Abstract
J Clin Hypertens (Greenwich). 2012; 14:346–352. ©2012 Wiley Periodicals, Inc.
Uric acid has been suspected to be a risk factor for hypertension since the 1870s. Numerous epidemiological studies demonstrate an association between uric acid and both incident and prevalent hypertension in diverse populations. Studies in elderly patients have had more variable results, raising the possibility that uric acid may be more significant to hypertension in the young. Animal models support a two‐phase mechanism for the development of hyperuricemic hypertension. Initially, uric acid induces vasoconstriction by activation of the renin‐angiotensin system and reduction of circulating nitric oxide, which can be reversed by lowering uric acid. Over time, uric acid uptake into vascular smooth muscle cells causes cellular proliferation and secondary arteriolosclerosis that impairs pressure natriuresis, causing sodium‐sensitive hypertension. Consistent with the animal model data, small clinical trials performed in adolescents with newly diagnosed essential hypertension demonstrate that at least in certain young patients, reduction of serum uric acid can mitigate blood pressure elevation. While more research is clearly necessary, the available data suggest that uric acid is likely causative in some cases of early‐onset hypertension.
The concept that uric acid might be associated with the development of hypertension is not a new one. Even in the earliest discussions of hypertension as a disease entity, uric acid was considered. In the 1870s, Frederick Mahomed 1 , 2 postulated that the problem of hypertension resulted from a circulating toxin that caused an increase in blood pressure (BP) and subsequently damaged the vasculature of the heart and kidneys. While he suggested several candidate molecules, he proposed uric acid as an important mediator and published the first sphymagraph tracings showing a patient with gout who had increased systemic BP. 2 A few years later, Alexander Haig 3 also linked uric acid with elevated BP and went so far as to write a textbook that suggested a diet that would lower uric acid and control BP in the general population. In 1897, Nathan Davis, 4 addressing the American Medical Association, argued that gout was a major cause of hypertension that manifested as arteriolar disease, interstitial renal injury, and myocardial hypertrophy. Henri Huchard, 5 a renowned cardiologist, hypothesized that arteriole sclerosis, the vascular lesion associated with hypertension, had three causes: uric acid, lead, and intake of fatty meats, the latter of which also yield increased uric acid. As early as 1913, experimental evidence supported a link between uric acid and hypertension. Injection of uric acid into rabbits was shown to increase BP. 6 In 1915, Urodonal, a drug consisting of theobromine and methenamine, was introduced in France as a treatment to lower uric acid and control BP; however, it was eventually proven to be ineffective. Nevertheless, at the end of the 19th century and the first two decades of the 20th century, uric acid was already linked with hypertension and cardiovascular diseases.
The investigation of a link between uric acid and hypertension made relatively little progress through much of the 20th century. While some of the cardiovascular risk trials measured uric acid and suggested an association between uric acid and hypertension, or cardiovascular disease (Table), the lack of plausible mechanistic evidence linking the two led most investigators to conclude that uric acid was an associated surrogate marker for more important risk factors such as obesity, diabetes, and chronic kidney disease (CKD). 7 In the 1980s, uric acid was removed from some of the common laboratory panels, markedly reducing the available epidemiologic data on uric acid in otherwise healthy patients and those with cardiovascular disease. The move was made after the majority of serious side effects from the urate‐lowering drug, allopurinol, were observed in patients with asymptomatic hyperuricemia, not gout. 8 The shift to minimize inadvertent diagnosis of hyperuricemia was thought to reduce risk of unnecessary medication side effects and reduced the awareness of the prevalence of hyperuricemia in the absence of symptomatic gout.
Table TABLE.
Epidemiology of Uric Acid and Hypertension
| Study (Year) | Population | Relative Risk of Hypertension | Reference |
|---|---|---|---|
| Israeli Heart (1972) | 10,000 Israeli men, age 17–25 y, enrolled at military induction | Two‐fold risk at 5 y | 9 |
| Fessel et al (1973) | 224 White men in Western United States, age >35 y | Greater increase in systolic blood pressure at 4 y | 60 |
| Gruskin (1985) | 55 Adolescents, racially mixed US population | Higher uric acid, higher blood pressure | 53 |
| Moscow Children’s Study (1985) | 145 Caucasian children in Moscow, age 8–17 | Uric acid >8 mg/dL predicts severe hypertension | 17 |
| Brand et al (1986) | 4286 Men and women age 35–50 y in the Framingham cohort | Uric acid, systolic blood pressure rise a linear relation | 61 |
| Hungarian Children’s (1990) | 17643 Hungarian children, age 6–19 y | Uric acid predicts adolescent hypertension | 16 |
| Kaiser Permanente (1990) | 2062 Adult men and women in the Kaiser Permanente Multiphasic Health Checkup cohort in Northern California | Two‐fold risk at 6 y | 62 |
| University of Utah (1991) | 1482 Adult men and women in 98 Utah pedigrees | Two‐fold risk at 7 y | 63 |
| NHANES (1993) | 6768 Healthy children age 6–17 y | Uric acid predicts adolescent hypertension | 18 |
| Olivetti Heart Study (1994) | 619 Adult men from Southern Italy | Two‐fold risk at 12 y | 64 |
| CARDIA study (1999) | 5115 Black men and women age 18–30 y | Increased risk at 10 y | 10 |
| Osaka Health Survey (2001) | 6356 Japanese men age 35–60 y | Two‐fold risk at 10 y | 15 |
| Hawaii‐LA‐Hiroshima Study (2001) | 140 Japanese American men age 40–69 y | 3.5‐fold risk at 15 y | 11 |
| Feig and Johnson (2003) | 175 Racially diverse children, age 6–18 y in Texas | Uric acid >5.5 mg/dL predicts hypertension | 54 |
| Osaka Factory Study (2003) | 433 Nonobese Japanese men age 18–40 y | 1.0 mg/dL, increased 27 mm Hg systolic blood pressure at 5 y | 12 |
| Osaka Health Survey (2003) | 2310 Male office workers in Japan, age 35–59 y | 1.6‐fold risk at 6 y | 14 |
| Okinawa (2004) | 4489 Japanese men and women, age >30 y | 1.7‐fold risk at 13 y | 13 |
| Bogalusa Heart (2005) | 577 Black (58%) and white (42%) children enrolled at age followed until age 18–35 y | Increased risk for diastolic hypertension at 11 y | 22 |
| Framingham (2005) | 3329 Men and women in the Framingham cohort | 1.6‐fold at 4 y | 23 |
| Normative Aging Study (2006) | 2062 Healthy men age 40–60 y at enrollment | 1.5‐fold at 21 y | 65 |
| ARIC (2006) | 9104 Mixed race (black and white) men and women age 45–64 y at enrollment | 1.5‐fold at 9 y | 66 |
| Beaver Dam Survey (2006) | 2520 White men (44%) and women (56%) age 43–84 y in Wisconsin | 1.65‐fold at 10 y | 67 |
| Health Professional Followup (2006) | 750 Mostly white men in Massachusetts | 1.08‐fold at 8 y | 68 |
| MRFIT (2007) | 3073 Men age 35–57 y | 1.8‐fold at 6 y | 69 |
| Nurses Health (2009) | 1496 Women, racially diverse, age 32–52 y | 1.9‐fold at 6 y | 70 |
| Qingdao Port Health (2009) | 7220 Men (74%) and women (26%) in Quingdoa China, mean age 37 y | 1.39 For men, 1.85 for women at 4 y | 71 |
| Jones et al (2009) | 141 Children age 7–18 y , 64% men, 71% black | 2.1‐fold risk in adolescence by ambulatory blood pressure monitoring | 72 |
| Leite et al (2010) | 1410 Men and women in Milan, Italy, young cohort 42–59 y, older cohort 60–74 | Increased risk in middle age, not elderly | 73 |
| Grayson et al (2010) | 55,607 Adults, meta‐analysis of 18 prospective studies | 1.41‐fold risk each 1 mg/dL uric acid | 74 |
| Silverstein et al (2011) | 108 Racially diverse children, age 6–18 in Texas and Washington, DC | Linear association between systolic blood pressure and uric acid in children on renal replacement therapy | 24 |
| GOCADAN (2012) | 1078 Alaskan native Americans with chronic kidney disease II or III | 1.2‐fold age‐adjusted risk | 75 |
| Fadrowski (2012) | 6036 Adolescents, age 11–17 y, evaluated in the National Health and Nutrition Examination Survey | Uric acid >5.5 mg/dL, 2.03‐fold risk | 76 |
Epidemiology
Numerous longitudinal cardiovascular risk trials have evaluated the possible relationship between serum uric acid and hypertension (Table). As early as 1972, in the Israeli Heart Trial, an evaluation of the medical data of young adults inducted into the armed services demonstrated that the highest tertile of uric acid was associated with double the risk of incident hypertension within 5 years. 9 The association is robust across racial groups, with similar findings in African Americans noted in the Coronary Artery Revascularization in Diabetes (CARDIA) trial 10 as well as several trials demonstrating the same association in Asians and Asian Americans. 11 , 12 , 13 , 14 , 15 Several studies in children and adolescents, particularly the Hungarian Children’s Study, 16 the Moscow Children’s trial, 17 and the National Health and Nutrition Examination Survey (NHANES) 18 in the 1980s and early 1990s demonstrated a particularly strong association between uric acid and hypertension despite the much lower incidence in children. Studies specifically of older and elderly patients have had much more variable results 7 , 19 , 20 , 21 and led many investigators to conclude that the association was spurious; however, an alternative explanation is that if uric acid leads to hypertension, there may be a preferential effect in the young.
In the past decade, new epidemiological studies have rekindled an interest in the link between uric acid and hypertension. Three longitudinal studies in Japanese patients showed an association between serum uric acid and incident hypertension. Nakanishi and colleagues 14 demonstrated a 1.6‐fold increased risk of new hypertension over 6 years in young adult office workers with serum uric acid in the highest tertile. Tanaguichi and associates 15 demonstrated a 2‐fold increased risk of new hypertension over 10 years associated with elevated uric acid in the Osaka Health Study. Masuo and coworkers 12 evaluated the linear association of serum uric acid and systolic BP, finding an average increase of 27 mm Hg per 1 mg/dL increase in serum uric acid among nonobese young men. In an ethnically diverse population within the Bogalusa Heart Study, higher childhood and young adult serum uric acid levels were associated with incident hypertension and progressive increase in BP even within the normal range. 22 A post hoc analysis from the Framingham Heart Study also suggested that a higher serum uric acid level is associated with increased risk of rising BP. 23 Taken together, the preponderance of evidence supports a close epidemiologic link between uric acid and hypertension that is robust across ethnic racial and anthropomorphic categories but may be attenuated in the elderly. One cautionary note that should be considered is that the paucity of recent reports of a lack of an association could be publication bias against negative studies.
Uric acid metabolism
The causes of mild to moderate hyperuricemia in the young are not well established; however, many possibilities exist and probably co‐exist. Increased uric acid can result from decreased renal function and in general, children with CKD and end‐stage renal disease have higher serum uric acid. 24 There are numerous medications that impair renal clearance of uric acid, even in the presence of normal glomerular filtration rate, including loop and thiazide diuretics. 25 Genetic polymorphisms in anion transporters such as uric acid anion transporter 1 (URAT‐1) 26 and the SLC2A9 that encodes for GLUT9, an anion transporter with affinity for uric acid, 27 can lead to hyperuricemia by altering proximal tubular urate clearance. Approximately 15% of uric acid clearance is through the GI tract; consequently, small bowel disease can also contribute increased serum uric acid. 28 Diets rich in fatty meats, seafood, and alcohol increase serum uric acid, 29 , 30 and obesity confers a 3‐fold increased risk of hyperuricemia. 31 Finally, as uric acid is the end point of the purine disposal pathway, impairment of the efficiency of purine recycling metabolism or overwhelming the recycling pathway with excessive cell death or cell turnover will increase serum uric acid. 32
Serum uric acid levels throughout the population correlate with sweetener consumption. 33 Sweetener consumption in the United States has dramatically increased since the introduction of high fructose corn syrup in the early 1970s. 34 Fructose raises uric acid rapidly via activation of the fructokinase pathway in hepatocytes. 35 Fructokinase consumes ATP, leading to an increased load of intracellular purines requiring metabolism and disposal through xanthine oxidase–mediated metabolism ending in uric acid. 35 The administration of large quantities of fructose to rats, 60% of their caloric intake, resulted in hyperuricema, elevated BP, the development of preglomerular arteriolopathy, 36 and features of the metabolic syndrome (elevated triglycerides, low high‐density lipoprotein cholesterol, abdominal obesity, and insulin resistance). 37 Furthermore, lowering uric acid prevents these changes despite ongoing fructose consumption. 34 The requirement for prodigious fructose intake in rats to raise uric acid may be because rats have uricase, an enzyme that metabolizes uric acid to allantoin, and is absent in humans.
Human studies also show that fructose loading leads to increased serum uric acid levels acutely, and that chronic increased fructose consumption leads to chronically increased serum uric acid and increases in BP. 38 With the nearly universal exposure to sweetened foods and beverages in the pediatric population, it is very likely that much of the hyperuricemia, especially that associated with obesity, is dietary rather than genetic in origin. 39 Consistent with this hypothesis, epidemiological studies have shown a relationship between fructose and serum uric acid in most but not all studies. 40 One reason some studies may be negative could reflect the action of fructose, as it tends to increase uric acid mostly in the postprandial setting and, since most studies use fasting uric acid levels, it is possible that an elevation in mean 24‐hour uric acid would be missed.
Jalal and colleagues 40 used the data from NHANES 2000–2003, which was a survey of healthy adults in the United States in which direct BP measurement was available as well as dietary intake of fructose as determined by dietary questionnaire. The major finding was that there was a strong independent relationship of fructose intake with elevated systolic BP. Interestingly, the relationship was independent of fasting serum uric acid. In a different study, Nguyen and colleagues 39 also found an independent relationship of sugary soft drinks with hypertension in adolescents. Perez‐Pozo and coworkers 41 administered 200 g of fructose per day to healthy overweight men with or without allopurinol during a 2‐week period. In this study, an increase in serum uric acid was observed in association with a significant increase in daytime systolic and both 24‐hour and daytime diastolic BP. Allopurinol reduced serum uric acid and blocked the BP rise. While the dose of fructose was very high, 25% of the NHANES cohort consumed similar quantities. 40
Animal Models of Hyperuricemic Hypertension
While significant epidemiological evidence supported the hypothesis that uric acid may be associated with hypertension, it was not until the experiments of Johnson and colleagues in 2001 that a plausible mechanism could be established using a rat model of hyperuricemia. Hyperuricemia results in hypertension within 2 weeks, with systolic BP and diastolic BP elevation proportional to serum uric acid. This effect can be ameliorated by uric acid–lowering drugs (allopurinol or benziodarone). Early hypertension is completely reversible with urate reduction, but prolonged hyperuricemia results in irreversible sodium‐sensitive hypertension that becomes uric acid–independent. 42 , 43 Early hypertension is mediated by increased renal renin and reduction of circulating plasma nitrates, 42 , 44 , 45 , 46 leading to a phenotype of excessive vasoconstriction that can be reversed by reduction of uric acid or renin‐angiotensin system blockade. The later, irreversible hypertension, is secondary to altered intra‐renal vascular architecture. Uric acid enters vascular smooth muscle cells via the URAT‐1 channel, resulting in activation of kinases, nuclear transcription factors, cyclo‐oxygenase 2 generation, and the production of growth factors (PDGF), and inflammatory proteins (C‐reactive protein, monocyte chemoattractant protein‐1), resulting in VSCM proliferation, shifted pressure natriuresis, and sodium‐sensitive hypertension 47 , 48 , 49 , 50 , 51 (Figure 1).
Figure 1.
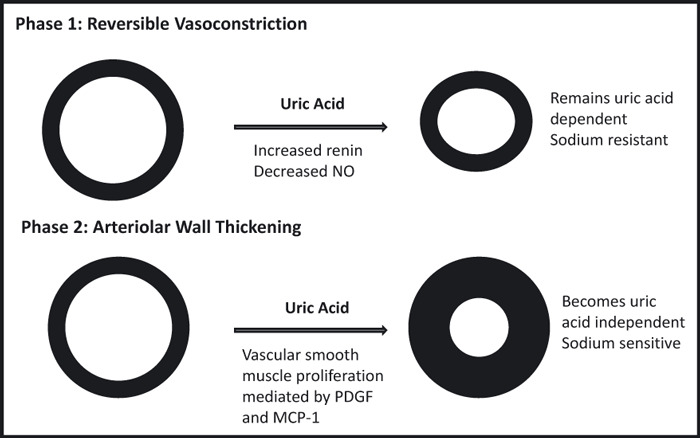
Animal model data suggest that hyperuricemia leads to hypertension in a stepwise fashion. The effects of uric acid on the blood vessel are shown. The first phase is direct, uric acid–dependent activation of the renin‐angiotensin system and down‐regulation of the nitric oxide (NO) production, leading to vasoconstriction. At this stage, uric acid reduction results in vascular relaxation and improved blood pressure. The second phase, which develops over time, is uric acid–mediated arteriolosclerosis. Uric acid uptake into vascular smooth muscle cells causes the activation and elaboration of production of growth factor (PDGF) and monocyte chemoattractant protein‐1 (MCP‐1). This results in the autocrine stimulation of vascular smooth muscle cell proliferation, vascular wall thickening, loss of vascular compliance, and a shift in pressure natriuresis. This process is not reversed by the late reduction of uric acid and causes permanent sodium‐sensitive hypertension.
These mechanistic studies, as well as the recent epidemiologic data described above, have led to a dramatic increase in the number of research publications addressing the link between uric acid and hypertension. The number had remained relatively constant from 1970 to 2000, but has been consistently rising since (Figure 2).
Figure 2.

Number of research articles published per year, 1970–2011, on the topic of the role of uric acid in hypertension. Articles were identified on PubMed using search terms uric acid, urate, hypertension, cardiovascular disease, fructose, sweetened beverages, xanthine oxidase inhibitors, allopurinol, uricosurics and probenecid. Reviews were excluded and abstracts were reviewed for relevance.
Pediatric Clinical Trials
In adolescents, there is a close association between elevated serum uric acid and the onset of essential hypertension. The Moscow Children’s Hypertension Study found hyperuricemia (>8.0 mg/dL) in 9.5% of children with normal BP, 49% of children with borderline hypertension, and 73% of children with moderate and severe hypertension. 52 The Hungarian Children’s Health Study followed 17,624 children born in Budapest in 1964 over 13 years and found that significant risk factors for the development of hypertension were elevated heart rate, early sexual maturity, and hyperuricemia. 16 These two studies did not separate the hypertensive children by underlying diagnosis, essential hypertension vs that caused by renal, cardiac, or endocrinologic causes independent of uric acid, so the relationship between serum uric and hypertension may be attenuated somewhat. In a small study, Gruskin 53 compared adolescents (13 to 18 years) who had essential hypertension with age‐matched healthy controls with normal BPs. The hypertensive children had both elevated serum uric acid (mean >6.5 mg/dL) and higher peripheral renin activity. In a racially diverse population referred for the evaluation of hypertension, Feig and Johnson 54 observed that the mean serum uric acid level children with white‐coat hypertension was 3.6±0.7 mg/dL, slightly higher in secondary hypertension (4.3±1.4 mg/dL, P=.008) and significantly elevated in children with primary hypertension (6.7±1.3mg/dL, P=.000004). There was a tight linear correlation between serum uric acid levels and systolic and diastolic BPs in the population referred for evaluation of hypertension (r=0.8 for systolic BP and r=0.6 for diastolic BP) (Figure 3). Each 1‐mg/dL increase in serum uric acid was associated with an average increase of 14 mm Hg in systolic BP and 7 mm Hg in diastolic BP. 54 Among patients referred for evaluation of hypertension, serum uric acid >5.5 mg/dL had an 89% positive predictive value for essential hypertension, while serum uric acid <5.0 had a negative predictive value for essential hypertension of 96%. 54
Figure 3.
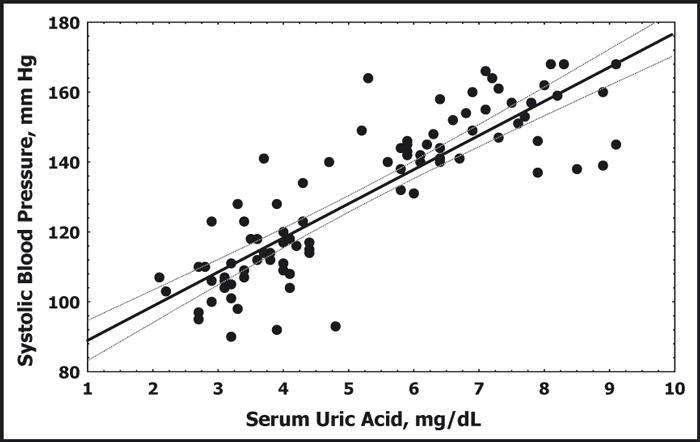
Serum uric acid is plotted against systolic blood pressure for children with normal blood pressure and children with primary hypertension. Data do not include individuals with secondary hypertension or white‐coat hypertension. The solid and dotted lines represent the best fit and 95% confidence intervals, respectively, and demonstrate the linear relation between uric acid and systolic blood pressure.54
The evaluation of a large‐referral population of children with essential hypertension and elevated uric acid reveal several observations that are consistent with the mechanisms of uric acid–mediated hypertension seen in the animal model. Among 513 children consecutively evaluated for hypertension, children with essential hypertension (n=206) had higher blood hemoglobin (14.6±1.3 g/dL) compared with those with secondary hypertension (n=176, hemoglobin 12.8±1.6) or white‐coat hypertension (n=135, hemoglobin 12.5±1.2 g/dL). In children with serum uric acid >6 mg/dL, the average hemoglobin is 15.4±1.4 g/dL. While the mechanism is not yet established, one possibility is that uric acid–mediated vasoconstriction and arteriolosclerosis, seen in the rat model, leads to decreased microvascular perfusion and increased serum erythropoetin. Polycythemia and hypertension have been previously described in patients with hyperuricemia and gout; however, it has been assumed that the elevation in uric acid was secondary to the hematopoietic disorder. 55 , 56 In obese patients with prehypertension and serum uric acid >5 mg/dL, systemic vascular resistance (measured by noninvasive bioimpedence) is elevated relative to obese children with normal uric acid and similar BP (2482±306 dynesec/cm5/m2 vs 1843±291 dynesec/cm5/m2). While these physiologic observations do not prove that the effects of increased serum uric acid are the same in humans as in rats, they are consistent with a vasoconstrictive effect due to uric acid.
Results from a small pilot study in children suggest that uric acid may directly contribute to the onset of hypertension in some humans. Five children, aged 14 to 17 years, with newly diagnosed and untreated essential hypertension were treated for 1 month with allopurinol as a solitary pharmacologic agent. All 5 children had a decrease in BP by both casual and ambulatory monitoring and 4 of the 5 were normotensive at the end of 1 month. 57 In a separate study, 30 adolescents with newly diagnosed essential hypertension were treated in a randomized, double‐blinded crossover trial with allopurinol vs placebo. Sixty‐seven percent of children taking allopurinol and 91% of children with serum uric acid <5.5 mg/dL on treatment had normal BPs compared with 3% of children taking placebo. 58 While these observations need to be confirmed in larger and more general populations, if serum uric acid is indeed directly causing renal arteriolopathy, altered regulation of natriuresis, and persistent systemic hypertension, it is a modifiable risk factor for CKD in the absence of other mechanisms.
Future Directions
The combination of epidemiological, animal model, and clinical trials support a causative role for uric acid in some patients with elevated BP. The controversy over its role stems from the lack of a plausible causative mechanism prior to 2001 and its overlap with other more conventional risk factors such as renal disease, diabetes, and obesity. More recent mechanistic studies, however, support uric acid–mediated activation of the renin‐angiotensin system, a process with rapid onset that can also be quickly controlled, followed by a more gradual alteration of renovascular geometry and sodium handling that results in chronic salt‐sensitive hypertension. The implications of this paired mechanism are two‐fold. First, it would explain the greater magnitude of effect seen in younger patients or at least the attenuation of affect in the elderly. Second, it may represent a unique opportunity in newly diagnosed hyperuricemic hypertension, in which metabolic control may delay or prevent irreversible vasculopathy and permanent future hypertension.
The link between fructose intake and serum uric acid may also hold important promise; however, while fructose loading clearly leads to increased serum uric acid and increased BP in clinical trials, the efficacy of fructose reduction has not been proven. A post hoc evaluation for the PRIMIER trial, a large trial of the efficacy of nonpharmacologic therapy for hypertension and cardiovascular risk mitigation, demonstrated that patients with the greatest reduction in sweetener consumption also had the greatest reduction in BP 59 ; however, the effect of sweetener intake reduction as monotherapy for BP has not been formally tested.
Conclusions
How best to approach mild to moderate hyperuricemia remains an open question. The currently available medications, especially allopurinol, are associated with significant, even life‐threatening, side effects that preclude its safe use in populations as large as those at risk for future hypertension. Consequently, the treatment of asymptomatic hyperuricemia or mild hypertension in the presence of hyperuricemia should not be treated with the currently available uric acid–lowering medications. As there are many classes of readily available antihypertensive medications with more optimal safety profiles, direct management of hypertension is preferable. The caveat to such an approach is the poor actual control rates in both adult and pediatric hypertension with current conventional therapies bespeak the need for novel therapeutics. Definitive, large‐scale studies, particularly randomized, double‐blinded, placebo‐controlled trials of urate‐lowering medications other than allopurinol, either xanthine oxidase inhibitors or uricosurics, are needed to prove, or refute, the general utility and safety of mitigating hypertension through uric acid control.
References
- 1. Mahomed F. The etiology of Bright’s disease and the prealbuminuric state. Med Chir Trans. 1874;39:197–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahomed FA. On chronic Bright’s disease, and its essential symptoms. Lancet. 1879;1:399–401. [Google Scholar]
- 3. Haig A. Uric Acid as a Factor in the Causation of Disease. 4th edn London, UK: J & A Churchill; 1897. [Google Scholar]
- 4. Davis NC. The cardiovascular and renal relations and manifestations of gout. JAMA. 1897;29:261–262. [Google Scholar]
- 5. Huchard H. Arteriolosclerosis: including its cardiac form. JAMA. 1909;53:1129. [Google Scholar]
- 6. Desgrez A. Influence de la constitution des corps puriques sure leur action vis‐a vis de la pression arterielle. Comptes Rendus de l’Academie des Sciences. 1913;156:93–94. [Google Scholar]
- 7. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- 8. Gutierrez‐Macias A, Lizarralde‐Palacios E, Martinez‐Odriozola P, Miguel‐De‐La‐Villa F. Fatal allopurinol hypersensitivity syndrome after treatment of asymptomatic hyperuricemia. Br Med J (Clin Res Ed). 2005;331:623–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahn HA, Medalie JH, Neufeld HN, et al. The incidence of hypertension and associated factors: the Israel ischemic heart study. Am Heart J. 1972;84:171–182. [DOI] [PubMed] [Google Scholar]
- 10. Dyer AR, Liu K, Walsh M, et al. Ten‐year incidence of elevated blood pressure and its predictors: the CARDIA study Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. [DOI] [PubMed] [Google Scholar]
- 11. Imazu M, Yamamoto H, Toyofuku M, et al. Hyperinsulinemia for the development of hypertension: data from the Hawaii‐Los Angeles‐Hiroshima Study. Hypertens Res. 2001;24:531–536. [DOI] [PubMed] [Google Scholar]
- 12. Masuo K, Kawaguchi H, Mikami H, et al. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. [DOI] [PubMed] [Google Scholar]
- 13. Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–841. [DOI] [PubMed] [Google Scholar]
- 14. Nakanishi N, Okamato M, Yoshida H, et al. Serum uric acid and the risk for development of hypertension and imparied fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530. [DOI] [PubMed] [Google Scholar]
- 15. Taniguchi Y, Hayashi T, Tsumura K, et al. Serum uric acid and the risk for hypertension and type 2 diabetes in Japanese men. The Osaka Health Survey. J Hypertens. 2001;19:1209–1215. [DOI] [PubMed] [Google Scholar]
- 16. Torok E, Gyarfas I, Csukas M. Factors associated with stable high blood pressure in adolescents. J Hypertens Suppl. 1985;3(Suppl 3):S389–S390. [PubMed] [Google Scholar]
- 17. Rovda Iu I. [Uric acid and arterial hypertension]. Pediatriia. 1992;10–12:74–78. [PubMed] [Google Scholar]
- 18. Goldstein HS, Manowitz P. Relation between serum uric acid and blood pressure in adolescents. Ann Hum Biol. 1993;20:423–431. [DOI] [PubMed] [Google Scholar]
- 19. Nefzger MD, Acheson RM, Heyman A. Mortality from stroke among U.S. veterans in Georgia and 5 western states I. Study plan and death rates. J Chronic Dis. 1973;26:393–404. [DOI] [PubMed] [Google Scholar]
- 20. Saito I, Folsom AR, Brancati FL, et al. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81–91. [DOI] [PubMed] [Google Scholar]
- 21. Staessen J. The determinants and prognostic significance of serum uric acid in elderly patients of the European Working Party on High Blood Pressure in the Elderly trial. Am J Med. 1991;90:50S–54S. [PubMed] [Google Scholar]
- 22. Alper AB Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. [DOI] [PubMed] [Google Scholar]
- 23. Sundstrom J, Sullivan L, D’Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. [DOI] [PubMed] [Google Scholar]
- 24. Silverstein D, Srivaths PR, Mattison P, et al. Serum uric acid is associated with high blood pressure in pediatric hemodialysis patients. Pediatr Nephrol. 2011;26:1123–1128. [DOI] [PubMed] [Google Scholar]
- 25. Reyes AJ. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur J Heart Fail. 2005;7:461–467. [DOI] [PubMed] [Google Scholar]
- 26. Graessler J, Graessler A, Unger S, et al. Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum. 2006;54:292–300. [DOI] [PubMed] [Google Scholar]
- 27. Parsa A, Brown E, Weir MR, et al. Genotype‐based changes in serum uric acid affect blood pressure. Kidney Int. 2012;81:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannella AC, Mikuls TR. Understanding treatments for gout. Am J Manag Care. 2005;11:S451–S458. [PubMed] [Google Scholar]
- 29. Lee JE, Kim YG, Choi YH, et al. Serum uric acid is associated with microalbuminuria in prehypertension. Hypertension. 2006;47:962–967. [DOI] [PubMed] [Google Scholar]
- 30. Schlesinger N. Dietary factors and hyperuricaemia. Curr Pharm Des. 2005;11:4133–4138. [DOI] [PubMed] [Google Scholar]
- 31. Hwang LC, Tsai CH, Chen TH. Overweight and obesity‐related metabolic disorders in hospital employees. J Formos Med Assoc. 2006;105:56–63. [DOI] [PubMed] [Google Scholar]
- 32. Masseoud D, Rott K, Liu‐Bryan R, Agudelo C. Overview of hyperuricaemia and gout. Curr Pharm Des. 2005;11:4117–4124. [DOI] [PubMed] [Google Scholar]
- 33. Rho YH, Zhu Y, Choi HK. The epidmeiology of uric acid and fructose. Semin Nephrol. 2011;31:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose‐induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. [DOI] [PubMed] [Google Scholar]
- 35. Fox IH, Kelley WN. Studies on the mechanism of fructose‐induced hyperuricemia in man. Metabolism. 1972;21:713–721. [DOI] [PubMed] [Google Scholar]
- 36. Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose‐induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. [DOI] [PubMed] [Google Scholar]
- 37. Johnson RJ, Perez‐Pozo SE, Sautin YY, et al. Excessive fructose intake, uric acid and the etiology of type 2 diabetes. Endocrin Rev. 2009;30:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown C, Culloo A, Yepuri G, Montani J. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R730–R737. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen S, Choi H, Lustig R, Hsu C. The association of sugar sweetened beverage consumption on serum uric acid and blood pressure in a nationally representative sample of adolescents. J Pediatr. 2009;154(6):807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jalal D, Smits G, Johnson RJ, Chonchol M. Increased fructose intake associates with elevated blood pressure. J Amer Soc Nephrol. 2010;21:1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perez‐Pozo SE, Schold J, Nakagawa T, et al. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). 2010;34(3):454–461. [DOI] [PubMed] [Google Scholar]
- 42. Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal‐independent mechanism. Hypertension. 2001;38:1101–1106. [DOI] [PubMed] [Google Scholar]
- 43. Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure‐independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. [DOI] [PubMed] [Google Scholar]
- 44. Sanchez‐Lozada LG, Tapia E, Lopez‐Molina R, et al. Effects of acute and chronic L‐arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol. 2007;292:F1238–F1244. [DOI] [PubMed] [Google Scholar]
- 45. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase‐mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. [DOI] [PubMed] [Google Scholar]
- 46. Gersch MS, Mu W, Cirillo P, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol. 2007;293:F1256–F1261. [DOI] [PubMed] [Google Scholar]
- 47. Watanabe S, Kang DH, Feng L, et al. Uric acid hominoid evolution and the pathogenesis of salt‐sensitivity. Hypertension. 2002;40:355–360. [DOI] [PubMed] [Google Scholar]
- 48. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid induced C‐reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. [DOI] [PubMed] [Google Scholar]
- 49. Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. [DOI] [PubMed] [Google Scholar]
- 50. Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein‐1 production in vascular smooth muscle cells via mitogen‐activated protein kinase and cyclooxygenase‐2. Hypertension. 2003;41:1287–1293. [DOI] [PubMed] [Google Scholar]
- 51. Price K, Sautin Y, Long D, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. [DOI] [PubMed] [Google Scholar]
- 52. Rovda IuI, Kazakova LM, Plaksina EA. [Parameters of uric acid metabolism in healthy children and in patients with arterial hypertension]. Pediatriia. 1990;(8):19–22. [PubMed] [Google Scholar]
- 53. Gruskin AB. The adolescent with essential hypertension. Am J Kidney Dis. 1985;6:86–90. [DOI] [PubMed] [Google Scholar]
- 54. Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Laszlo J. Current management of polycythemia vera and related diseases. Postgrad Med. 1974;55:168–173. [DOI] [PubMed] [Google Scholar]
- 56. Szmigiel Z, Pawlicka G, Sowa M. [Clinical effects of purine metabolism disorders in hematopoietic proliferative diseases]. Pol Tyg Lek. 1976;31:2119–2122. [PubMed] [Google Scholar]
- 57. Feig DI, Nakagawa T, Karumanchi SA, et al. Hypothesis: Uric acid, nephron number and the pathogenesis of essential hypertension. Kidney Int. 2004;66:281–287. [DOI] [PubMed] [Google Scholar]
- 58. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen L, Caballer B, Mitchell DC, et al. Reducing consumption of sugar‐sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fessel WJ, Siegelaub AB, Johnson ES. Correlates and consequences of asymptomatic hyperuricemia. Arch Intern Med. 1973;132:144–154. [PubMed] [Google Scholar]
- 61. Brand FN, McGee DL, Kannel WB, et al. Hyperuricemia as a risk factor of coronary heart disease: the Framingham Study. Am J Epidemiol. 1985;121:11–18. [DOI] [PubMed] [Google Scholar]
- 62. Selby JV, Friedman GD, Quesenberry CP Jr. Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol. 1990;131:1017–1027. [DOI] [PubMed] [Google Scholar]
- 63. Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah. A screening analysis. Hypertension. 1991;17:969–976. [DOI] [PubMed] [Google Scholar]
- 64. Jossa F, Farinaro E, Panico S, et al. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 65. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48:1031–1036. [DOI] [PubMed] [Google Scholar]
- 66. Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. [DOI] [PubMed] [Google Scholar]
- 67. Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long‐term incidence of hypertension: population‐based cohort study. J Hum Hypertens. 2006;20:937–945. [DOI] [PubMed] [Google Scholar]
- 68. Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18:287–292. [DOI] [PubMed] [Google Scholar]
- 69. Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. [DOI] [PubMed] [Google Scholar]
- 70. Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med. 2009;169:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang W, Sun K, Yang Y, et al. Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clin Chem. 2009;55:2026–2034. [DOI] [PubMed] [Google Scholar]
- 72. Jones DP, Richey PA, Alpert BS. Comparison of ambulatory blood pressure reference standards in children evaluated for hypertension. Blood Pressure Monit. 2009;14:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leite MA. Uric acid and fibrinogen: age‐modulated relationships with blood pressure components. J Human Hypertens. 2011;25:476–483. [DOI] [PubMed] [Google Scholar]
- 74. Grayson PC, Kim SY, Lavalley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta‐analysis. Arthritis Care Res. 2011;63:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jolly SE, Mete M, Wang H, et al. Uric acid, hypertension and chronic kidney disease among Alaska Eskimos: the Genetics of Coronary Artery Disease in Alaska natives (GOCADAN) Study. J Clin Hypertens. 2012;14:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Loeffler LF, Navas‐Acien A, Brady TM, et al. Uric acid level and elevated blood pressure in US adolescents. Hypertension. 2012;59:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]


