Abstract
J Clin Hypertens (Greenwich). 2012; 14:848–854. ©2012 Wiley Periodicals, Inc.
The authors investigated the effects of moderate‐intensity resistance, aerobic, or combined exercise on blood pressure and arterial stiffness in overweight and obese individuals compared with no exercise. Participants were randomized to 4 groups: control, aerobic, resistance, and combination. Assessments were made at baseline, week 8, and week 12. In participant‐designated responders, those in the intervention groups who had improved levels of systolic blood pressure (SBP) or augmentation index (AI), we observed a significant decrease of SBP in aerobic (−4%, P=.027), resistance (−5.1%, P=.04), and combination groups (−6.3%, P=.000) at week 8 and in the combination group (−6.3%, P=.005) at week 12, compared with baseline. AI was significantly lower at week 12 in the aerobic (−12%, P=.047), resistance (−9.5%, P=.036), and combination (−12.7%, P=.003) groups compared with baseline, as well as in the combination group (−10.7%, P=.047) compared with the control group. We did not observe significant changes in SBP, DBP, or AI between the interventions when assessing the entire cohort, although there were significant improvements in a subgroup of responders. Thus, some but not all overweight and obese individuals can improve blood pressure and arterial stiffness by participating in regular combination exercise, decreasing the risk of developing cardiovascular disease.
Hypertension is associated with increased risk of coronary heart disease (CHD), stroke, heart failure, and kidney failure. 1 In Australia, hypertension is a major health problem and data from the 1999–2000 AusDiab study 2 indicated that 30% of the population 25 years and older had high systolic (≥140 mm Hg) or diastolic (≥90 mm Hg) blood pressure (BP) or were taking medication to control hypertension. Many people with hypertension go untreated, as there are rarely signs or symptoms of the condition. 3
Higher levels of physical activity are associated with decreased prevalence of hypertension. 3 Endurance training decreases BP due to lower systemic vascular resistance involving the sympathetic nervous system and the renin‐angiotensin system. 4 Regular aerobic exercise has also been demonstrated to significantly decrease BP in healthy sedentary normotensive and/or hypertensive adults, 5 while other studies have confirmed that resistance training is also beneficial in reducing BP. 6 , 7 There are limited data, however, on the effect of a combination of aerobic and resistance exercise training on BP.
Endurance exercise training is associated with lower levels of stiffness in central arteries, which suggests that regular exercise may be able to delay or prevent age‐related increases in arterial stiffness. 8 A single bout of aerobic exercise can improve endothelial function in sedentary 9 and physically active individuals. 10 In addition, the beneficial effects of aerobic training on arterial stiffness and endothelial function have also been observed in numerous studies. 11 , 12 In contrast, the effect of resistance training has not been studied as extensively as aerobic training, although current data indicate a role in improving endothelial dysfunction. 7 Resistance training has also been associated with lower levels of arterial compliance. 13 However, a cross‐sectional study by Cooks and colleagues 14 observed that rowing, which has both aerobic and resistance exercise components, has an overall positive effect on arterial stiffness compared with sedentary controls.
There is much evidence supporting the beneficial effects of aerobic exercise on BP and arterial stiffness, but limited data on the effect of other exercise types such as resistance or a combination of aerobic and resistance training. Thus, the aim of this study was to investigate the chronic effects of resistance, aerobic, or combined exercise at moderate intensity on BP and arterial stiffness in overweight and obese individuals compared with no exercise.
Materials and Methods
Participants
The current investigation was part of a larger study, 15 which can be referred to for full details of study methods. Ninety‐seven overweight or obese men and women, between the ages of 40 and 66 years with a body mass index (BMI) between 25 kg/m2 and 40 kg/m2 were recruited from the community of Perth, Australia. Participants were required to be sedentary to lightly active, participating in <1 hour of moderate‐intensity physical activity each week during the past 3 months. Interested individuals were screened via telephone. Exclusion criteria included diabetes mellitus, pre‐existing heart conditions, use of lipid‐lowering medications or β‐blockers, pregnant or lactating women, smokers, gastrointestinal tract surgery, and major illness (acute or chronic) including any that would limit the ability to perform the necessary exercises. Informed written consent was obtained from all participants. All study procedures were appr‐oved by the Curtin University Ethics Committee (HR166/2004).
Study Design
This 12‐week study was a randomized parallel‐group design examining the effects of different exercise regimens on BP and arterial stiffness in overweight and obese individuals compared with individuals who did no exercise. Participants were randomized to 4 different groups as they were recruited by the researcher (using a randomization sequence generated from http://www.randomization.com): the control group performed no exercise and received a placebo dietary supplement only (participants were asked to take a teaspoon of supplement in a glass of water once per day; the supplement contained approximately 2 g of breadcrumbs and 0.1 g of Equal artificial sweetener); the aerobic group performed 30 minutes of aerobic exercise 5 d/wk, which consisted of treadmill walking; the resistance group performed 30 minutes of resistance exercise 5 d/wk using weight resistance machines; and the combination group performed 15 minutes of aerobic and 15 minutes of resistance exercise 5 d/wk.
Assessment visits at baseline, week 8, and week 12 were conducted for vascular measurements in clinical rooms at Curtin University. Individuals in the control group were requested to continue their normal physical activity while those in the exercise groups completed baseline measurements over 1 week before attending an initiation session at the Curtin Fitness Centre, where they were instructed to follow an additional exercise program as demonstrated by centre staff.
Exercise Interventions
The exercise interventions were either 30 minutes of aerobic exercise on a treadmill, 30 minutes of resistance exercise (4 sets of 8–12 repetitions at 10 RM level of leg press, leg curl, leg extension, bench press, rear deltoid row) or a combination of 15 minutes of aerobic exercise and 15 minutes of resistance exercise. Full details on the exercise intervention can be found in Ho and colleagues. 15
Measurement of Brachial BP
Systolic BP (SBP) and diastolic BP (DBP) were measured on the left arm of participants with an automated sphygmomanometer (Dinamap ProCare; GE Healthcare, Chalfont St. Giles, UK) with participants resting in a supine position with the arm at the level of the heart for at least 10 minutes before and during measurements. 16 Measurements were taken in the fasting state in the morning, between 7 am and 11 am at baseline, week 8, and week 12. Three readings were taken at 2‐minute intervals and the average was calculated.
Pulse Wave Analysis
Aortic root pressure waveform measurements were performed as previously described, 17 utilizing a pressure‐sensitive probe attached to the Sphygmocor machine (AtCor Medical, Sydney, New South Wales, Australia). Measurements were taken in the fasting state at baseline, week 8, and week 12. For each assessment, at least 3 measurements were taken and results were averaged. Results were expressed using the central augmentation index (AI), which is a measure of the stiffness of arteries. The greater the arterial stiffness is, the higher the value for AI.
Anthropometric Measures
Anthropometric measures were completed and BMI calculated. Weight was measured using electronic scales (Tanita Corporation, Tokyo, Japan). Waist circumference was measured at the mid‐point between the bottom of the rib cage and the iliac crest and hip circumference was measured at the widest point at the hip. Abdominal and total body fat was measured by dual‐energy x‐ray absorptiometry (DXA) 18 at baseline and 12 weeks (GE Lunar Prodigy DXA scanner; GE Healthcare, Waukesha, WI).
Statistical Analysis
A sample size of 16 participants was predicted to provide sufficient power (80%) at the 5% significance level to detect a 15% difference; however, we aimed to recruit 20 participants to accommodate for a 20% attrition rate.
Data were analyzed using the General Linear Model to assess the effects of the groups after adjusting for baseline values. We also carried out a further subgroup analysis of data from the study. Those in the exercise intervention groups who had either improved levels of SBP or AI at either week 8 or week 12 compared with baseline were designated responders. Those who did not show improvements were designated nonresponders. We excluded data from nonresponders and analyzed the remaining data sets from participants designated as responders using the General Linear Model and adjusting for baseline values.
Only significant between‐group effects within the General Linear Model were further investigated using post hoc comparisons. Statistical analyses were carried out using SPSS for Windows (SPSS Inc, Chicago, IL).
This clinical trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR), registration number: ACTRN12609000684224.
Results
Vascular Changes in All Participants
Ninety‐seven overweight or obese participants were recruited and 80 commenced the study (Figure 1). There were 10 hypertensive participants at the beginning of the study (SBP >140 mm Hg or taking medication), of whom 5 were taking antihypertensive medication (1 in the control group, 1 in the aerobic group, 2 in the resistance group, and 1 in the combination group). The baseline characteristics of participants in the 4 intervention groups are shown in Table I.
Figure 1.
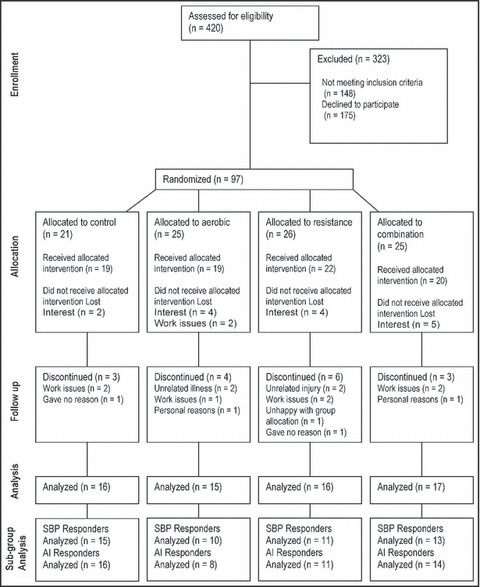
Participant flow diagram. SBP indicates systolic blood pressure; AI, augmentation index.
Table I.
Characteristics of All Participants at Baseline
| No Exercise (n=16) | Aerobic (n=15) | Resistance (n=16) | Combination (n=17) | |
|---|---|---|---|---|
| Age, y | 52 (40–66) | 55 (44–62) | 52 (43–59) | 53 (43–64) |
| Weight, kg | 85.1 (64.8–123.0) | 91.9 (65.9–124.1) | 89.3 (71.9–127.5) | 90.0 (62.2–122.3) |
| BMI, kg/m2 | 32.4 (26.0–48.0) | 32.7 (25.0–45.6) | 33.0 (25.8–44.6) | 33.3 (23.4–40.2) |
| Body fat, % | 46.5 (35.9–59.9) | 44.6 (30.7–52.5) | 43.7 (34.6–52.2) | 45.8 (28.8–55.5) |
| WC, cm | 100.3 (80.0–131.0) | 103.7 (82.0–118.0) | 104.0 (83.5–135.5) | 102.2 (81.5–124.5) |
| WHR | 0.85 (0.75–1.01) | 0.87 (0.76–1.00) | 0.88 (0.78–1.03) | 0.86 (0.74–1.02) |
| SBP, mm Hg | 120.0 (108–134) | 119.9 (96–159) | 125.9 (96–160) | 117.7 (102–150) |
| DBP, mm Hg | 65.4 (48–79) | 67.4 (55–86) | 70.9 (60–92) | 66.4 (58–79) |
| AI, % | 32.0 (14–50) | 33.3 (14–46) | 30.7 (16–53) | 31.5 (11–47) |
Abbreviations: AI, central augmentation index; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; WC, waist circumference; WHR, waist hip ratio. Values are mean and range (in parentheses) for 64 participants at baseline.
Resting BP was measured in the fasting state at baseline and at weeks 8 and 12. SBP decreased significantly in the control group by 3.3% (P=.038) and in the combination group by 4.2% (P=.034) at week 12 compared with baseline (Table II). DBP decreased significantly in the control group by 3.3% (P=.039) at week 12 compared with baseline (Table II). Although DBP decreased at the end of the study from baseline by 4.3% in the combination exercise group, this difference was not statistically significant (P=.055).
Table II.
Vascular Changes of All Participants
| Baseline | Week 8 | Week 12 | |
|---|---|---|---|
| SBP, mm Hg | |||
| No exercise | 120.06±1.707 | 120.19±2.958 | 116.06±2.584a |
| Aerobic | 119.93±4.064 | 117.67±3.727 | 120.53±4.076 |
| Resistance | 125.94±4.757 | 122.69±4.434 | 124.25±4.704 |
| Combination | 117.71±3.284 | 113.82±2.596 | 112.76±3.623a |
| DBP, mm Hg | |||
| No exercise | 65.38±1.893 | 65.75±1.687 | 63.19±1.701a |
| Aerobic | 67.40±2.054 | 66.40±2.408 | 67.60±2.244 |
| Resistance | 70.94±2.274 | 69.13±2.093 | 69.94±2.578 |
| Combination | 66.41±1.544 | 65.12±1.550 | 63.53±1.978 |
| AI, % | |||
| No exercise | 32.00±2.045 | 31.38±2.010 | 31.31±1.997 |
| Aerobic | 33.27±2.032 | 31.73±2.025 | 32.87±2.550 |
| Resistance | 30.69±2.526 | 29.25±2.489 | 29.56±2.543 |
| Combination | 31.47±2.319 | 30.29±1.746 | 29.59±2.018 |
| RHR, beats per min | |||
| No exercise | 59.75±2.407 | 60.13±2.587 | 59.88±2.434 |
| Aerobic | 60.40±1.804 | 63.67±1.703 | 58.87±1.457 |
| Resistance | 60.38±2.680 | 62.38±2.631 | 59.56±2.045 |
| Combination | 60.71±1.557 | 58.29±1.153 | 60.35±1.150 |
Abbreviations: AI, central augmentation index; DBP, diastolic blood pressure; RHR, resting heart rate; SBP, systolic blood pressure. aStatistically significant difference within group from baseline is indicated by P<.05. Values are mean and standard error of the mean for 64 participants (control group, n=16; aerobic group, n=15; resistance group, n=16; combination group, n=17).
Measures of the AI did not change significantly in any group during the 12‐week study period (Table II), and there were no significant differences between exercise interventions at any time.
Vascular Changes in Responders
When analyzing data from all 64 participants, we did not observe significant changes in SBP, DBP, or AI between the interventions. We then designated the term responders to those in the exercise intervention groups who had either improved levels (decrease >1) of SBP or AI at either week 8 or week 12 compared with baseline and those who did not show improvements were designated nonresponders. Excluding data from participants who did not respond to the treatments (nonresponders), data were then re‐analyzed for the remaining data set from participants designated as responders. The numbers were as follows: SBP, control group (n=15), aerobic group (n=10), resistance group (n=11), combination group (n=13); and AI, control group (n=16), aerobic group (n=8), resistance group (n=11), combination group (n=14).
We observed that SBP decreased significantly in the aerobic (−4%, P=.027), resistance (−5.1%, P=.04), and combination groups (−6.3%, P=.000) at week 8 compared with baseline and also at week 12 compared with baseline in the combination group (−6.3%, P=.005) in participants who were designated as responders (Figure 2). When comparing changes between groups, SBP decreased significantly more in the combination group compared with the control week at week 8 (P=.027) but was not different from the aerobic and resistance groups.
Figure 2.
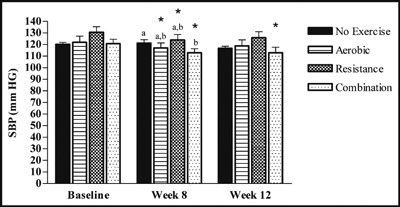
Changes of responders for systolic blood pressure (SBP). SBP was measured in the fasted state at baseline, week 8, and week 12 (control, n=15; aerobic, n=10; resistance, n=11; combination, n=13). Values are mean±standard error of the mean. Statistically significant difference from baseline indicated by *P<.05. Different letters above bar graphs indicate significant difference between groups at P<.05.
Changes to arterial stiffness were also reassessed to take into account responders. Measures of AI were significantly lower at week 8 compared with baseline in the resistance (−11.5%, P=.006) and combination (−9.3%, P=.048) groups and at week 12 compared with baseline in the aerobic (−12%, P=.047), resistance (−9.5%, P=.036), and combination (−12.7%, P=.003) exercise groups (Figure 3). When comparing between‐group changes to AI there was a significant improvement in the combination exercise group (−10.7%, P=.047) compared with the control group at week 12.
Figure 3.
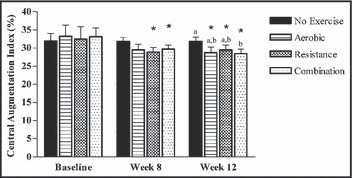
Changes of responders for central augmentation index (AI). AI was measured in the fasted state at baseline, week 8, and week‐12 (control, n=16. aerobic, n=8; resistance, n=11; combination, n=14). Values are mean±standard error of the mean. Statistically significant difference from baseline indicated by *P<.05. Different letters above bar graphs indicate significant difference between groups at P<.05.
Discussion
The aim of this study was to investigate whether 12 weeks of training with aerobic exercise, resistance exercise, or combined exercise at moderate intensity for 30 minutes, 5 d/wk would induce and sustain improvements to BP and arterial stiffness in overweight and obese individuals compared with no exercise. We did not observe any significant changes to BP or arterial stiffness between the control and exercise groups at 12 weeks when analyzing data from all 64 participants. However, some participants responded to exercise training with lower levels of SBP or AI at either week 8 or week 12 compared with baseline. These participants were designated as responders (n=49) and the data were then reanalyzed, excluding the nonresponders. In participants designated as responders, we observed a significant decrease of 6.3% in SBP in the combination group at week 12 compared with baseline. AI was significantly lower by 12% at week 12 compared with baseline in the aerobic group, significantly lower by 9.5% in the resistance group, and significantly lower by 12.7% in the combination group, as well as significantly lower by 10.7% in the combination group compared with control. These results suggest that 12 weeks of moderate training with aerobic, resistance, or combined exercise is not sufficient for improvements in vascular function in overweight and obese adults. However, some overweight and obese individuals who participate in regular combination exercise training can have improvements in BP and arterial stiffness, decreasing the risk of developing cardiovascular disease.
A study by King and colleagues 19 highlights the importance of examining subgroups in intervention studies to consider individual differences. Due to individual variability, not every person responds to a given intervention to the same extent. It is believed that some individuals voluntarily or involuntarily initiate compensatory behavior or metabolic responses to oppose the negative energy balance caused by exercise. 20 We believe the same interindividual variability may affect other measures such as BP. Therefore, for our study, patients who responded to the interventions (responders) were subjected to a separate secondary analysis.
After analyzing the responders, we observed significant within‐group decreases in SBP in the aerobic, resistance, and combination groups after 8 weeks of exercise compared with baseline and also after 12 weeks in the combination group compared with baseline (Figure 2). Our results are similar to those from previous studies who also observed significant decreases in BP compared with baseline after aerobic, 5 , 21 , 22 resistance, 22 or combination of aerobic and resistance exercise training. 23 A study by Murtagh and colleagues 24 found that 60 minutes of walking each week for 12 weeks was not sufficient for any significant changes in BP post‐intervention or compared with control. Other studies with greater amounts of aerobic or resistance exercise training reported only significant within‐group decreases in BP from initial levels. 5 , 22 Moreau and colleagues 21 measured a significant decrease in SBP from baseline in postmenopausal women with hypertension after 12 weeks of daily walking. The results from these studies suggest that improvements in BP are dependent on the amount of exercise performed.
Arterial stiffening is an independent risk factor for cardiovascular disease. 25 As people age, the central arteries gradually stiffen, 26 with the rate of progression influenced by hypertension, diabetes, and atherosclerosis. 27 The AI is an indicator of arterial stiffness and has been shown to be higher in those with hypercholesterolaemia. 28 Studies have shown an inverse relationship between habitual aerobic exercise and arterial stiffness. 29 When analyzing for responders (those who had improved measurements of AI at week 8 or week 12 compared with baseline) we found a significant between‐group difference, with a lower AI in the combination group compared with the control group at week 12 and significant within‐group decreases in arterial stiffness in the resistance, and combination exercise groups at week 8 compared with baseline and in the aerobic, resistance, and combination exercise groups at week 12 compared with baseline. Cross‐sectional studies have found a relationship between habitual aerobic exercise and a lower AI or arterial stiffness. 12 , 29 While prospective studies have also observed significant improvements in AI after exercise training, 11 , 25 most studies have found no changes, similar to our initial results. 30 , 31 However, Okamoto and colleagues 23 found that in young, healthy participants, aerobic exercise performed after resistance exercise significantly decreased arterial stiffness compared with sedentary controls, but there was no significant change in the group who performed aerobic exercise before resistance training. This may explain why we observed improvements in the combination exercise group in our study despite the resistance exercise component; however, we did not control or record whether aerobic exercise was performed before or after resistance training.
Limitations
The present study had a number of limitations. The majority of participants were women despite a higher prevalence of overweight and obesity in men in the Australian setting. 32 Since the participants were not representative of the general population, care must be taken when generalizing results to the entire Australian population. As a result of limited sample size, our study may have been underpowered to detect significant changes in some variables. For an 80% power sample, 16 participants were required in each group, but we did not achieve this number in all 4 intervention groups, since 12% of participants withdrew after the study commenced, mainly for personal reasons. In addition, since the study took place with several groups of participants staggered over a 15‐month period, seasonal changes may have been a factor because some people tend to change diet, eating patterns, and lifestyle depending on the weather. This study was demanding for participants, with a significant time commitment associated with the exercise prescription and travel to and from the university gym. Monitoring the intensity and frequency of exercise was another challenge, as participants completed exercise at different times and places. Despite the completion of exercise diaries and regular contact with the research team, we were reliant on participant honesty and accuracy in their self‐reported information. Control group participants received a non‐training–oriented intervention, which may have biased the results. Individual differences in fitness level, physical ability, and trainability may have also influenced study outcomes despite all participants being recruited as sedentary or only participating in low levels of activity.
Conclusions
Despite no significant changes in analyses including the total cohort (64 participants), a significantly greater decrease in arterial stiffness was seen in the combination exercise group compared with the control at week 12 when considering responders vs nonresponders. There was also a significant decrease in SBP in the combination group compared with the control group at week 8, although this change was no longer significant at week 12. From the results of our study, 12 weeks of moderate‐intensity exercise training did not improve vascular function in overweight and obese adults; however, we did observe that a subgroup of responders did have a favorable impact on high BP and arterial stiffness by participating in regular exercise training, which combined aerobic and resistance activity. By improving these components of vascular health, patients can decrease the risk of developing cardiovascular disease.
Acknowledgments
Acknowledgments and disclosures: SH coordinated the trial, conducted data collection, and statistical analysis and had input into the manuscript. APH and SRB had input into the writing of the manuscript and SSD provided statistical oversight. SP conceived and designed the study, supervised the study and the statistical analysis, and mentored SH. The authors declare that they have no conflicts of interest. This trial was partially funded by Curtin DRG grant and ATN Centre for Metabolic Fitness.
References
- 1. Australian Institute of Health and Welfare . Australia’s Health, 2006. Canberra, ACT: Australian Institute of Health and Welfare; 2006. [Google Scholar]
- 2. Barr ELM, Magliano DJ, Zimmet PZ, et al. AusDiab 2005: The Australian Diabetes, Obesity and Lifestyle Study. Melbourne, Vic., Australia: International Diabetes Institute; 2006. [Google Scholar]
- 3. Wassertheil‐Smoller S, Anderson G, Psaty BM, et al. Hypertension and its treatment in postmenopausal women: baseline data from the women’s health initiative. Hypertension. 2000;36:780–789. [DOI] [PubMed] [Google Scholar]
- 4. Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;14:12–17. [DOI] [PubMed] [Google Scholar]
- 5. Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE family study. Med Sci Sports Exerc. 2003;35:1703–1709. [DOI] [PubMed] [Google Scholar]
- 6. Collier SR, Kanaley JA, Carhart R Jr, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre‐ and stage‐1 hypertensives. J Hum Hypertens. 2008;22:678–686. [DOI] [PubMed] [Google Scholar]
- 7. Umpierre D, Stein R. Hemodynamic and vascular effects of resistance training: implications for cardiovascular disease. Arq Bras Cardiol. 2007;89:233–239. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka H, DeSouza CA, Seals DR. Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. [DOI] [PubMed] [Google Scholar]
- 9. Harvey PJ, Morris B, Kubo T, et al. Hemodynamic after‐effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens. 2005;23:285–292. [DOI] [PubMed] [Google Scholar]
- 10. Padilla J, Harris RA, Fly AD, et al. The effect of acute exercise on endothelial function following a high‐fat meal. Eur J Appl Physiol. 2006;98:256–262. [DOI] [PubMed] [Google Scholar]
- 11. Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–2718. [DOI] [PubMed] [Google Scholar]
- 12. Tabara Y, Yuasa T, Oshiumi A, et al. Effect of acute and long‐term aerobic exercise on arterial stiffness in the elderly. Hypertens Res. 2007;30:895–902. [DOI] [PubMed] [Google Scholar]
- 13. Miyachi M, Donato AJ, Yamamoto K, et al. Greater age‐related reductions in central arterial compliance in resistance‐trained men. Hypertension. 2002;41:130–135. [DOI] [PubMed] [Google Scholar]
- 14. Cook JN, DeVan AE, Schleifer JL, et al. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol. 2006;209:H1596–H1600. [DOI] [PubMed] [Google Scholar]
- 15. Ho SS, Dhaliwal SS, Hills A, Pal S. Effect of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese. BMC Public Health. 2012; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pal S, Ellis V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity. 2010;18:1354–1359. [DOI] [PubMed] [Google Scholar]
- 17. Ho SS, Dhaliwal SS, Hills A, Pal S. Acute exercise improves postprandial cardiovascular risk factors in overweight and obese individuals. Atherosclerosis. 2011;214:178–184. [DOI] [PubMed] [Google Scholar]
- 18. Pal S, Khossousi A, Binns C, et al. The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin and other metabolic syndrome risk factors in overweight and obese individuals. Br J Nutr. 2011;105:90–100. [DOI] [PubMed] [Google Scholar]
- 19. King NA, Hopkins M, Caudwell P, et al. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise‐induced weight loss. Int J Obes. 2008;32:177–184. [DOI] [PubMed] [Google Scholar]
- 20. King NA, Caudwell P, Hopkins M, et al. Metabolic and behavioural compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15:1373–1383. [DOI] [PubMed] [Google Scholar]
- 21. Moreau KL, Degarmo R, Langley J, et al. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc. 2001;33:1825–1831. [DOI] [PubMed] [Google Scholar]
- 22. Sarsan A, Ardic F, Özgen M, et al. The effects of aerobic and resistance exercises in obese women. Clin Rehabil. 2006;20:773–782. [DOI] [PubMed] [Google Scholar]
- 23. Okamoto T, Masuhara M, Ikuta K. Combined aerobic and resistance training and vascular function: effect of aerobic exercise before and after resistance training. J Appl Physiol. 2007;103:1655–1661. [DOI] [PubMed] [Google Scholar]
- 24. Murtagh EM, Boreham CAG, Nevill A, et al. The effects of 60 minutes of brisk walking per week, accumulated in two different patterns, on cardiovascular risk. Prev Med. 2005;41:92–97. [DOI] [PubMed] [Google Scholar]
- 25. Casey DP, Pierce GL, Howe KS, et al. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol. 2007;100:403–408. [DOI] [PubMed] [Google Scholar]
- 26. Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. [DOI] [PubMed] [Google Scholar]
- 27. Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. [DOI] [PubMed] [Google Scholar]
- 28. Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–1011. [DOI] [PubMed] [Google Scholar]
- 29. Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol. 2006;41:501–507. [DOI] [PubMed] [Google Scholar]
- 30. Stewart KJ, Bacher AC, Turner K, et al. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005;28:9–18. [DOI] [PubMed] [Google Scholar]
- 31. Rakobowchuk M, McGowan CL, de Groot PC, et al. Effect of whole body resistance training on arterial compliance in young men. Exp Physiol. 2005;90:645–651. [DOI] [PubMed] [Google Scholar]
- 32. Australian Institute of Health and Welfare . Obesity Trends in Older Australians. Canberra, ACT: Australian Institute of Health and Welfare; 2004. [Google Scholar]


