Abstract
J Clin Hypertens (Greenwich). 2011;13:774–779. ©2011 Wiley Periodicals, Inc.
The purpose of the study was to investigate blood pressure (BP) distribution, prevalence of hypertension, and correlation between BP and body mass index (BMI) in 9‐ to 10‐year‐old Icelandic children. Two manual and two automated BP measurements were performed in 1071 Icelandic children. Children with elevated BP underwent a second BP screening, and a third screening was performed if the BP was elevated at the second visit. Hypertension was defined as BP ≥95th percentile at all three visits. White‐coat hypertension was diagnosed in hypertensive children with normal 24‐hour ambulatory BP. Of 970 children with complete data, 489 were girls (50.4%). The mean BP was 111/63 mm Hg in girls and 112/64 mm Hg in boys (P<.001). The prevalence of elevated BP was 13.1%, 6.0%, and 3.1% after the first, second, and third screen, respectively. The prevalence of sustained hypertension was 2.5% and an additional 0.6% had white‐coat hypertension. A significant correlation between BMI and BP was observed (r=0.338, P<.001) and 8.6% of the obese children had hypertension. The prevalence of hypertension in 9‐ to 10‐year‐old Icelandic children is lower than indicated in recent reports and is associated with obesity.
The prevalence of childhood hypertension appears to have increased in Western societies during the past 2 decades, in parallel with the obesity epidemic. 1 , 2 , 3 Studies carried out in the United States in the 1970s showed a prevalence of hypertension in children of approximately 2%, 4 , 5 , 6 whereas a recent US study 3 found a prevalence of 4.5% in children and adolescents aged 10 to 19 years. The latter study indicated a significantly greater risk of hypertension in boys and overweight children and a rising prevalence of hypertension that was associated with higher body mass index (BMI). Another recent study conducted in Italy 7 found the prevalence of hypertension to be 4.2% in 6‐ to 11‐year‐old children and also identified overweight as a risk factor for hypertension. In a more recent study among 11‐ to 17‐year‐old adolescents in Houston schools, 8 3.2% had hypertension and 15.7% had prehypertension.
The association between overweight and blood pressure (BP) is particularly important because overweight children tend to become overweight adults 9 , 10 , 11 and childhood BP has been found to be a strong predictor of both adult hypertension 12 , 13 and coronary artery calcifications in young adults. 14 This notion is further supported by a recent study from our group indicating that elevated systolic BP (SBP) in children and adolescents may increase the risk of clinically significant coronary artery disease in adult life. 15 The observed increase in the prevalence of childhood obesity and hypertension may, therefore, have significant implications for future cardiovascular health in affected children. The objective of the present study was to investigate the BP distribution, prevalence of hypertension, and association between BP and BMI in healthy 9‐ to 10‐year‐old Icelandic school children.
Methods
Study Sample
Nine‐ to 10‐year‐old students (fourth grade, born in 1999) in 70 schools in the greater Reykjavik area were recruited for school‐based BP screening from January to March 2009. Principals in 39 of the 70 schools accepted an invitation to participate in the study. Parents of all participating children signed an informed consent and answered a questionnaire on prior history of hypertension diagnosed by a physician and medication use.
The study was approved by the National Bioethics Committee (No. 08‐138) and the Icelandic Data Protection Authority.
Measurements and Definitions
During each of the school screenings, a total of 4 seated BP measurements, 2 manual (Accoson Green Light 300 Sphygmomanometer; Accoson, Harlow, Essex, UK) and 2 automated readings (IntelliVue MP50 Patient Monitor; Philips, Andover, MA), were carried out at least 30 seconds apart, following a minimum of 2 minutes of rest. Two trained physicians, wearing casual clothing, performed all of the BP measurements in a quiet room close to the classroom. BP was measured with a cuff appropriate for the size of the child’s upper arm. Manual BP readings were approximated to the nearest 2 mm Hg. SBP was determined by the appearance of the first Korotkoff sound and diastolic BP (DBP) by the disappearance of Korotkoff sounds (the fifth Korotkoff sound) with the manual BP device. The average of the 4 BP measurements was used to determine each child’s BP level. BP percentiles were defined according to normative BP data published by the National High Blood Pressure Education Program (NHBPEP) Working Group on High Blood Pressure in children and adolescents. 16 Children with elevated mean SBP and/or DBP (BP greater than or equal to the age, sex, and height‐percentile–specific 95th BP percentile) at the first screening session had their BP measured again in the same manner within a period of 2 weeks. A third BP screening was carried out if the mean SBP and/or DBP was elevated at the second visit. The third screening was performed at the Hypertension Clinic of the Children’s Medical Center at Landspitali–The National University Hospital of Iceland in Reykjavík with the parents present. Hypertension was defined as a mean BP ≥95th percentile at all 3 visits. Prehypertension was defined as BP ≥90th percentile but <95th percentile. Twenty‐four–hour ambulatory BP monitoring (ABPM) (SpaceLabs, Inc, Redmond, WA) was carried out in children with persistently elevated BP to rule out white‐coat hypertension (WCHTN). Normative values published by Wuhl and co‐workers 17 were used to define elevated 24‐hour ambulatory BP levels. Children with a mean BP ≥95th percentile at all 3 visits and normal 24‐hour ambulatory BP levels were diagnosed with WCHTN. All children with hypertension were referred to the Children’s Medical Center’s Hypertension Clinic for further evaluation.
Age was defined as the child’s age on the day of the first BP screening. School nurses recorded the sex of each child and measured height and weight. BMI was calculated for each child. The most current anthropometric reference data for sex and age from the Centers for Disease Control and Prevention (CDC) were used to establish height and weight percentiles. BMI Z scores were generated from equations provided by the CDC, and BMI percentiles were calculated for each child. BMI quartiles and the 5th, 5th to 10th, 10th to 25th, 25th to 50th, 50th to 75th, 75th to 90th, 90th to 95th, and >95th percentile categories were generated by rounding the exact BMI percentile to the nearest categorical threshold. Overweight was defined as a BMI between the 85th and 95th percentile, and children with BMI >95th percentile were considered obese.
Statistical Analysis
Data is presented as percentage or mean±standard deviation (SD) as appropriate. The data was examined with regard to distribution and tested for normality using the Shapiro–Wilk test. Chi‐square test and the Somers’ d for ordinal variables were used to examine the association between categorical variables. Spearman correlation coefficient was used to examine the association between continuous variables. A P value <.05 was considered statistically significant. Statistical analysis was performed with the computer software SPSS 17.0 (SPSS, Chicago, IL).
Results
Of 1472 students in the 39 participating schools, 1071 accepted an invitation to participate in the study. A total of 970 students, 489 girls (50.4%) and 481 boys (49.6%), successfully completed the first BP screening. The mean age was 9.6±0.3 years. Forty‐four children were excluded from the study because they did not show up for the BP measurement (29 children were absent from school due to illness, 8 did not attend school on the day of measurement or were unavailable due to other school activities, and parents of 7 children refused participation in the study), 55 children were excluded because height and weight measurements were not available, 1 child fainted during the first BP measurement and did not undergo further measurements, and 1 child was excluded because of severe disability. Participation rates ranged from 30% to 100% in different schools.
The clinical characteristics of the study participants are displayed in Table I. No child had a history of hypertension diagnosed by a physician prior to entering the study. Distribution of the mean BP at the first school screening is illustrated in Figure 1, showing SBP markedly skewed to the right. At the initial screening, 73.4% had normal BP, 13.5% had BP in the prehypertensive range, and 13.1% had BP in the hypertensive range. When the BP at the first screening was divided into quartiles based on normative data, 0.4%, 9.8%, 29.2%, and 60.6% of the children had a mean SBP in the 1st, 2nd, 3rd, and 4th quartiles, respectively, and 3.1%, 32.7%, 49.3%, and 14.9% of the children had a mean DBP in the same respective quartiles. The mean SBP at the first screening was 110.7±7.9 mm Hg in girls and 112.0±7.4 mm Hg in boys (P=.008) and the mean DBP at the first screening was 62.7±5.9 mm Hg in girls and 63.5±5.1 mm Hg in boys (P=.02). The mean SBP obtained with the automated and the manual devices was nearly identical, 111.4±8.7 mm Hg vs 111.3±8.1 mm Hg, respectively (P=.73), whereas the mean DBP was significantly lower with the automated device, 61.5±7.0 mm Hg vs 64.7±6.7 mm Hg (P<.001).
Table I.
Clinical Characteristics of the Study Patients
| First Screening (N=970) | Boys (n=481) | Girls (n=489) | P Value |
|---|---|---|---|
| Height, cm | 139.3±6.0 | 138.8±6.1 | .19 |
| Weight, kg | 34.0±6.5 | 34.5±7.5 | .21 |
| BMI, kg/m2 | 17.4±2.5 | 17.8±3.0 | .03 |
| BMI percentile | 56.9±28.0 | 57.0±28.3 | .95 |
| SBP/DBP, mm Hg | 112.0±7.4/63.5±5.0 | 110.7±7.9/62.7±5.9 | .008/.02 |
| Second Screening (N=275) | n=135 | n=140 | |
|---|---|---|---|
| Height, cm | 139.4±5.7 | 140.0±6.4 | .38 |
| Weight, kg | 34.9±6.1 | 38.0±8.7 | .001 |
| BMI, kg/m2 | 17.9±2.6 | 19.2±3.5 | <.001 |
| BMI percentile | 63.0±24.5 | 69.2±27.5 | <.001 |
| SBP/DBP, mm Hg | 115.0±8.0/64.3±6.3 | 113.5±6.7/63.4±4.7 | .09/.16 |
| Third Screening (N=53) | n=34 | n=19 | |
|---|---|---|---|
| Height, cm | 140.8±6.2 | 141.7±5.4 | .60 |
| Weight, kg | 36.0±6.4 | 40.9±8.4 | .03 |
| BMI, kg/m2 | 18.1±2.7 | 20.2±3.2 | .02 |
| BMI percentile | 65.3±25.1 | 78.8±21.0 | .42 |
| SBP/DBP, mm Hg | 119.6±6.0/65.7±4.9 | 122.8±6.2/67.1±6.7 | .06/.40 |
Values are expressed as mean±standard deviation. Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 1.
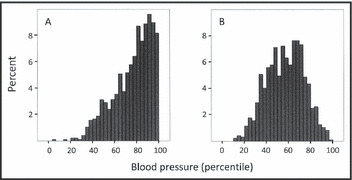
Distribution of systolic (panel A) and diastolic (panel B) blood pressure in 970 nine‐ to 10‐year‐old Icelandic children at the first blood pressure screening session. Blood pressure percentiles were defined according to normative blood pressure data published by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. 16
The prevalence of elevated SBP was 13.1% (n=127) among the children who completed the first screening, 6.0% (n=58) after the second, and 3.1% (n=30) following the third screening session. Thus, the overall prevalence of hypertension was 3.1% (n=30). Only 5 children (0.5%) had elevated DBP at the first screening, 2 of whom had normal SBP. Twenty‐four–hour ABPM confirmed sustained systolic hypertension in 2.4% (n=23) of the children and WCHTN in 0.6% (n=6) or 20% of the hypertensive children. One additional child who had elevated BP at the third screening session but did not undergo ABPM was considered hypertensive. Therefore, the total prevalence of sustained hypertension was 2.5%. The results of the ABPM showed that 6 children had isolated systolic hypertension, 16 had both systolic and diastolic hypertension, and only 1 had isolated diastolic hypertension. Although boys had a slightly higher prevalence of elevated BP than girls (13.9% vs 12.3%, P=.44) at the first screening, no sex differences (3.1% vs 3.1%, P=.96) were noted at the third screening. Eight of 127 children with elevated BP at the first screening session and 11 of 58 children who had elevated BP at the second session did not show up for the last BP screening for various reasons.
The overall prevalence of obesity (BMI >95th percentile) was 7.2% (n=70), and the prevalence was higher in females, 8.2% vs 6.2% in males (P=.24). The prevalence of overweight and obesity (BMI ≥85th percentile) was 20.1% (n=195). At the first screening, a significant positive correlation was found between BMI and SBP (r=0.34, P≤.001; Figure 2), BMI percentiles and SBP (r=0.34, P≤.001), and BMI percentiles and SBP percentiles (r=0.27, P≤.001), with further analysis yielding an adjusted R 2 of 6.4% to 11.3% (P<.001). Correlation between BMI or BMI percentiles and DBP or DBP percentiles was also significant, with the correlation coefficient ranging from 0.174 to 0.222 (P<.001).
Figure 2.
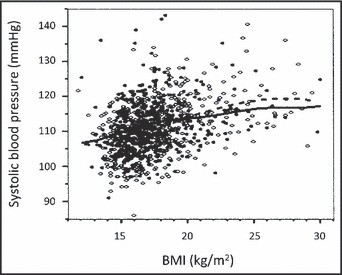
Correlation between body mass index (BMI) and mean systolic blood pressure (Spearman’s r=0.34, P≤.001). The open circles and dashed line represent girls and the black dots and solid line represent boys. The lines indicate fit by nonparametric smoothing.
The prevalence of elevated BP at first screening among children in the 90th to 95th and ≥95th percentile BMI categories was 22.6% and 31.4%, respectively. Prevalence of elevated BP in these categories at the second screening was 4.8% and 20.0%, respectively, and 4.8% and 8.6%, respectively, at the third screening session (Figure 3). Children with BMI in the highest quartile were almost 4 times more likely to have a mean SBP ≥95th percentile at the first screening than those with BMI in the lowest quartile (21.5% vs 6.4%, P<.001; Table II). As expected, the overall prevalence of hypertension after the third screening was lower for each BMI quartile when compared with the first screening (Table II, Figure 3). A more than 8‐fold increase in the prevalence of systolic hypertension was found as the BMI increased from <25th (prevalence 0.6%) to >75th percentiles (prevalence 5.4%; P=.003), as is demonstrated in Table II. Children with BP in the prehypertension range at the first visit were more likely to be obese than those with normal BP (9.2% vs 5.1%), but the difference was only borderline significant (P=.06).
Figure 3.
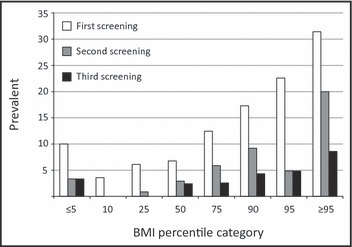
Prevalence of elevated blood pressure at the first, second, and third screening sessions, categorized by body mass index (BMI) percentiles (Somers’ d test for linear trend; first screening, P<.001; second screening, P=.002; third screening, P=.007).
Table II.
Frequency of Systolic Blood Pressure ≥95th Percentile at the First and Third Screenings by Quartiles of BMI
| BMI Quartiles | P Valuea | |||||
|---|---|---|---|---|---|---|
| <25th | 25th–49th | 50th–74th | ≥75th | |||
| First BP screening | ||||||
| All patients | SBP ≥95% | 6.4 | 6.8 | 12.5 | 21.5 | <.001 |
| Boys | SBP ≥95% | 9.5 | 6.9 | 17.9 | 17.3 | .013 |
| Girls | SBP ≥95% | 3.4 | 6.6 | 6.8 | 25.5 | <.001 |
| Third BP screening | ||||||
| All patients | SBP ≥95% | 0.6 | 2.4 | 2.6 | 5.4 | .003 |
| Boys | SBP ≥95% | 1.2 | 3.0 | 3.6 | 3.8 | .26 |
| Girls | SBP ≥95% | 0 | 1.9 | 1.5 | 6.8 | .003 |
Values are expressed as percentages. Abbreviations: BMI, body mass index; SBP, systolic blood pressure. aSomers’ d for ordinal variables.
Sixty‐four children (6.6%) were taking prescription drugs, 13 (20.3%) of whom were taking >1 medication. Twenty‐nine children (3.0%) were taking medications that may raise BP, including methylphenidate (n=22), aripiprazole (n=6), and atomoxetine (n=6); 5 children were taking 2 stimulants simultaneously. Two of the children (3.1%) with sustained hypertension were taking methylphenidate, 1 of whom was also treated with aripiprazole. Two children (0.2%) were taking a medication known to decrease BP (propranolol) but neither one of them had hypertension. Thirty‐three children (3.4%) were taking medications that are not known to affect BP. Information on medication use was not available for 1.3% of the children.
Discussion
In this population‐based study, the prevalence of sustained hypertension in 9‐ to 10‐year‐old Icelandic school children was 2.5% and an additional 0.6% were found to have WCHTN. There was a significant positive correlation between BMI and SBP at the first BP screening session, with BMI accounting for approximately 7% to 12% of the variation in SBP. Moreover, children in the highest BMI quartile were almost 8 times more likely to have hypertension than those in the lowest BMI quartile.
The 2.5% prevalence of sustained hypertension in our study is similar to the prevalence of approximately 2% observed in several US studies from the 1970s. 4 , 5 , 6 Our findings are also in concert with the results of two more recent studies comprising more than 6000 healthy 12‐year‐old Swiss school children 18 and more than 2000 Oklahoma school children, 19 yielding a prevalence of hypertension of 2.2% and 2.8%, respectively. However, two recent cross‐sectional studies from the United States 3 and Italy 7 that applied an identical BP measurement strategy to the one used in our study, showed a significantly higher prevalence of hypertension. In the US study, the prevalence of hypertension was 4.5% in 10‐ to 19‐year‐old Texas school children, while in the Italian study the prevalence was 4.2% in a population of 6‐ to 11‐year‐old school children. Hypertension was more common in girls in the Italian study and in boys in the US study, while no sex difference was noted in our study.
The prevalence of WCHTN was not evaluated in any of the aforementioned epidemiologic studies. In the present study, 20% of the hypertensive children had confirmed WCHTN, which is similar to previous reports in children with elevated office BP. 20 , 21 , 22 The lack of 24‐hour ABPM in the studies from Switzerland, 18 United States,3,19 and Italy 7 is likely to have caused an overestimation of the true prevalence of sustained hypertension in these populations.
The distribution of mean SBP obtained at the first of the 3 screening sessions in our study was significantly skewed to the right when compared with the US reference values 16 that are based on single BP measurements. Our results are, however, in line with the recently reported high prevalence of BP in the prehypertensive and hypertensive range in US children and adolescents at the time of first screening. 8 , 23 The skewness was further exaggerated if we included only the first measurement for each child at the first screening. Despite this shift to the right in BP distribution at the first school screening, the prevalence of sustained hypertension was only 2.5%. The reason for the discrepancy between the BP distribution at the first screening and the relatively low prevalence of hypertension in our study is not clear. The absence of parents during the first and second BP measurements may have increased the level of stress and anxiety among the children, potentially resulting in higher BP values. The parents were present at the third screening session, at which time the children had also become more familiar with the BP measurements. The fact that the US normative values are largely based on BP measurements obtained before the onset of the obesity epidemic may have further accentuated the shift of the SBP curve to the right in our study.
We observed a significant positive correlation between SBP and BMI, with roughly 7% to 12% of the variation in SBP accounted for by BMI. This association has previously been reported by a number of investigators. 3 , 7 , 18 , 19 , 24 , 25 In our study, the overall prevalence of elevated BP at the third BP screening was 3.1% compared with 2.2%, 4.2%, and 4.5% in studies from Switzerland, 18 Italy, 7 and the United States, 3 respectively. Furthermore, the prevalence of elevated BP in children with BMI ≥95% after the first and third BP screenings in our study was comparable to that found in the Swiss, US, and Italian studies. In all these studies, the prevalence of hypertension appears to parallel the prevalence of obesity, which was 7.2% in our study, 3.6% in the Swiss study, and approximately 20% in the US and Italian studies. Moreover, the prevalence of hypertension in obese children was quite high in all 4 studies, 8.6% in the present study, and 10.8%, 11%, and 15% in the Italian, US, and Swiss studies, respectively. The mean BMI in our study was also lower than was observed in two previous studies 3 , 19 that yielded a higher prevalence of elevated BP and hypertension. Thus, it appears that the variation in the prevalence of hypertension between studies can largely be explained by differences in the prevalence of overweight and obesity. The increasing prevalence of obesity in children may therefore have a negative impact on future cardiovascular health in Western societies, as hypertension in children and young adults has been shown to be associated with increased risk of cardiovascular disease in adult life. 1 , 15
Strengths and Limitations
Two of the hypertensive children in our study were taking the central nervous system stimulant methylphenidate, which may have contributed to the BP elevation. However, a recent study in children with chronic multiple tic disorder and attention deficit hyperactivity disorder 26 suggests that elevation of SBP in these children may primarily be associated with comorbid anxiety rather than the medications used to treat the disorders. Furthermore, another study by the same investigators 27 found no correlation between plasma levels of methylphenidate and BP.
The strengths of our study include the population‐based design and a large sample size, as the 970 participants with complete data account for approximately 20% of all Icelandic children born in 1999 and 1.3% of all Icelandic children who were 0 to 17 years of age during the year the study was conducted. Thus, the results of the study are likely to be representative of the age‐matched general population. Another strong feature of the study is that all BP measurements were carried out by the same two physicians and practically all of our hypertensive children underwent 24‐hour ABPM to exclude WCHTN. However, the measurement of height and weight by different nurses in the participating schools can be viewed as a limitation of the study.
Conclusions
This study shows that hypertension is present in a significant proportion of 9‐ to 10‐year‐old Icelandic children and the strong positive correlation between hypertension and BMI. The increasing prevalence of overweight and obesity has become a serious public health problem in Western countries that requires urgent intervention by responsible authorities. Failure to do so will likely result in increasing prevalence of essential hypertension and other cardiovascular risk factors in children, leading to an epidemic of cardiovascular complications in early adult life and reduced life expectancy of affected individuals. Comprehensive BP and weight screening programs in the school system should be strongly considered.
Acknowledgements and disclosures:
Preliminary results of this study appeared in an abstract form at the Biennial Meeting of the Nordic Society of Nephrology in Helsinki, Finland, August 2009, and the Annual Meeting of the European Society of Pediatric Nephrology in Birmingham, UK, in September of 2009. We would like to thank the participants and all the principals, teachers, nurses, and secretaries at the participating schools for their support. We are also grateful to Vistor Inc (Horgatun 2, 210 Gardabaer, Iceland) for kindly providing the automated Philips blood pressure monitor. Finally, we thank Loftur I. Bjarnason, Computer Science student at the University of Iceland, for data management and programming. The study was supported by the Landspitali–The National University Hospital of Iceland Research Fund. There are no financial disclosures and the authors have no conflicts of interest.
Sandra D. Steinthorsdottir and Sigridur B. Eliasdottir contributed equally to this work.
References
- 1. Din‐Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 2. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437–1439. [DOI] [PubMed] [Google Scholar]
- 3. Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school‐aged children. Pediatrics. 2004;113:475–482. [DOI] [PubMed] [Google Scholar]
- 4. Fixler DE, Laird WP, Fitzgerald V, et al. Hypertension screening in schools: results of the Dallas study. Pediatrics. 1979;63:32–36. [PubMed] [Google Scholar]
- 5. Lauer RM, Connor WE, Leaverton PE, et al. Coronary heart disease risk factors in school children: the Muscatine study. J Pediatr. 1975;86:697–706. [DOI] [PubMed] [Google Scholar]
- 6. Silverberg DS, Nostrand CV, Juchli B, et al. Screening for hypertension in a high school population. Can Med Assoc J. 1975;113:103–108. [PMC free article] [PubMed] [Google Scholar]
- 7. Genovesi S, Giussani M, Pieruzzi F, et al. Results of blood pressure screening in a population of school‐aged children in the province of Milan: role of overweight. J Hypertens. 2005;23:493–497. [DOI] [PubMed] [Google Scholar]
- 8. McNiece KL, Poffenbarger TS, Turner JL, et al. Prevalence of hypertension and pre‐hypertension among adolescents. J Pediatr. 2007;150:640–644. [DOI] [PubMed] [Google Scholar]
- 9. Serdula MK, Ivery D, Coates RJ, et al. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–177. [DOI] [PubMed] [Google Scholar]
- 10. Whitaker RC, Wright JA, Pepe MS, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. [DOI] [PubMed] [Google Scholar]
- 11. Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3 Pt 2):497–504. [PubMed] [Google Scholar]
- 12. Bao W, Threefoot SA, Srinivasan SR, et al. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8:657–665. [DOI] [PubMed] [Google Scholar]
- 13. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 14. Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. [DOI] [PubMed] [Google Scholar]
- 15. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target‐organ damage later in life. Pediatr Nephrol. 2010;25:323–328. [DOI] [PubMed] [Google Scholar]
- 16. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 17. Wuhl E, Witte K, Soergel M, et al. Distribution of 24‐h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 18. Chiolero A, Cachat F, Burnier M, et al. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25:2209–2217. [DOI] [PubMed] [Google Scholar]
- 19. Moore WE, Stephens A, Wilson T, et al. Body mass index and blood pressure screening in a rural public school system: the Healthy Kids Project. Prev Chronic Dis. 2006;3:A114. [PMC free article] [PubMed] [Google Scholar]
- 20. Sorof JM, Portman RJ. White coat hypertension in children with elevated casual blood pressure. J Pediatr. 2000;137:493–497. [DOI] [PubMed] [Google Scholar]
- 21. Lande MB, Meagher CC, Fisher SG, et al. Left ventricular mass index in children with white coat hypertension. J Pediatr. 2008;153:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pickering TG, James GD, Boddie C, et al. How common is white coat hypertension? JAMA. 1988;259:225–228. [PubMed] [Google Scholar]
- 23. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–879. [DOI] [PubMed] [Google Scholar]
- 24. Nur N, Cetinkaya S, Yilmaz A, et al. Prevalence of hypertension among high school students in a middle Anatolian province of Turkey. J Health Popul Nutr. 2008;26:88–94. [PMC free article] [PubMed] [Google Scholar]
- 25. Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–447. [DOI] [PubMed] [Google Scholar]
- 26. Gadow KD, Nolan EE. Methylphenidate and comorbid anxiety disorder in children with both chronic multiple tic disorder and ADHD. J Atten Disord. 2011;15:246–256. [DOI] [PubMed] [Google Scholar]
- 27. Stevens JR, George RA, Fusillo S, et al. Plasma methylphenidate concentrations in youths treated with high‐dose osmotic release oral system formulation. J Child Adolesc Psychopharmacol. 2010;20:49–54. [DOI] [PubMed] [Google Scholar]


