are shown in Figure 2 (last observation carried forward for patients who started but did not complete the double‐blind treatment period). The change with the nebivolol + lisinopril combination was significantly greater than with placebo, nebivolol alone, and lisinopril alone. Since the difference between the combination and placebo was significant, it was appropriate to compare the combination effects with those of the individual treatments. The least‐square differences for DBP between the combination and these 3 other groups were 9.0 mm Hg, 3.3 mm Hg, and 5.1 mm Hg.
Figure 2.
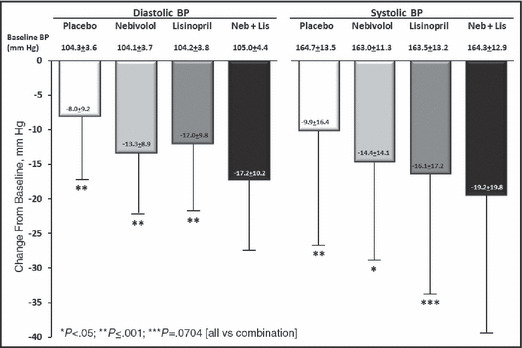
Baseline diastolic and systolic blood pressure (BP) values and changes in BP during 6 weeks of treatment in hypertensive patients randomized to treatment with placebo, nebivolol (Neb) 20 mg daily, lisinopril (Lis) 40 mg daily, or the combination of nebivolol 20 mg daily + lisinopril 20 mg daily. Values are mean±standard deviation.
The results of the changes in SBP are also shown in Figure 2. The changes in SBP with the combination were significantly greater than with placebo or nebivolol monotherapy, but did not quite reach significance (P=.0704) when compared with lisinopril monotherapy. The least‐square differences between the combination and placebo, nebivolol and lisinopril, were 10.0 mm Hg, 3.5 mm Hg, and 3.2 mm Hg.
Response rates (achievement of BPs <130/80 mm Hg in diabetic patients, <140/90 mm Hg in all others) were also calculated. For the combination, the response rate in these patients with baseline stage 2 hypertension was 33.9%, which was significantly greater than with placebo (7.5%, P<.0001), nebivolol (21.6%, P=.0030), and lisinopril (21.7%, P=.0031).
Adverse Events
There were no deaths in the trial, and the rates of serious adverse events were 1.1% for the combination group, 2.1% for placebo, 1.6% for nebivolol, and 2.1% for lisinopril.
A detailed listing of the most common (≥2%) adverse events during the trial is shown in Table II for the safety population. In general, the active treatments were well tolerated and there was no evidence of a higher event rate in the combination treatment group than with either of the monotherapies. There were no meaningful changes in clinical laboratory measurements during the 6 weeks of treatment in the 4 patient groups.
Table II.
Common (≥2%) Adverse Events During 6 Weeks of Treatment With Placebo or Active Therapy
| Category | Placebo (n=95) | Nebivolol (n=188) | Lisinopril (n=189) | Nebivolol + Lisinopril (n=189) |
|---|---|---|---|---|
| n (%) | ||||
| Patients with at least one adverse event | 29 (30.5) | 51 (27.1) | 58 (30.7) | 57 (30.7) |
| Upper respiratory tract infection | 3 (3.2) | 6 (3.2) | 5 (2.6) | 7 (3.7) |
| Headache | 7 (7.4) | 5 (2.7) | 6 (3.2) | 6 (3.2) |
| Bradycardia | 0 | 4 (2.1) | 0 | 4 (2.1) |
| Nasopharyngitis | 1 (1.1) | 8 (4.3) | 5 (2.6) | 4 (2.1) |
| Cough | 1 (1.1) | 4 (2.1) | 6 (3.2) | 3 (1.6) |
| Fatigue | 1 (1.1) | 6 (3.2) | 1 (0.5) | 3 (1.6) |
| Dizziness | 2 (2.1) | 2 (1.1) | 3 (1.6) | 2 (1.1) |
| Nasal congestion | 1 (1.1) | 1 (0.5) | 4 (2.1) | 1 (0.5) |
| Neck pain | 2 (2.1) | 0 | 0 | 0 |
| Sinus congestion | 1 (1.1) | 0 | 6 (3.2) | 0 |
Discussion
This study has demonstrated that the β‐blocker nebivolol adds significantly to the DBP‐reducing effect of the ACE inhibitor lisinopril in patients with stage 2 hypertension. Specifically, this combination therapy was significantly more effective than nebivolol alone and lisinopril alone in producing this effect.
A directionally similar finding was observed for the secondary end point of change in SBP; however, while the combination treatment was more effective than nebivolol alone, it narrowly missed being statistically superior to lisinopril. The combination therapy was significantly more effective than the two monotherapies in achieving clinical BP treatment targets (<130/80 mm Hg for patients with diabetes, <140/90 mm Hg for all others). With the two‐drug combination, more than one third of patients with stage 2 hypertension satisfied these response criteria.
It is not clear why this combination of nebivolol plus lisinopril appeared to be more effective (as compared with its component monotherapies) than the recently reported experience with carvedilol and lisinopril, 15 in which lisinopril—as in the present study—was tested in its 40‐mg dose. We could speculate that nebivolol’s antihypertensive mechanism of action is sufficiently different from that of other β‐blockers to provide additional effects when administered with a blocker of the RAS. Both nebivolol and carvedilol are regarded as vasodilating β‐blockers, but while carvedilol is believed to exert this action through α‐blockade, nebivolol instead appears to vasodilate by enhancing availability of vascular endothelial nitric oxide. At this point, however, we do not have sufficient mechanistic information to reach conclusions regarding the differential BP effects of these agents when combined with an ACE inhibitor.
Despite the positive finding in this study, the additional effect of nebivolol when combined with lisinopril (as compared with lisinopril alone) appeared to be slightly less than reported previously. 17 In the earlier study, though, nebivolol was added to patients whose BPs were elevated despite already receiving a RAS blocker, so there might have been a selection bias favoring the effectiveness of the added drug. Moreover, in that earlier study, the underlying blockers of the RAS might not all have been administered in maximum doses, again creating the opportunity for greater efficacy of an added agent.
In the present relatively short‐term study, adverse event rates with the combination treatment were not meaningfully different from those in the placebo or single‐drug groups. Similarly, there did not appear to be any untoward laboratory findings during the 6‐week treatment period. These results add to the feasibility of planning larger and more definitive future studies of nebivolol combined with blockers of the RAS.
Study Limitations
One of the unexpected findings in this study was the large placebo effect on BP. Even though all of the active treatment groups exhibited BP reductions significantly greater than placebo, it is possible that the large BP change in the placebo group was indicative of a substantial regression‐to‐the‐mean effect whereby patients selected on the basis of a high BP at one particular moment may more typically have lower BPs at other times. This phenomenon could potentially dilute the findings of a study such as the present one. In fact, just considering the patient group receiving nebivolol alone in this trial, the placebo‐subtracted BP reductions were of lesser amplitude than those reported for the same dose of nebivolol during the registration studies for this drug. 19
An important characteristic of the patient cohort in this study was that one third was African American. Even though nebivolol has been shown to be effective in black patients, it is known that lisinopril (as with ACE inhibitors in general) is less effective in black patients than in other ethnicities. Again, this could have exerted a small diluting effect on this study’s ability to compare efficacies among the treatment groups.
Another possible limitation of the study was that patients 65 or older were not enrolled, reflecting concern at exposing patients at relatively higher cardiovascular risk to placebo treatment for up to 10 weeks. Since so many hypertensive patients are now in older age groups, we should be somewhat cautious in extrapolating from the present findings into broad medical practice. There could also be some criticism that we did not use the maximum US‐approved dose of nebivolol, which is 40 mg daily. In reality, this highest dose is only marginally, if at all, more effective than the 20‐mg dose, 19 and since the 40‐mg dose requires taking two 20‐mg tablets, it is used only rarely. It can also be noted that lisinopril has been administered in a maximum dose of 80 mg, although this dose has not been shown to be more effective than the recommended maximum dose of 40 mg that was used in this study.
Conclusions
This study has shown that the combination of nebivolol and a RAS blocker is more effective in reducing BP than either agent alone. This combination also appears to be well tolerated, laying the foundation for further exploration of this therapeutic approach.
Disclosures: Dr Weber provides consulting and speaking services to Forest Laboratories, Takeda, Novartis, Daiichi Sankyo, and Boehringer Ingelheim. Dr Basile provides consulting services to Daiichi Sankyo, Forest Laboratories, and Eli‐Lilly and speaking services to Daiichi Sankyo, Forest Laboratories, Eli‐Lilly, Boehringer Ingelheim, and Takeda. Dr Stapff, Dr Zhou, and Ms Khan are employees of Forest Laboratories, which funded the study and participated in its design.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure: the JNC7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Bakris GL. An approach to achieving recommended blood pressure goals in diabetic patients. Arch Intern Med. 2001;161:2661–2667. [DOI] [PubMed] [Google Scholar]
- 3. Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benzazepril HCl versus comparable component‐based therapy. Chem Herit. 2003;9:324–332. [DOI] [PubMed] [Google Scholar]
- 4. Jamerson K, Weber MA, Bakris GL, et al.; ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 5. Weinberger MH. Blood pressure and metabolic responses to hydrochlorothiazide, captopril, and the combination in black and white mild‐to‐moderate hypertensive patients. J Cardiovasc Pharmacol. 1985;7(Suppl 1):S22–S55. [DOI] [PubMed] [Google Scholar]
- 6. Cappuccio FP, Markandu ND, Tucker FA, et al. Does a diuretic cause a further fall in blood pressure in hypertensive patients already on nifedipine? J Clin Hypertens. 1986;4:346–353. [PubMed] [Google Scholar]
- 7. Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. J Am Soc Hypertension. 2010;4(2):90–98. [DOI] [PubMed] [Google Scholar]
- 8. Huttunen M, Lampainen E, Lilja M, et al. Which antihypertensive to add to a beta‐blocker: ACE inhibitor or diuretic? J Hum Hypertens. 1992;6:121–125. [PubMed] [Google Scholar]
- 9. Bursztyn M, Gavras I, Gourley L, et al. Effect of combination therapy with atenolol and the angiotensin‐converting enzyme inhibitor benazepril. Clin Ther. 1994;16:429–436. [PubMed] [Google Scholar]
- 10. Wing LM, Chalmers JP, West MJ, et al. Treatment of hypertension with enalapril and hydrochlorothiazide or enalapril and atenolol: contrasts in hypotensive interactions. J Hypertens. 1987;5:S603–S606. [PubMed] [Google Scholar]
- 11. Drayer JLM, Weber MA, Lipson JL, et al. Differential effects of diuresis and betaadrenoreceptor blockade during angiotensin converting enzyme inhibition in patients with severe hypertension. J Clin Pharmacol. 1982;22:179–186. [DOI] [PubMed] [Google Scholar]
- 12. Staessen J, Fagard R, Lijnen P, et al. Double‐blind comparison between propranolol and bendroflumethiazide in captopril‐treated resistant hypertensive patients. Am Heart J. 1983;106:321–328. [DOI] [PubMed] [Google Scholar]
- 13. Ruddy MC, Bialy GB, Kostis JB. Intrinsic sympathomimetic activity counteracts beta‐blocker inhibition of renin activation. Angiology. 1989;40:45–50. [DOI] [PubMed] [Google Scholar]
- 14. Wright JT Jr., Bakris GL, Bell DS, et al. Lowering blood pressure with beta‐blockers in combination with other renin‐angiotensin system blockers in patients with hypertension and type 2 diabetes: results from the GEMINI Trial. J Clin Hypertens. 2007;9:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bakris GL, Iyengar M, Lukas MA, et al. Effect of combining extended‐release carvedilol and lisinopril in hypertension: results of the COSMOS study. J Clin Hypertens. 2010;12:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss R. Nebivolol: a novel beta‐blocker with nitric oxide‐induced vasodilation. Vasc Health Risk Manag. 2006;2:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neutel JM, Smith DHG, Gradman AH. Adding nebivolol to ongoing antihypertensive therapy improves blood pressure and response rates in patients with uncontrolled stage 1‐2 hypertension. J Hum Hypertens. 2010;24:64–73. [DOI] [PubMed] [Google Scholar]
- 18. Kong L, Koch G, Liu T, et al. Performance of some multiple testing procedures to compare three doses of a test drug and placebo. Pharmaceut Statist. 2005;4:25–35. [Google Scholar]
- 19. Forest laboratories, Inc . Bystolic: Full Prescribing Information. St Louis, MO: Forest laboratories, Inc; 2011. [Google Scholar]


