Abstract
Higher prevalence of both hypertension and obesity in African Americans is associated with a disproportionately greater burden of cardiovascular diseases in this ethnic group. The purpose of this study was to examine whether there is an interaction between hypertension and obesity that significantly increases the expression of metabolic risk factors for cardiovascular disease. Four groups of young adult African Americans were recruited based on their weight and blood pressure (BP). The effects of weight and BP on metabolic risk factors were analyzed based on data obtained from 484 patients. Results demonstrated that high BP and obesity were independently associated with increased odds of abnormal glucose tolerance, 1.8‐ and 2.2‐fold, respectively. The coexistence of both high BP and obesity further increased the odds of abnormal glucose tolerance 4‐fold. In addition, the geometric mean of homeostasis model assessment, an estimate of insulin resistance, increased by 18% with high BP, 60% with obesity, and 90% with the presence of both high BP and obesity. Although no statistically significant interaction between high BP and obesity was detected, the relationships of both high BP and obesity with metabolic risk factors were clearly additive.J Clin Hypertens (Greenwich). 2011;13:397–403. ©2011 Wiley Periodicals, Inc.
Hypertension is a major health problem and a well‐established risk factor for cardiovascular (CV) events. 1 Obesity is also a significant public health issue due to its metabolic burden leading to diabetes mellitus. 2 Both hypertension and obesity are prevalent in African Americans, and the excess rates of these conditions are parallel with disproportionately greater rates of CV events in African Americans compared with Caucasians. 3 While it has been established that both hypertension and obesity contribute to adverse outcomes, the extent to which high blood pressure (HBP) contributes to abnormal glucose metabolism and other metabolic risk factors is less clear. We conducted a study to examine the relationships of HBP and obesity with metabolic risk factors in young adult African Americans prior to clinical manifestation of CV disease or overt diabetes. The purpose of the study was to determine whether there is an interaction between HBP and obesity that significantly increases the expression of metabolic risk factors for CV disease.
Methods
Study Population
Healthy young African American adults were recruited in urban Philadelphia through local advertisements between 2006 and 2009. Inclusion criteria for enrollment included adults with or without obesity (defined as body mass index [BMI] ≥30 kg/m2) and with or without HBP (defined as blood pressure [BP] ≥130/85 mm Hg or taking any antihypertensive medications). Exclusion criteria included known secondary hypertension, diabetes, renal disease, CV disease, autoimmune disease, thyroid disease, sickle cell disease, eating disorders, and use of steroids. The study protocol was approved by the institutional review board of Thomas Jefferson University. Written informed consent was obtained from each participant at enrollment.
Study Procedures
Each participant was given instructions to collect a timed overnight urine sample on the morning of the visit to the clinical research center. Data on health status, medication use, and health‐related behaviors were obtained by self‐report of each participant. Clinical assessments were obtained from all participants upon their arrival to the research center in the early morning, including BP and anthropometric measurements (height, weight, and waist circumference). BP measurements were obtained from each patient following a 10‐minute rest period in a seated position using auscultation with an aneroid sphygmomanometer. The average of 4 separate measurements of systolic BP (SBP) and diastolic BP (DBP) was used as the BP value for each participant. BMI was calculated as weight (kg) divided by height squared (m2).
An oral glucose tolerance test (OGTT) was conducted after a 12‐hour overnight fast. A fasting blood sample was obtained for plasma glucose, insulin, and lipids. Following the ingestion of 75 g of glucose solution (Glucola; Ames Diagnostics, Elkhart, IN), blood samples were then obtained at 30, 60, and 120 minutes post‐ingestion and assayed for plasma glucose and insulin concentrations. Plasma glucose concentration was analyzed with the glucose oxidase technique (YS Model 27; Glucostat, Yellow Springs, OH). Plasma insulin concentration was determined with a solid phase radioimmunoassay (RIA) (Coat‐a‐Count; Diagnostic Products Corp, Los Angeles, CA). Coefficients of variation for intra‐assay and inter‐assay variability for glucose and insulin assays were <5%. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA). 4 Fasting lipids including total cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), and triglycerides were measured using the Hitachi 704 standard enzymatic method in the Lipid Laboratory of Thomas Jefferson University. Urine albumin excretion (UAE) was measured by ELISA (Exocell, Philadelphia, PA) and corrected for creatinine (mg/g cr). These assays were highly reliable, with a consistently low coefficient of variation (<5%).
Statistical Analyses
HBP was defined as SBP ≥130 mm Hg or DBP ≥85 mm Hg or being on any antihypertensive medication, while normal BP (NBP) was defined as BP <130/85 mm Hg and not on any antihypertensive medication. Patients were stratified into 4 different groups based on BMI and BP status: nonobese normal BP (N‐NBP), nonobese HBP (N‐HBP), obese normal BP (O‐NBP), and obese HBP (O‐HBP). Obese was defined as BMI ≥30 kg/m2 while nonobese includes both overweight (BMI 25 kg/m2 to <30 kg/m2) and normal weight (BMI <25 kg/m2). Glucose tolerance status was determined using fasting and 2‐hour OGTT glucose values: normal glucose tolerance was defined as fasting blood glucose <100 mg/dL and 2‐hour post‐OGTT glucose <140 mg/dL; impaired glucose tolerance was defined as fasting blood glucose 100 mg/dL to 125 mg/dL or 2‐hour glucose of 140 mg/dL to 199 mg/dL; and diabetic was defined as fasting blood glucose >125 mg/dL or 2‐hour post‐OGTT glucose >199 mg/dL. The term abnormal glucose tolerance included both impaired glucose tolerance and diabetes (based on OGTT results). The metabolic syndrome was defined as having any 3 of the following 5 criteria: waist circumference ≥102 cm for men or ≥88 cm for women; BP ≥130/85 mm Hg or taking antihypertensive medication; HDL <40 mg/dL for men or <50 mg/dL for women; triglycerides ≥150 mg/dL; fasting glucose ≥110 mg/dL. 5
Statistical Modeling
Categoric variables available for analysis included glucose tolerance (normal vs abnormal glucose tolerance), sex, obesity, HBP, alcohol consumption (response to “do you drink alcohol? yes or no”) and smoking status (response to “do you smoke cigarettes? yes or no”). Continuous variables included age, HDL, LDL, triglycerides, and HOMA. Due to skewed distributions, triglyceride and HOMA data were log‐transformed for statistical analysis. The central tendency and dispersion of these two variables were summarized for each BMI‐BP group by geometric means and the first and third quartiles on their original scales. All other continuous variables were summarized by arithmetic means and standard deviations. Similarly, counts and percentages were tabulated for the categoric variables. Unadjusted analysis of variance (ANOVA) F tests for differences in means across the BMI‐BP groups were conducted for continuous variables, and Fisher exact tests were conducted for detecting dependencies between categoric variables and BMI‐BP group.
The dependent variables investigated for association with obesity and HBP were glucose tolerance status, HDL, LDL, triglycerides, and HOMA. Abnormal glucose tolerance was modeled by logistic regression and HDL and LDL were modeled by linear regression. Triglycerides and HOMA were modeled by linear regression after log‐transformation. The regression parameter estimates for these two variables were subsequently anti–log‐transformed back to the original scale and presented as geometric mean ratios (GMRs). Each model was adjusted for age, sex, smoking, and alcohol consumption. The results are presented for obesity, HBP, or both conditions by linear contrast in terms of odds ratios (glucose tolerance), expected differences (HDL and LDL), and expected GMRs (triglycerides and HOMA) with 95% confidence intervals (CIs). The significance of an interaction term between obesity and HBP was tested by a one degree of freedom likelihood ratio test for each dependent variable model. Diuretic use was introduced into models to determine whether it was significantly related to the risk factors. The significance level for all tests was set at α=0.05.
Results
A total of 505 young adult African Americans were enrolled into the study. Twenty‐one participants were removed from data analysis for either incomplete data (n=10), inability to verify reported antihypertensive medication use (n=7), or suspected renal disease based on albuminuria ≥300 mg/gm creatinine and/or serum creatinine ≥1.5 mg/dL (n=4). Data analysis was conducted for 484 study participants with complete data. These participants were stratified into 4 groups based on their BMI and BP status: N‐NBP, N‐HBP, O‐NBP, and O‐HBP. As shown in Table I, four groups were clearly separated by BMI and BP according to the study protocol. More women were stratified to the obese groups (O‐NBP and O‐HBP) while more men were stratified to the nonobese groups (N‐NBP and N‐HBP). Although no known diabetics were enrolled in the study, based on the results of the OGTT, 9% of participants were found to be diabetic and 29% met criteria for impaired glucose tolerance. Three percent of participants in the N‐NBP group were found to be diabetic, and 9% to 13% of participants in the remaining groups (N‐HBP, O‐NBP, O‐HBP) were found to be diabetic. Compared with the N‐NBP group, the N‐HBP and O‐NBP groups had higher but comparable percentages (40%–41%) of abnormal glucose tolerance (impaired and diabetic), and the O‐HBP group had the highest percentage (54%) of abnormal glucose tolerance. More than two thirds of participants in HBP groups (N‐HBP and O‐HBP) reported taking antihypertensive medications. The percentages of participants who reported a positive family history of diabetes ranged from 27% to 37% across the 4 groups, and there was no statistically significant difference among the 4 groups.
Table I.
Characteristics of Study Patients
| Nonobese/NBP (n=121) | Nonobese/HBP (n=120) | Obese/NBP (n=117) | Obese/HBP (n=126) | P Valuea | |
| Sex | |||||
| Female | 51 (42) | 33 (28) | 82 (70) | 76 (60) | <.01 |
| BMI group | |||||
| Normal | 64 (53) | 39 (33) | N/A | N/A | <.01 |
| Overweight | 57 (47) | 81 (68) | N/A | N/A | |
| Obese | N/A | N/A | 117 (100) | 126 (100) | |
| HTN medication | N/A | 78 (65) | N/A | 91 (72) | <.01 |
| Diuretics | N/A | 34 (28) | N/A | 40 (32) | <.01 |
| Glucose tolerance | |||||
| Normal | 98 (81) | 71 (59) | 70 (60) | 58 (46) | <.01 |
| Impaired | 19 (16) | 38 (32) | 32 (27) | 53 (42) | |
| Diabetic | 4 (3) | 11 (9) | 15 (13) | 15 (12) | |
| Metabolic syndromeb | 1 (1) | 25 (21) | 22 (19) | 78 (62) | <.01 |
| Family history of diabetesc | 33 (27) | 37 (31) | 41 (35) | 46 (37) | .40 |
Values are expressed as number (percentage). Abbreviations: BMI, body mass index; HBP, high blood pressure; HTN, hypertension; N/A, not applicable; NBP, normal blood pressure. aFisher exact test. bMetabolic syndrome is defined as ≥3 of following: waist circumference ≥102 cm (for men), ≥88 cm (for women); systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or use of hypertension medications; high‐density lipoprotein <40 mg/dL (for men), <50 mg/dL (for women); triglycerides ≥150 mg/dL; fasting glucose ≥110 mg/dL. cFamily history of diabetes: parent or sibling.
Table II lists the CV risk factor parameters within each of the 4 groups. Patients in 2 HBP groups (N‐HBP and O‐HBP) were somewhat older than those in the NBP groups (N‐NBP and O‐NBP). Mean fasting glucose was lowest in the N‐NBP group (97.1 mg/dL), intermediate in the N‐HBP group (102.5 mg/dL), and highest in the obese groups (107.8 mg/dL for O‐NBP and 107.3 mg/dL for O‐HBP). Mean levels of fasting insulin, HDL, LDL, triglycerides, and HOMA differed across BMI‐BP grouping (P<.01 for each). Compared with the N‐NBP group, the N‐HBP, O‐NBP, and O‐HBP groups had progressively higher mean fasting insulin levels. Obese groups (O‐NBP and O‐HBP) had lower mean HDL levels and higher mean LDL levels compared with the nonobese groups (N‐NBP and N‐HBP). Mean triglyceride levels were higher in the HBP groups (N‐HBP and O‐HBP) compared with the NBP groups. Compared with the N‐NBP group, the N‐HBP, O‐NBP, and O‐HBP groups had progressively greater insulin resistance measured by HOMA.
Table II.
Cardiovascular Risk Factor Parameters of Study Patients
| Parameters, mean (SD)a | Nonobese/NBP (n=121) | Nonobese/HBP (n=120) | Obese/NBP (n=117) | Obese/HBP (n=126) | P Valueb |
|---|---|---|---|---|---|
| Age, y | 35.3 (8.0) | 40.3 (6.1) | 35.4 (7.8) | 39.7 (6.6) | <.01 |
| BMI, kg/m2 | 24.7 (3.2) | 26.2 (2.4) | 37.4 (6.7) | 37.8 (7.2) | <.01 |
| Waist circumference, cm | 83.5 (8.6) | 88.0 (7.7) | 109.8 (15.1) | 111.9 (15.8) | <.01 |
| BP systolic, mm Hg | 111.4 (9.0) | 130.7 (17.8) | 114.2 (8.7) | 130.2 (19.0) | <.01 |
| BP diastolic, mm Hg | 69.8 (7.8) | 83.0 (12.9) | 68.9 (8.0) | 79.8 (12.3) | <.01 |
| Fasting glucose, mg/dL | 97.1 (11.7) | 102.5 (13.7) | 107.8 (24.3) | 107.3 (21.0) | <.01 |
| Fasting insulin, μU/mL | 6.5 (7.2) | 7.2 (8.4) | 9.5 (10.9) | 10.9 (8.7) | <.01 |
| HDL, mg/dL | 50.2 (14.8) | 51.3 (15.2) | 44.8 (14.1) | 45.6 (14.0) | <.01 |
| LDL, mg/dL | 103.8 (30.1) | 103.7 (26.7) | 114.0 (26.8) | 112.5 (31.5) | <.01 |
| Triglycerides, mg/dL | 70.6 (39.1) | 86.7 (47.3) | 74.5 (46.9) | 97.0 (54.1) | <.01 |
| Total cholesterol, mg/dL | 168.2 (32.9) | 172.3 (29.6) | 173.2 (30.6) | 177.2 (36.0) | .24 |
| UAE, mg/g creatininec | 3.8 [1.5, 10.0] | 6.0 [1.9, 18.1] | 4.8 [2.2, 11.8] | 5.8 [2.2, 14.6] | .07 |
| HOMAc | 1.1 [0.7, 1.6] | 1.3 [0.8, 1.7] | 1.8 [1.0, 2.9] | 2.2 [1.3, 3.7] | <.01 |
Abbreviations: BMI, body mass index; BP, blood pressure; HBP, high blood pressure; HDL, high‐density lipoprotein; HOMA, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; NBP, normal blood pressure; UAE, urinary albumin excretion. aApplied to all parameters except for UAE and HOMA. bAnalysis of variance F test. cData natural log‐transformed: geometric means with [1st quartile, 3rd quartile] presented.
We then stratified the entire cohort according to BMI status. Table III provides the participant characteristics for men and women separately according to 4 BMI categories representing normal weight, overweight, obese, and severely obese. The results demonstrated a clear increase in prevalence of abnormal glucose tolerance (impaired and diabetic) with each increment in BMI category in men. In women, the trend was less clear, and the sample size of women in the normal BMI subgroup was smaller. There was a progressive increase in prevalence of metabolic syndrome with increasing BMI category among both men and women (0%–53% for men and 6%–44% for women).
Table III.
Characteristics of Men and Women by Different BMI Groups
| Male Patients | BMI <25 (n=71) | ≥25 BMI <30 (n=86) | ≥30 BMI <35 (n=45) | BMI ≥35 (n=40) | P Valuea |
|---|---|---|---|---|---|
| Blood pressure | |||||
| HBP | 31 (44) | 56 (65) | 25 (56) | 25 (63) | .05 |
| Hypertension medications | 17 (24) | 37 (43) | 16 (36) | 17 (43) | .07 |
| Diuretics | 6 (8) | 17 (20) | 6 (13) | 9 (23) | .12 |
| Glucose tolerance status | <.01 | ||||
| Normal | 58 (82) | 55 (64) | 26 (58) | 9 (23) | |
| Impaired | 10 (14) | 27 (31) | 15 (33) | 20 (50) | |
| Diabetic | 3 (4) | 4 (5) | 4 (9) | 11 (28) | |
| Metabolic syndromea | 0 (0) | 9 (10) | 12 (27) | 21 (53) | <.01 |
| Family history of diabetesb | 19 (27) | 28 (33) | 15 (33) | 16 (40) | .55 |
| female patients | BMI <25 (n=32) | ≥25 bmi <30 (n=52) | ≥30 bmi <35 (n=68) | BMI ≥35 (n=90) | P value c |
| Blood pressure groups | |||||
| HBP | 8 (25) | 25 (48) | 34 (50) | 42 (47) | .10 |
| Hypertension medications | 6 (19) | 18 (35) | 29 (43) | 29 (32) | .13 |
| Diuretics | 2 (6) | 9 (17) | 12 (18) | 13 (14) | .46 |
| Glucose tolerance status | .06 | ||||
| Normal | 21 (66) | 35 (67) | 48 (71) | 45 (50) | |
| Impaired | 10 (31) | 10 (19) | 15 (22) | 35 (39) | |
| Diabetic | 1 (3) | 7 (13) | 5 (7) | 10 (11) | |
| Metabolic syndromea | 2 (6) | 15 (29) | 27 (40) | 40 (44) | <.01 |
| Family history of diabetesb | 7 (22) | 16 (31) | 28 (41) | 28 (31) | .26 |
Values are expressed as number (percentage). Abbreviations: BMI, body mass index; HBP, high blood pressure. aMetabolic syndrome is defined as ≥3 of following: waist circumference ≥102 cm (men), ≥88 cm (women); systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or taking hypertension medication; high‐density lipoprotein <40 mg/dL (men), <50 mg/dL (women); triglycerides ≥150 mg/dL; fasting glucose ≥110 mg/dL. bFamily history of diabetes: parent or sibling. cFisher exact test
We detected no statistically significant interaction terms between obesity and HBP in models with main effects adjusted for age, sex, smoking, and alcohol consumption. The P values from the likelihood ratio tests for these terms were .17 (glucose tolerance), .94 (HDL), .66 (LDL), .43 (triglyceride), and .73 (HOMA). Therefore, the interaction terms were removed from the final models. The association estimates for obesity and HBP with metabolic risk factors are summarized in Table IV. Obesity was associated with a 2.2‐fold increase in the odds of having abnormal glucose tolerance (odds ratio, 2.22; 95% CI, 1.47–3.34) while HBP was associated with a 1.8‐fold increase in the odds (odds ratio, 1.84; 95% CI, 1.23–2.74). When both obesity and HBP were present, the odds of having abnormal glucose tolerance increased by 4.1‐fold (odds ratio, 4.07; 95% CI, 2.29–7.23). The Figure provides a graphic depiction of the association between abnormal glucose tolerance and obesity, HBP, or both. Obesity was associated with significantly lower HDL levels (−5.68 mg/dL; 95% CI, −8.37 to −2.99) and higher LDL levels (8.78 mg/dL; 95% CI, 3.41–14.15), but no significant increase in triglyceride levels (GMR, 1.08; 95% CI, 0.99–1.18). HBP was associated with significantly higher triglyceride levels (GMR, 1.21; 95% CI, 1.10–1.32), but no significant difference in both HDL (0.31 mg/dL; 95% CI −2.39 to 3.00) and LDL levels (−0.43 mg/dL; 95% CI, −5.81 to 4.96). Obesity and HBP were both associated with higher insulin resistance index measured by HOMA (GMR, 1.60; 95% CI, 1.39–1.86 for obesity and GMR, 1.18; 95% CI, 1.02–1.37 for HBP). We considered the possibility that diuretic use could contribute to abnormal glucose tolerance. When diuretic use was added as a variable in the final models, no statistically significant associations between diuretics and glucose tolerance state, HDL, LDL, triglycerides, or HOMA were detected.
Table IV.
Estimated Association of Obesity and Hypertension on Metabolic Risk Factors
| Obesity | HBP | Obesity and HBP | |
|---|---|---|---|
| Abnormal glucose tolerance, OR (95% CI)a | 2.22 (1.47–3.34) | 1.84 (1.23–2.74) | 4.07 (2.29–7.23) |
| Expected difference in HDL, mean (95% CI), mg/dLb | −5.68 (−8.37 to −2.99) | 0.31 (−2.39 to 3.00) | −5.38 (−9.09 to −1.66) |
| Expected difference in LDL, mean (95% CI), mg/dLb | 8.78 (3.41–14.15) | −0.43 (−5.81 to 4.96) | 8.35 (0.94–15.77) |
| Expected relative difference in triglycerides, GMR (95% CI)c | 1.08 (0.99–1.18) | 1.21 (1.10–1.32) | 1.31 (1.16–1.48) |
| Expected relative difference in HOMA, GMR (95% CI)c | 1.60 (1.39–1.86) | 1.18 (1.02–1.37) | 1.90 (1.55–2.32) |
Abbreviations: CI, confidence interval; HDL, high‐density lipoprotein; HBP, high blood pressure; HOMA, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein. aOdds ratios (ORs) modeled from cumulative odds model increasing levels of glucose tolerance status (0=normal, 1=impaired/diabetic). bAdditive regression model coefficients. cThese dependent variable data were natural log‐transformed to correct for skewness in regression models. The results are presented as geometric mean ratios (GMRs) representing multiplicative increases in the dependent variable (eg, triglycerides) associated with the independent variable (eg, obesity). All models include age, sex, smoking, and alcohol use.
Figure FIGURE.
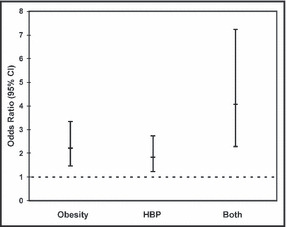
The odds ratio for abnormal glucose tolerance, represented by the small square, and 95% confidence interval (CI), represented by the vertical line, is depicted for obesity alone, for high blood pressure (HBP) alone, and for both obesity and high blood pressure.
Discussion
Data from this study demonstrate that both HBP and obesity are individually associated with an increase in odds for abnormal glucose tolerance by 1.8‐ and 2.2‐fold, respectively. The odds for abnormal glucose tolerance increase 4‐fold with coexistence of both HBP and obesity. In addition, the geometric mean of HOMA, an estimate of insulin resistance, increases by 18% with HBP, 60% with obesity, and 90% with the presence of both HBP and obesity. Although no statistically significant interaction between HBP and obesity was detected, the relationships of both conditions with the metabolic risk factors are clearly additive.
Both obesity and hypertension occur at higher rates in African Americans and the prevalence continues to rise. 6 , 7 There are extensive data available on the link between obesity and abnormal glucose metabolism. 8 , 9 Data that demonstrated improved glucose metabolism following weight reduction support the causal effect of obesity on abnormal glucose metabolism. 10 , 11 , 12 An independent association between hypertension and abnormal glucose metabolism has also been reported. Data from the San Antonio Heart Study showed that baseline hypertension was associated with an increase in 8‐year incidence of impaired glucose tolerance in both Mexicans and non‐Hispanic whites. 13 Feskens and colleagues 14 performed a cross‐sectional analysis on longitudinal data obtained over 30 years on the Finnish and Dutch cohorts of the Seven Countries Study. These investigators showed that men with diabetes and impaired glucose tolerance at the 30‐year follow‐up examination had significantly higher SBP and higher prevalence of hypertension than men with normal glucose tolerance, and the differences in BP between the glucose tolerance groups could be seen 5, 20, and 30 years earlier. Brancati and colleagues 15 also reported that higher BP was associated with greater risk of developing type 2 diabetes in African Americans based on analysis of data from the Atherosclerosis Risk in Communities Study. A previous study by our group in a different cohort of young adult African Americans reported a highly significant inverse correlation of BP and insulin sensitivity quantified by insulin clamp, and higher BP was associated with abnormal glucose metabolism. 16 The results of our current study are consistent with previous findings that HBP and obesity were individually associated with higher risk of abnormal glucose metabolism. The coexistence of HBP and obesity increase the risk of abnormal glucose metabolism further than either condition alone.
Rates of obesity are much higher in African Americans than in Caucasians. 7 Although the association between excessive body weight and adverse outcome is clear, controversy remains on the level of excess body weight or BMI that confers heightened risk for adverse outcome. Several studies reported that adverse outcomes are mostly associated with BMI in excess of 30 kg/m2, and there has been little or no evidence of adverse outcomes associated with BMI in the range of 25 kg/m2 to 30 kg/m2. 17 , 18 , 19 , 20 However, using data from the National Institutes of Health/American Association of Retired Persons cohorts, Adams and colleagues. 21 reported that BMI in both the overweight and obese ranges were associated with higher mortality in healthy lifetime nonsmokers. When the analyses were restricted to BMI during midlife (50 years), the association became stronger, with mortality rates increasing 20% to 40% in those with BMI from ≥25 to <30 kg/m2, and 2‐ to 3‐fold in those with BMI ≥30 kg/m2. Our data from this study on young adult African Americans demonstrate a progressive increase in prevalence of abnormal glucose tolerance as BMI exceeds 25 kg/m2 in men while the trend was less clear in women.
Despite their greater rates of CV events, African Americans appear to have a more favorable lipid profile compared with Caucasians. In both men and women, mean plasma triglyceride concentration is reported to be approximately 20 mg/dL lower in African Americans compared with their Caucasian counterparts. 22 Our previous study in a different cohort of young adult African Americans reported a significant correlation of insulin resistance measured by insulin clamp with triglyceride level, and this relationship extended below the triglyceride threshold for metabolic syndrome. 23 The association between HBP and higher triglyceride level has also been reported in previous studies. 23 , 24 Compared with Caucasians, HDL levels are higher in African Americans. 25 The higher HDL levels among African Americans can not be explained by race differences in BMI or other factors that have an effect on plasma HDL concentration such as alcohol intake, physical activity, or smoking. 26 Although African Americans appear to have a more favorable plasma lipid pattern compared with Caucasians and Mexican Americans, it is not clear whether the lipid patterns in African Americans are protective or whether the lipid threshold at which risk increases is at a lower level of triglyceride and higher level of HDL in African Americans. In our current study of young adult African Americans, HBP was associated significantly with higher triglyceride level while obesity was associated significantly with lower HDL and higher LDL levels.
Our results confirm previous findings that both obesity and HBP contribute to the increased risk of abnormal glucose tolerance. In addition, our results in this group of young adult African Americans demonstrate that the effect of obesity and hypertension on metabolic risk factors start at a young age.
Limitations
There are some limitations in this study. First is the cross‐sectional design. While the results of our data analysis suggested an additive relationship for both HBP and obesity with deterioration of glucose metabolism, these findings represent an association and do not permit us to draw conclusions on causation. Secondly, there were more nonobese men with HBP and more obese women with NBP enrolled in the study. Although the rates of obesity among African American men and women in our cohort are consistent with national data, the lack of sex balance could have potentially skewed the results. Due to the clinical concern about diuretics on abnormal glucose metabolism, we conducted analyses to determine whether diuretic use among hypertensives contributed to the adverse effect on glucose metabolism. The results of our analysis found that diuretic use did not contribute to the additive effect of hypertension on abnormal glucose tolerance. We did not find any effect of antihypertensive medication on abnormal glucose tolerance. Due to smaller numbers of cases using other classes of medication and frequent use of combinations of drug classes, we did not have sufficient power to conduct a separate analysis for each class of antihypertensive medication. Finally, all participants in this study were African American, which limits the generalizability of the results to other populations.
Conclusions
Obesity and HBP are both independently associated with greater prevalence of abnormal glucose metabolism. The presence of both conditions together was associated with additive increases in the prevalence or levels of metabolic risk factors. These results indicate that the high rates of HBP and obesity in African Americans may place this ethnic group at a disproportionately high risk for abnormal glucose tolerance and associated CV disease.
Disclosures:
This work was supported by a grant from the Pennsylvania Department of Health. The department disclaims responsibility for any analyses, interpretations, or conclusions. There is no conflict of interest.
References
- 1. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 3. Hozawa A, Folsom AR, Sharrett AR, et al. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects – Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. [DOI] [PubMed] [Google Scholar]
- 4. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 5. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 6. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 7. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;29:2847–2850. [DOI] [PubMed] [Google Scholar]
- 8. Folsom AR, Burke GL, Byers CL, et al. Implications of obesity for cardiovascular disease in blacks: the CARDIA and ARIC studies. Am J Clin Nutr. 1991;53:1604S–1611S. [DOI] [PubMed] [Google Scholar]
- 9. Poirier P, Giles TD, Bray GA, et al; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism . Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 10. Dengel DR, Hagberg JM, Pratley RE, et al. Improvements in blood pressure, glucose metabolism, and lipoprotein lipids after aerobic exercise plus weight loss in obese, hypertensive middle‐aged men. Metab Clin Exp. 1998;47:1075–1082. [DOI] [PubMed] [Google Scholar]
- 11. Racette SB, Weiss EP, Hickner RC, et al. Modest weight loss improves insulin action in obese African Americans. Metab Clin Exp. 2005;54:960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batsis JA, Sarr MG, Collazo‐Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morales PA, Mitchell BD, Valdez RA, et al. Incidence of NIDDM and impaired glucose tolerance in hypertensive subjects. The San Antonio Heart Study. Diabetes. 1993;42:154–161. [DOI] [PubMed] [Google Scholar]
- 14. Feskens EJ, Tuomilehto J, Stengard JH, et al. Hypertension and overweight associated with hyperinsulinaemia and glucose tolerance: a longitudinal study of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetologia. 1995;38:839–847. [DOI] [PubMed] [Google Scholar]
- 15. Brancati FL, Kao WH, Folsom AR, et al. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. [DOI] [PubMed] [Google Scholar]
- 16. Falkner B, Sherif K, Sumner AE, et al. Blood pressure increase with impaired glucose tolerance in young adult American blacks. Hypertension. 1999;34:1086–1090. [DOI] [PubMed] [Google Scholar]
- 17. Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 18. Romero‐Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 19. Flegal KM, Graubard BI, Williamson DF, et al. Cause‐specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. [DOI] [PubMed] [Google Scholar]
- 20. Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–3271. [DOI] [PubMed] [Google Scholar]
- 21. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 22. Hutchinson RG, Watson RL, Davis CE, et al. Racial differences in risk factors for atherosclerosis. The ARIC study. Atherosclerosis Risk in Communities. Angiology. 1997;48:279–290. [DOI] [PubMed] [Google Scholar]
- 23. Stein E, Kushner H, Gidding S, et al. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metab Clin Exp. 2007;56:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Natali A, Muscelli E, Casolaro A, et al. Metabolic characteristics of prehypertension: role of classification criteria and gender. J Hypertens. 2009;27:2394–2402. [DOI] [PubMed] [Google Scholar]
- 25. Hall WD, Clark LT, Wenger NK, et al; for the African‐American Lipid and Cardiovascular Council (AALCC) . The metabolic syndrome in African Americans: a review. Ethn Dis. 2003;13:414–428. [PubMed] [Google Scholar]
- 26. Sprafka JM, Norsted SW, Folsom AR, et al. Life‐style factors do not explain racial differences in high‐density lipoprotein cholesterol: the Minnesota Heart Survey. Epidemiology. 1992;3:156–163. [DOI] [PubMed] [Google Scholar]


