Abstract
The blood pressure (BP) effects of naproxcinod and naproxen were assessed in an 8‐week, double‐blind, crossover study in 131 hypertensive patients aged 50 to 74 years. Patients received naproxcinod 750 mg twice daily or naproxen 500 mg twice daily, then the alternate treatment, each for 14 days, with placebo run‐in/washout before each active treatment period and 24‐hour ambulatory BP monitoring conducted before and after each active treatment period. Mean change from baseline in average 24‐hour systolic BP (SBP) after 2 weeks of treatment numerically favored naproxcinod 750 mg twice daily (least‐squares [LS] mean for naproxcinod minus naproxen: −1.6 mm Hg; P=.12). Post hoc analyses showed statistically significant SBP differences favoring naproxcinod for the 8 elapsed hours (LS mean: −4.4 mm Hg; P<.0001) and the 24 hours following morning dosing (LS mean: −2.4 mm Hg; P=.006). Naproxcinod may be a beneficial alternative for patients with osteoarthritis requiring nonsteroidal anti‐inflammatory drugs. J Clin Hypertens (Greenwich). 2011;13:376–384. ©2011 Wiley Periodicals, Inc.
Patients with osteoarthritis often have comorbid conditions, including hypertension, coronary artery disease, diabetes mellitus, and chronic kidney disease. Hypertension affects approximately 40% to 50% of patients with osteoarthritis 1 and places patients at risk for premature renal and cardiovascular disease (CVD). 2 Nonsteroidal anti‐inflammatory drugs (NSAIDs) commonly used to treat osteoarthritis have the potential to increase blood pressure (BP), 3 , 4 , 5 , 6 , 7 as well as to disrupt BP control in patients treated with antihypertensive agents. 8 , 9
Inhibition of cyclooxygenase (COX) 2 enzymes by selective COX‐2 inhibitors or traditional NSAIDs provides anti‐inflammatory efficacy, but also results in a reduction of prostaglandin synthesis, which is associated with both antinatriuretic and vasoconstrictive effects. 10 , 11 , 12 The resulting imbalance between vasodilation and vasoconstriction may lead to increased systemic vascular resistance, which can elevate BP, interfere with the action of antihypertensive agents, and potentially increase hypertension‐related morbidity. 3 , 4 , 6 , 8 NSAIDs (both selective and nonselective) may increase BP, adversely affect kidney function, and increase cardiovascular risk. Indeed, these agents are associated with significant increases in the risk of serious cardiovascular events such as cardiovascular death, myocardial infarction, and stroke. 13 This was particularly evident with the selective COX‐2 inhibitor rofecoxib, which was removed by its manufacturer in September 2004 because of concerns of increasing risk for serious cardiovascular events. 14 Following this, the US Food and Drug Administration released a supplemental request to all manufacturers of NSAIDs and COX‐2 inhibitors, prescription or over‐the‐counter, to add information to the label that highlights the potential for cardiovascular events when using these agents. 15 Thus, caution is recommended when they are used in patients with hypertension, diabetes, kidney disease, or other conditions associated with increased cardiovascular risk. 16
Naproxcinod is a COX‐inhibiting nitric oxide donator (CINOD) with analgesic, anti‐inflammatory, antipyretic, and nitric oxide (NO)–donating effects. NO exerts many beneficial effects on the cardiovascular system, including modulation of BP and vascular tone, inhibition of platelet aggregation and leukocyte adhesion, and prevention of smooth muscle cell proliferation. 17 Reduced bioavailability of NO is thought to be one of the central common factors in CVD, 18 although the exact relationship is not fully understood. Provision of NO, through its effects on vascular tone and other NO‐mediated mechanisms, may be expected to ameliorate some of the BP increase commonly associated with NSAID use. This is supported by evidence from preclinical studies 19 , 20 , 21 and a clinical study 22 that demonstrated a lesser effect on BP for naproxcinod 375 mg twice daily and 750 mg twice daily compared with naproxen 500 mg twice daily. Therefore, naproxcinod may represent a potentially safer alternative therapy for patients who require NSAIDs, particularly those who have existing hypertension or borderline high BP levels.
Although cuff BP measurements are commonly used to assess BP in the clinical setting, ambulatory BP monitoring (ABPM) has been shown to be a better predictor of cardiovascular risk. 23 , 24 In the present study, we investigated the pharmacodynamic effect of NO donation on BP by comparing the effect of naproxcinod 750 mg twice daily with that of the equimolar dose of naproxen, 500 mg twice daily, on 24‐hour BP measured by ABPM. Once absorbed, naproxcinod is rapidly cleaved to release naproxen and an NO‐donating moiety. Therefore, equimolar doses of naproxcinod and naproxen were used to determine the effect of the NO released by naproxcinod.
Methods
The study was conducted in accordance with the protocol, the International Conference on Harmonization Consolidated Guideline for Good Clinical Practice, 21 Code of Federal Regulations, and the Declaration of Helsinki. All patients provided dated and signed written informed consent prior to initiation of any study procedures. The protocol and study materials were approved by a central institutional review board (Mid*Lands IRB, Leawood, KS).
Study Design
This was an 8‐week, exploratory phase 1, double‐blind, randomized, crossover study. The primary objective was to characterize the 24‐hour arterial BP profile of naproxcinod 750 mg twice daily as measured by ABPM, compared with baseline and naproxen 500 mg twice daily after 14 days of administration in volunteers with stable essential hypertension who were not chronic NSAID users. The secondary objective was to evaluate the general safety and tolerability of naproxcinod 750 mg twice daily.
After a 14‐day placebo run‐in period, patients were randomly assigned in a 1:1 ratio to one of two treatment sequences (Figure 1). Patients received naproxcinod 750 mg twice daily or naproxen 500 mg twice daily for 14 days, then placebo twice daily for 14 days, followed by a 14‐day period of treatment with the alternate active therapy. Arterial BP was measured using 24‐hour ABPM at the beginning and end of each active treatment period.
Figure 1.
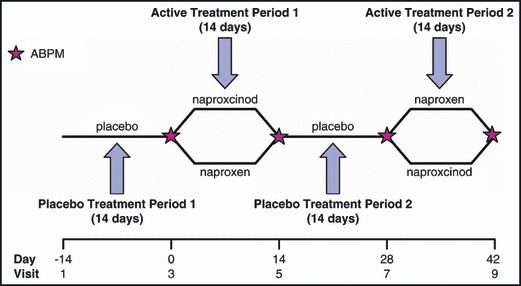
Study design. ABPM indicates ambulatory blood pressure monitoring.
Participants and investigators were blinded to study treatment assignments. To ensure blinding, oral capsules of each active treatment and the corresponding placebo were identical in appearance, smell, taste, and packaging. Patients took capsules of the active agent and the alternate placebo at each dose. They were instructed to take study medication with food and other prescribed medications in the morning and evening, preferably with a 12‐hour interval between doses.
The primary end point was the mean change from baseline in the average 24‐hour SBP at the end of 2 weeks of treatment. Secondary end points included the mean change from baseline after 2 weeks of treatment in the average 24‐hour diastolic BP (DBP) and the average daytime (6 am–10 pm) and average nighttime (10:01 pm–5:59 am) SBP and DBP.
Patients
Male and female volunteers aged 50 to 74 years with controlled hypertension (defined in this study as SBP <150 mm Hg and DBP<95 mm Hg at screening and baseline) were enrolled. Patients could be receiving ≤2 classes of antihypertensive drugs at stable doses (no dose changes of ≥50% or changes due to lack of efficacy or worsening of disease within the previous 3 months) at screening and could not be currently taking NSAIDs, including aspirin (unless ≤162 mg/d), on a long‐term basis. Patients were also excluded if their antihypertensive medication dose exceeded the recommended dose, they had nondominant arm circumference <24 cm or >42 cm, they were expected to require any new medications during the study, or had uncontrolled diabetes, hepatic dysfunction, or history of an acute cardiovascular event within the past year. Other exclusion criteria included a history of gastroduodenal bleeding or ulceration within the past 5 years, clinically relevant abnormalities on electrocardiography, hypersensitivity or contraindications to NSAIDs or organic nitrate drugs, history of renal impairment, history of alcohol or drug abuse within the past 2 years, and current use of phosphodiesterase type‐5 inhibitors, nitrates, other NO‐donating drugs, or anticoagulants.
Assessments
Ambulatory BP Monitoring. ABPM was performed using a Spacelabs Medical Model 90207 ABPM device (Issaquah, WA). A total of 4 ABPM assessments were specified by the protocol, which were performed at the beginning and end of each active treatment period, with the first ABPM performed at the time of study drug intake (approximately 8:30 am [±45 minutes]). BP measurements were recorded every 20 minutes during the daytime (6 am–10 pm) and hourly during the nighttime (10:01 pm–5:59 am).
Ideally, each patient had 56 BP measurements per 24‐hour ABPM (48 daytime, 8 nighttime). An acceptable ABPM consisted of >45 valid readings during a session of at least 23.5 hours, with ≤3 consecutive invalid daytime readings and ≤2 consecutive invalid nighttime readings. Unacceptable ABPM assessments were repeated only once. Treatment compliance was monitored by accounting for the amounts of study drug dispensed and returned before and after each active treatment period.
Safety. Safety was assessed by monitoring for adverse events (AEs) from the screening visit to 7 days after the last dose of study drug. Investigators documented the seriousness, severity, action taken, and relationship to study drug for each AE. Heart rate, electrocardiography, body mass index, and laboratory parameters were also routinely evaluated throughout the study.
Statistical Methods
The modified intent‐to‐treat (mITT) population, which included all randomized patients who had ≥1 acceptable follow‐up ABPM, was used for the ABPM analysis. The average 24‐hour ABPM data were based on an average of the hourly means recorded during the 24‐hour period, based on clock hours. An analysis of variance (ANOVA) model for a 2‐treatment, 2‐period crossover design, with fixed effects for treatment and period (as within‐subject terms), fixed effects of treatment sequence (as a between‐subject factor), and random effects for subject nested within sequence, was used to determine the difference in the primary end point between the two treatments. A P value ≤.05 for treatment effect determined statistical significance. Carryover effect was assessed by testing the treatment sequence effect against the between‐subject error term. A P value ≤.10 was considered statistically significant for this comparison. With no carryover effect between the active treatment periods, ABPM data from both active treatment periods could be used to compare the effects of the two treatments.
The analysis of the primary end point was repeated using descriptive statistics for the following subgroups: site, age group (younger than 65 vs 65 years and older), sex, race (black, African American, or of African heritage vs all other races), ethnicity (Hispanic vs non‐Hispanic), antihypertensive drug classes, and low‐dose aspirin use.
To further investigate the BP profile of naproxcinod relative to naproxen, the following post hoc analyses were performed to assess the differences between treatments: (1) the average 24‐hour SBP at the end of 2 weeks of treatment using the average of the hourly means for each elapsed hour following the morning dose, and (2) the average 8‐hour SBP at the end of 2 weeks of treatment using the average of the hourly means for the 8 elapsed hours following the morning dose.
For each of these variables, an ANOVA model with treatment sequence, period, and treatment as fixed effects, and subject within sequence as a random effect was conducted. Carryover effect was assessed as described above.
A sample size of 102 patients was required to detect a statistically significant difference in the primary end point between the 2 treatments, assuming a difference of 3 mm Hg, with a standard deviation (SD) of 10 mm Hg. These calculations were based on 1‐way repeated measures with an α level of 0.05 and a power of 85%. Assuming a drop‐out rate of 15% over the course of the study, 120 patients were needed (60 in each treatment sequence).
Safety analyses were based on the safety population (all patients receiving ≥1 dose of investigational product) and were primarily performed using standard summary statistics.
Results
Patient Disposition
Participants were recruited from 15 sites in the United States between April 27 and September 13, 2006. A total of 131 patients were randomized and included in the safety population, and 117 completed the study (117 of 131 [89.3%]). The mITT population comprised 121 patients, and 116 of these (95.9%) completed the study. In the safety population, 4 patients assigned to the naproxcinod to naproxen sequence and 10 patients assigned to the naproxen to naproxcinod sequence discontinued study treatment. Reasons for discontinuation in the naproxcinod to naproxen sequence were AE (n=1), withdrew consent (n=2), and other (n=1). In the naproxen to naproxcinod sequence, reasons for discontinuation were AE (n=1), withdrew consent (n=4), and other (n=5).
Demographic and Baseline Characteristics. Patient baseline characteristics are shown in Table I. Overall, approximately half of the patients were men, about one quarter were 65 years and older, about one quarter had diabetes, and about one third were taking low‐dose aspirin. In the mITT population, there were no statistically significant differences between treatment sequences in demographic or other baseline characteristics. A total of 5 patients, 1 in the naproxen to naproxcinod sequence and 4 in the naproxcinod to naproxen sequence, were taking >2 antihypertensive drug classes at baseline, which were treated as protocol deviations, and the patient data was included in the analysis.
Table I.
Baseline Characteristics (Modified Intent‐to‐Treat Population)
| Characteristic | Naproxcinod to Naproxen (n=61) | Naproxen to Naproxcinod (n=60) | Total (N=121) | P Value |
|---|---|---|---|---|
| Men, No. (%) | 34 (55.7) | 25 (41.7) | 59 (48.8) | .15 |
| Mean age, y (SD) | 59.7 (6.7) | 60.2 (7.0) | 59.9 (6.8) | .66 |
| Age ≥65 y, No. (%) | 17 (27.9) | 15 (25.0) | 32 (26.4) | .84 |
| Mean weight, kg (SD) | 89.7 (23.0) | 86.7 (17.5) | 88.2 (20.4) | .43 |
| Mean body mass index, kg/m2 (SD) | 31.0 (6.1) | 30.8 (6.2) | 30.9 (6.1) | .90 |
| Low‐dose aspirin use, No. (%) | 22 (36.1) | 17 (28.3) | 39 (32.2) | .44 |
| Diabetes, No. (%) | 16 (26.2) | 14 (23.3) | 30 (24.8) | .83 |
| Race, No. (%) | ||||
| White | 51 (83.6) | 53 (88.3) | 104 (86.0) | .53 |
| Black/African American | 9 (14.8) | 7 (11.7) | 16 (13.2) | |
| Native Hawaiian/Pacific Islander | 1 (1.6) | 0 (0.0) | 1 (0.8) | |
| Antihypertensive drug classes, No. (%) | ||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | .10 |
| 1 | 27 (44.3) | 19 (31.7) | 46 (38.0) | |
| 2 | 30 (49.2) | 40 (66.7) | 70 (57.9) | |
| ≥3 | 4 (6.6) | 1 (1.7) | 5 (4.1) | |
Abbreviation: SD, standard deviation.
ABPM Results
For naproxcinod 750 mg twice daily, the mean±SD 24‐hour average SBP was 125.1±10.92 mm Hg at baseline and 127.2±10.34 mm Hg at the end of active treatment. The least‐squares (LS) mean change from baseline after 2 weeks of treatment was 2.0 mm Hg (95% confidence interval [CI]: 0.29–3.75). For naproxen 500 mg twice daily, the mean±SD 24‐hour average SBP was 126.0±12.11 mm Hg at baseline and 130.4±13.36 mm Hg at the end of active treatment. The LS mean change from baseline after 2 weeks of treatment was 3.7 mm Hg (95% CI, 1.91–5.43) (Table IIa). The LS mean difference between the two treatments in the change from baseline in the average 24‐hour SBP after 2 weeks of treatment (the primary end point) numerically favored naproxcinod 750 mg twice daily, but did not reach statistical significance (LS mean difference, −1.6 mm Hg; 95% CI, −3.75 to 0.45; P=.12) (Table IIa).
Table II.
Baseline and Mean Change From Baseline in the 24‐Hour Average Systolic BP and Diastolic BP at the End of Active Treatment by Treatment (Modified Intent‐to‐Treat Population)
| Naproxcinod 750 mg twice daily (n=121) | Naproxen 500 mg twice daily (n=121) | Differencea | P Value | |
|---|---|---|---|---|
| Primary Variable | ||||
| 24‐Hour systolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean systolic BP, mm Hg (SD) | 125.1 (10.92) | 126.0 (12.11) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean systolic BP, mm Hg (SD) | 127.2 (10.34) | 130.4 (13.36) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | 2.0 (0.29–3.75) | 3.7 (1.91–5.43) | −1.6 (−3.75 to 0.45) | .12 |
| Carryover effect | .49 | |||
| Secondary Ambulatory BP Monitoring Variables | ||||
| 24‐Hour diastolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean diastolic BP, mm Hg (SD) | 73.8 (8.54) | 73.7 (8.25) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean diastolic BP, mm Hg (SD) | 73.9 (8.26) | 76.0 (8.83) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | 0.2 (−0.92 to 1.22) | 2.0 (0.88–3.05) | −1.8 (−3.13 to −0.50) | .008 |
| Carryover effect | .73 | |||
| Daytime systolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean systolic BP, mm Hg (SD) | 128.7 (11.07) | 129.8 (12.34) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean systolic BP, mm Hg (SD) | 131.5 (10.73) | 135.1 (13.48) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | 2.8 (0.94–4.61) | 4.5 (2.63–6.35) | −1.7 (−3.96 to 0.54) | .13 |
| Carryover effect | .33 | |||
| Daytime diastolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean diastolic BP, mm Hg (SD) | 76.7 (9.11) | 77.0 (9.09) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean diastolic BP, mm Hg (SD) | 77.2 (8.92) | 79.6 (9.27) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | 0.6 (−0.57 to 1.77) | 2.2 (1.01–3.38) | −1.6 (−3.12 to −0.07) | .04 |
| Carryover effect | .50 | |||
| Nighttime systolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean systolic BP, mm Hg (SD) | 117.7 (12.78) | 118.1 (13.47) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean systolic BP, mm Hg (SD) | 118.2 (11.89) | 120.8 (15.27) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | 0.5 (−1.56 to 2.55) | 2.2 (0.09–4.25) | −1.7 (−4.36 to 1.01) | .22 |
| Carryover effect | .87 | |||
| Nighttime diastolic BP | ||||
| Baseline | ||||
| No. | 110 | 111 | ||
| Mean diastolic BP, mm Hg (SD) | 67.8 (9.01) | 66.8 (8.21) | ||
| End of treatment | ||||
| No. | 109 | 108 | ||
| Mean diastolic BP, mm Hg (SD) | 67.1 (8.52) | 68.6 (9.56) | ||
| Change from baseline at the end of treatment | ||||
| No. | 103 | 100 | ||
| LS mean, mm Hg (95% CI) | −0.7 (−2.01 to 0.59) | 1.5 (0.18–2.81) | −2.2 (−3.82 to −0.58) | .009 |
| Carryover effect | .83 | |||
Abbreviations: bid, twice per day; BP, blood pressure; CI, confidence interval; daytime, 6 am–10 pm; LS, least‐squares; nighttime, 10:01 pm–5:59 am; SD, standard deviation. aNaproxcinod group – naproxen group.
For naproxcinod 750 mg twice daily, the mean±SD 24‐hour average DBP was 73.8±8.54 mm Hg at baseline and 73.9±8.26 mm Hg at the end of active treatment. The LS mean change from baseline after 2 weeks of treatment was 0.2 mm Hg (95% CI, −0.92 to 1.22). For naproxen 500 mg twice daily, the mean±SD 24‐hour average DBP was 73.7±8.25 mm Hg at baseline and 76.0±8.83 mm Hg at the end of active treatment. The LS mean change from baseline after 2 weeks of treatment was 2.0 mm Hg (95% CI, 0.88–3.05). There was a statistically significant difference in favor of naproxcinod 750 mg twice daily as compared with naproxen 500 mg twice daily (LS mean difference, −1.8 mm Hg; 95% CI, −3.13 to −0.50; P=.008) (Table IIb). No carryover effect between treatment periods was observed for SBP or DBP.
There was a statistically significant difference in favor of naproxcinod 750 mg twice daily as compared with naproxen 500 mg twice daily for the mean change from baseline after 2 weeks of active treatment in the average daytime DBP and average nighttime DBP recorded during 24‐hour ABPM (Table IIb). The LS mean change in daytime and nighttime SBP was numerically smaller for naproxcinod 750 mg twice daily compared with naproxen 500 mg twice daily, but the difference between the groups did not reach statistical significance (Table IIb).
Analysis of the primary end point using descriptive statistics by age, sex, antihypertensive drug class (except angiotensin II antagonists), diuretic use, and low‐dose aspirin use also revealed a smaller increase in 24‐hour SBP for the naproxcinod group than with the naproxen group. The analyses by race and ethnicity did not reveal the same trend (data not shown).
The SBP profiles of naproxcinod 750 mg twice daily and naproxen 500 mg twice daily were evaluated graphically in a post hoc analysis using the 24‐hour average SBP values (based on elapsed hours following the morning dose) obtained after 2 weeks of treatment (Figure 2a). Examination of the mean changes from baseline revealed that patients treated with naproxcinod 750 mg twice daily had lower average hourly SBP values for 19 of the 24 elapsed hours following the morning dose compared with those treated with naproxen 500 mg twice daily (Figure 2b).
Figure 2.
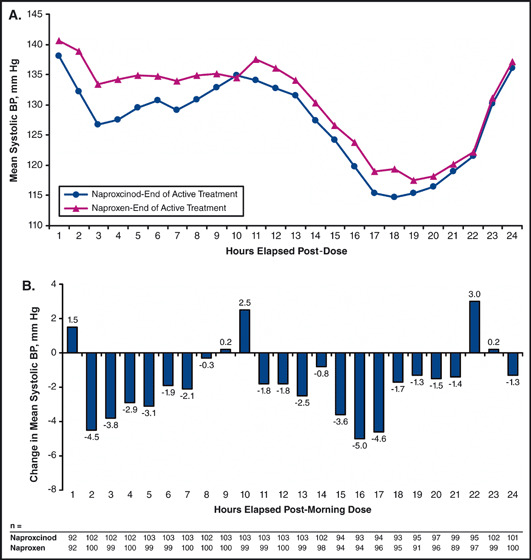
End‐of‐treatment systolic blood pressure (BP) profiles for naproxcinod and naproxen (A) and mean hourly differences between the end‐of‐treatment systolic BP profiles after adjustment for baseline differences (modified intent‐to‐treat population) (B). End of treatment baseline, naproxcinod – naproxen.
At the end of 2 weeks of active treatment, the LS mean SBP for the 24 elapsed hours following the morning dose was 128.0 mm Hg (95% CI, 125.8–130.3) with naproxcinod 750 mg twice daily and 130.5 mm Hg (95% CI, 128.2–132.7) with naproxen 500 mg twice daily. There was a difference of −2.4 mm Hg (95% CI, −4.16 to −0.71; P=.006) in favor of naproxcinod 750 mg twice daily compared with naproxen 500 mg twice daily (Figure 3). The LS mean hourly SBP for the 8 elapsed hours following the morning dose was lower with naproxcinod 750 mg twice daily than with naproxen 500 mg twice daily after 2 weeks of active treatment: 131.2 mm Hg (95% CI, 128.9–133.5) for naproxcinod and 135.7 mm Hg (95% CI, 133.4–138.0) for naproxen, a difference of −4.4 mm Hg (95% CI, −6.40 to −2.49; P<.0001) (Figure 3) in favor of naproxcinod 750 mg twice daily.
Figure 3.
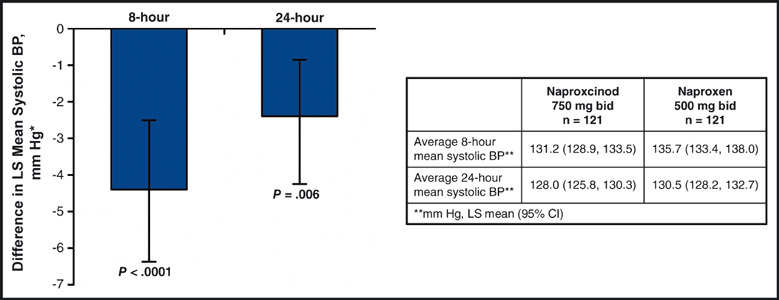
Difference in least‐squares (LS) mean average 24‐hour systolic blood pressure (BP) and LS mean average 8‐hour post‐morning dose (elapsed hours) systolic BP between naproxcinod and naproxen at the end of active treatment (modified intent‐to‐treat population). *Between‐group difference in average hourly systolic BP at the end of active treatment (naproxcinod – naproxen). Error bars indicate 95% confidence interval (CI). bid indicates twice daily.
Safety Results
Compliance and Exposure. Compliance to study medication was high throughout the study. All patients took ≥80% of naproxcinod or naproxen treatment, except for 1 patient in the naproxcinod to naproxen sequence. Study drug exposure in the safety population was similar between the treatments. The mean durations of exposure for naproxcinod and naproxen were 14.7 days and 15.1 days, respectively.
Adverse Events. The overall incidence of patients who experienced ≥1 AE was 13.0% (16 of 123) during treatment with naproxcinod 750 mg twice daily and 9.4% (12 of 128) during naproxen 500 mg twice‐daily treatment. Most AEs were of mild or moderate severity. The most frequently reported AEs by organ system were gastrointestinal system disorders. Three patients in each treatment group experienced ≥1 treatment‐related AE. Treatment‐related diarrhea and dyspepsia were experienced by 1 patient in each of the treatment groups. Fluid retention was observed in 1 patient receiving naproxcinod, and an increase in BP of moderate severity was observed in 1 patient during naproxen treatment. During naproxen treatment, 2 patients experienced dizziness and 1 patient each experienced dry mouth, flatulence, and malaise. The only serious AE was an acute myocardial infarction during the second placebo washout period in a patient assigned to the naproxcinod to naproxen treatment sequence. This AE was considered unrelated to study treatment and led to discontinuation from the study. One other AE led to study discontinuation: an increase in BP during naproxen treatment that was moderate in severity and related to study treatment. There were no deaths during the study, and standard clinical and laboratory assessments did not reveal any safety concerns with naproxcinod or naproxen.
Discussion
In this study, naproxcinod 750 mg twice daily appeared to have less of an effect on BP, as measured by ABPM, compared with naproxen 500 mg twice daily after 2 weeks of treatment in volunteer patients with stable, controlled hypertension who were not long‐term NSAID users. The mean change from baseline in the average 24‐hour SBP showed an increase for both treatment groups, but the increase was lower for naproxcinod 750 mg twice daily compared with naproxen 500 mg twice daily. After 2 weeks of treatment, the mean BP levels observed with naproxcinod 750 mg twice daily were consistently lower than those observed with naproxen 500 mg twice daily through most of the 24‐hour period. Statistically significant differences between the treatment groups favoring naproxcinod 750 mg twice daily were observed in the mean change from baseline in the average 24‐hour, daytime, and nighttime DBP. In a post hoc analysis, the average SBP observed with naproxcinod 750 mg twice daily during the 8 elapsed hours following the morning dose was statistically significantly lower than that seen with naproxen 500 mg twice daily, and the average SBP observed during 24 hours after the morning dose (elapsed hours) was also statistically significantly lower with naproxcinod 750 mg twice daily compared with naproxen 500 mg twice daily. Although pain relief was not addressed in this study, the doses of naproxen 500 mg twice daily and naproxcinod 750 mg twice daily have previously been shown to provide equal analgesic potency in a study of patients with osteoarthritis. 25
The potential for developing hypertension or worsening BP control in patients who receive NSAID therapy is an important clinical concern. An NSAID that effectively relieves the signs and symptoms of osteoarthritis with less effect on BP would be an important addition to current treatment options, particularly for patients with hypertension. Renal inhibition of COX‐2 by NSAIDs results in decreased prostaglandin synthesis and is associated with both antinatriuretic and vasoconstrictor effects as well as a decrease in renal function. 10 , 11 , 12 These effects can disrupt BP control and are especially relevant in patients who have preexisting hypertension, edema, or congestive heart failure. 26 Meta‐analyses of clinical trials of NSAIDs in patients with arthritis have demonstrated that many agents within the class (eg, ibuprofen, indomethacin, and naproxen) may increase mean arterial BP by approximately 5 mm Hg to 6 mm Hg in patients with hypertension. 4 , 5 , 6 In older patients, sustained BP elevations are associated with increased risk for both ischemic and hemorrhagic stroke, congestive heart failure, renal dysfunction, and ischemic cardiac events. 27 , 28
This study evaluated the effect of NO donation on BP in patients receiving NSAIDs. Naproxcinod, an investigational drug, was compared with naproxen, an approved NSAID commonly used in the management of osteoarthritis. The selected naproxcinod dose of 750 mg was chosen to evaluate the effect of the NO moiety, as this dose has been shown to be effective and well tolerated in patients with osteoarthritis in phase 2 studies. 22 , 29 In addition, this dose of naproxcinod is equimolar in its naproxen moiety to the active comparator naproxen 500 mg twice daily, which is a commonly recommended dose for the treatment of signs and symptoms of osteoarthritis. A crossover design was employed to decrease the impact of interindividual variability on the results. The study was powered for a 3‐mm Hg difference in SBP, which was not observed. Although the P value for the differences in the 24‐hour SBP end point was encouraging (.12) it did not reach statistical significance, although several secondary end points, including 24‐hour DBP, were significant. The estimate of 3 mm Hg was based on differences noted in office‐based BP evaluations. The use of ABPM often results in lower BP readings when compared with office‐based measures. 30 The patients in this study were relatively young (mean age, younger than 60 years) and had well controlled BP on their antihypertensive regimens, which may also have blunted the degree of SBP increase that was anticipated since older patients are prone to BP increases. 31 , 32
ABPM was used to compare the effect of naproxcinod and naproxen on BP profiles. Although cuff BP measurements are typically employed in clinical practice, they are often not representative of BP fluctuations that occur during the course of the day and night. The multiple BP readings generated by ABPM are obtained during normal activities and provide an estimate of the BP burden over a 24‐hour period. 33 ABPM has been shown to be a more valuable predictor of cardiovascular risk than office‐based BP monitoring in patients with treated 34 and untreated 23 , 24 hypertension.
Limitations
The nature of the phase 1, exploratory study warrants caution against extrapolating the results to the clinical setting. The short duration of the active treatment periods (2 weeks) does not provide information about the long‐term BP effects of naproxcinod, which may be prescribed for relief of chronic pain and inflammation due to osteoarthritis. In addition, the study population excluded patients who were regular users of NSAIDs; therefore, the BP results may not be completely applicable to a population of patients with osteoarthritis who have been exposed to NSAIDs on a long‐term basis.
Conclusions
In this short‐term study, the incidence of AEs and serious AEs was low for both active treatments. Naproxcinod 750 mg twice daily and naproxen 500 mg twice daily were safe and well tolerated. The low incidence of treatment‐related AEs suggests that the BP effects of naproxcinod do not come at a cost to patient safety. The results of this exploratory study suggest that naproxcinod exhibits less effect on BP relative to naproxen in patients with stable, treated hypertension who are not long‐term NSAID users and supports studies that assess the BP effects of longer‐term use of naproxcinod in patients with hypertension.
Acknowledgments
Acknowledgments and disclosures: The authors thank Janet Manfre of in Science Communications, a Wolters Kluwer business, for editorial assistance in the preparation of this manuscript. This assistance was funded by NicOx S.A. Study Investigators: Robert Broker, Hillcrest Clinical Research LLC, Simpsonville, SC; Deanna Cheung, Memorial Research Medical Clinic, Long Beach, CA; John E. Ervin, The Center for Pharmaceutical Research, Kansas City, MO; Thomas M. Hyers, C.A.R.E. Clinical Research, St Louis, MO; Dean Kereiakes, The Lindner Clinical Trial Center, Cincinnati, OH; Thomas W. Littlejohn, III, Piedmont Medical Research Associates, Winston‐Salem, NC; Andres Patron, Andres Patron, DO, PA, Pembroke Pines, FL; Henry A. Punzi, Trinity Hypertension Research Institute, Punzi Medical Center, Carrollton, TX; Sherwyn Schwartz, Diabetes & Glandular Disease Research Associates PA, San Antonio, TX. This study was supported by NicOx, S.A. The authors disclose the following potential conflicts of interest: Raymond Townsend, MD, has served as a consultant for NicOx. Neville Bittar, MD, was a paid investigator for the study discussed in this manuscript. Jeffrey Rosen, MD, and William Smith, MD, received a clinical grant from NicOx to perform the trial discussed in this manuscript. Andrea Ramsay, MD, has nothing to disclose. Steven G. Chrysant, MD, has received funds from the company regarding the conduct of the study and has received consultation fees from NicOx. Robert Weiss, MD, has nothing to disclose. Brigitte Duquesroix, MD, and Jacques Djian, MD, are employees of NicOx S.A, Sophia‐Antipolis, France, and have stock options. Aldina Pivodic, MSc, is a former employee of NicOx S.A, Sophia‐Antipolis, France.
References
- 1. Singh G, Miller JD, Lee FH, et al. Prevalence of cardiovascular disease risk factors among US adults with self‐reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8(15 suppl):S383–S391. [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 3. Johnson AG. NSAIDs and blood pressure. Clinical importance for older patients. Drugs Aging. 1998;12:17–27. [DOI] [PubMed] [Google Scholar]
- 4. Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti‐inflammatory drugs affect blood pressure? A meta‐analysis. Ann Intern Med. 1994;121:289–300. [DOI] [PubMed] [Google Scholar]
- 5. Morrison A, Ramey DR, van Adelsberg J, Watson DJ. Systematic review of trials of the effect of continued use of oral non‐selective NSAIDs on blood pressure and hypertension. Curr Med Res Opin. 2007;23:2395–2404. [DOI] [PubMed] [Google Scholar]
- 6. Pope JE, Anderson JJ, Felson DT. A meta‐analysis of the effects of nonsteroidal anti‐inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–484. [PubMed] [Google Scholar]
- 7. Whelton A, White WB, Bello AE, et al. Effects of celecoxib and rofecoxib on blood pressure and edema in patients > or =65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol. 2002;90:959–963. [DOI] [PubMed] [Google Scholar]
- 8. Chrischilles EA, Wallace RB. Nonsteroidal anti‐inflammatory drugs and blood pressure in an elderly population. J Gerontol. 1993;48:M91–M96. [DOI] [PubMed] [Google Scholar]
- 9. Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am J Hypertens. 2000;13:1161–1167. [DOI] [PubMed] [Google Scholar]
- 10. Brater DC. Effects of nonsteroidal anti‐inflammatory drugs on renal function: focus on cyclooxygenase‐2‐selective inhibition. Am J Med. 1999;107:65S–70S; discussion 70S–71S. [DOI] [PubMed] [Google Scholar]
- 11. Whelton A. Nephrotoxicity of nonsteroidal anti‐inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. [DOI] [PubMed] [Google Scholar]
- 12. Whelton A, Schulman G, Wallemark C, et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med. 2000;160:1465–1470. [DOI] [PubMed] [Google Scholar]
- 13. Fosbol EL, Folke F, Jacobsen S, et al. Cause‐specific cardiovascular risk associated with nonsteroidal antiinflammatory drugs among healthy individuals. Circ Cardiovasc Qual Outcomes. 2010;3:395–405. [DOI] [PubMed] [Google Scholar]
- 14. Simon LS, White WB. COX‐2 selective inhibitors and heart health. Postgrad Med. 2005;117(1 suppl):7–20. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . COX‐2 Selective (includes Bextra, Celebrex, and Vioxx) and Non‐Selective Non‐Steroidal Anti‐Inflammatory Drugs (NSAIDs). 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM103420. Accessed January 4, 2011.
- 16. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. [DOI] [PubMed] [Google Scholar]
- 17. Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. [DOI] [PubMed] [Google Scholar]
- 18. Pepine CJ. Why vascular biology matters. Am J Cardiol. 2001;88:5K–9K. [DOI] [PubMed] [Google Scholar]
- 19. Muscara MN, McKnight W, Del Soldato P, Wallace JL. Effect of a nitric oxide‐releasing naproxen derivative on hypertension and gastric damage induced by chronic nitric oxide inhibition in the rat. Life Sci. 1998;62:PL235–PL240. [DOI] [PubMed] [Google Scholar]
- 20. Muscará MN, McKnight W, Lovren F, et al. Antihypertensive properties of a nitric oxide‐releasing naproxen derivative in two‐kidney, one‐clip rats. Am J Physiol Heart Circ Physiol. 2000;279:H528–H535. [DOI] [PubMed] [Google Scholar]
- 21. Presotto C, Bolla MI, Olivieri R, et al. HCT 3012 reduces blood pressure in the spontaneously hypertensive rat model. Arthritis Rheum. 2006;54(suppl 9):S147. Presentation 231. [Google Scholar]
- 22. Schnitzer TJ, Kivitz AJ, Lipetz RS, et al. Comparison of the COX‐inhibiting nitric oxide donator AZD3582 and rofecoxib in treating the signs and symptoms of osteoarthritis of the knee. Arthritis Rheum. 2005;53:827–837. [DOI] [PubMed] [Google Scholar]
- 23. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 24. Ohkubo T, Imai Y, Tsuji I, et al. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens. 1997;15:357–364. [DOI] [PubMed] [Google Scholar]
- 25. Schnitzer TJ, Kivitz A, Frayssinet H, Duquesroix B. Efficacy and safety of naproxcinod in the treatment of patients with osteoarthritis of the knee: a 13‐week prospective, randomized, multicenter study. Osteoarthritis Cartilage. 2010;18:629–639. [DOI] [PubMed] [Google Scholar]
- 26. White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408–418. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 28. White WB. Benefits of antihypertensive therapy in older patients with hypertension. Arch Intern Med. 2000;160:149–150. [DOI] [PubMed] [Google Scholar]
- 29. Karlsson J, Pivodic A, Aguirre D, Schnitzer TJ. Efficacy, safety, and tolerability of the cyclooxygenase‐inhibiting nitric oxide donator naproxcinod in treating osteoarthritis of the hip or knee. J Rheumatol. 2009;36:1290–1297. [DOI] [PubMed] [Google Scholar]
- 30. Gorostidi M, Sobrino J, Segura J, et al. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross‐sectional analysis of a 20,000‐patient database in Spain. J Hypertens. 2007;25:977–984. [DOI] [PubMed] [Google Scholar]
- 31. Gurwitz JH, Avorn J, Bohn RL, et al. Initiation of antihypertensive treatment during nonsteroidal anti‐inflammatory drug therapy. JAMA. 1994;272:781–786. [PubMed] [Google Scholar]
- 32. Wilson SL, Poulter NR. The effect of non‐steroidal anti‐inflammatory drugs and other commonly used non‐narcotic analgesics on blood pressure level in adults. J Hypertens. 2006;24:1457–1469. [DOI] [PubMed] [Google Scholar]
- 33. White WB. Ambulatory blood‐pressure monitoring in clinical practice. N Engl J Med. 2003;348:2377–2378. [DOI] [PubMed] [Google Scholar]
- 34. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. [DOI] [PubMed] [Google Scholar]


