Abstract
J Clin Hypertens (Greenwich). 2012;14:799–801. ©2012 Wiley Periodicals, Inc.
Sympathetic overactivity plays a crucial pathogenetic role in the maintenance and aggravation of arterial hypertension in patients with end‐stage renal disease (ESRD). Renal denervation has been shown to be effective and safe in reducing blood pressure (BP) in patients with treatment‐resistant hypertension; however, there are only case reports in hypertensive patients with ESRD and data are lacking about possibility of renal denervation in small renal arteries. A woman with uncontrolled treatment‐resistant hypertension on chronic hemodialysis underwent bilateral native kidney, catheter‐based renal denervation. Both native renal arteries were <4 mm. After 6 months without any change of antihypertensive medication or hemodialysis parameters, the authors observed a remarkable BP reduction of 38/30 mm Hg (from baseline 172/100 mm Hg to 134/70 mm Hg) as evaluated by 24‐hour ambulatory BP monitoring. The authors report that renal denervation seems to be effective in controlling hypertension in patients with ESRD, even in cases of small renal arteries.
Arterial hypertension is one of the leading pathogenetic factors contributing to the worldwide increasing incidence of end‐stage renal disease (ESRD) and likely contributes to the high rates of cardiovascular (CV) disease in chronic hemodialysis patients. The rates of CV disease in patients with ESRD remains high despite the availability of hemodialysis (5.1% dying of acute myocardial infarction, an additional 5% dying of heart failure, and 26.1% dying of arrythmia or cardiac arrest (USRDS atlas: http://www.usrds.org/2011/pdf/v2_ch04_11.pdf). Animal experiments and human studies have demonstrated increased sympathetic nervous system activity contributing to the development, maintenance, and progression of both hypertension and chronic kidney disease. 1 Nevertheless, the contribution of the native nonfunctioning kidney to hypertension in ESRD patients remains speculative. Nephrectomy of native kidney in patients undergoing hemodialysis has been reported to reduce blood pressure (BP) and muscle sympathetic nerve activity. 2 Spinal rhizotomy of rats with hypertension following partial nephrectomy has been shown to simultaneously reduce BP and hypothalamic noradrenaline content, confirming that the kidneys are neurologically active and contribute to sympathetic‐mediated hypertension. 3 A more recent rat model of uninephrectomy and chronic aortic regurgitation showed that activation of the sympathetic nervous system augments kidney renin‐angiotensin system and oxidative stress, which act as crucial cardiorenal mediators. 4 Previous studies in the 1950s reported encouraging effects on hypertension control after extensive nonselective surgical sympathectomy, which likely included the splanchnic and renal vascular beds. Despite improved mortality rates in these patients, the procedures were associated with significant comorbidity and adverse events. In recent clinical studies, renal denervation has been shown to be effective and safe in reducing BP in treatment‐resistant hypertension. However, these studies excluded patients with chronic kidney disease (estimated glomerular filtration rate <45 mL/min/1.73 m2) and renal arteries <4 mm in diameter. 5 , 6 The potential safety and efficacy of therapeutic denervation of native nonfunctioning kidney in patients with ESRD are limited to two case reports. 7 , 8 The potential to reduce CV risk in this population without jeopardizing renal function offers a unique benefit risk of therapeutic renal denervation. Frequently, however, the renal arteries in ESRD patients are atrophic and below the renal artery diameter required by the Symplicity‐HTN clinical trials. Thus, the safety of the device in this patient population remains unexplored. We therefore initiated a study named “Renal Nerve Ablation in Chronic Kidney Disease Patients” (NCT01442883) to address these questions. Here we report our experience in the first enrolled participant, a hypertensive patient with ESRD sustained on hemodialysis and with renal artery diameter of 3 mm.
Case Report
A 29‐year‐old anuric woman (158 cm, dry weight 57 kg) with ESRD on chronic hemodialysis (for 3 years) and treatment‐resistant hypertension was admitted to our university hospital. In history, renal biopsy for evaluation of underlying disease was not performed because the patient already had stage 4 chronic kidney disease at first referral and small kidneys were found by sonography. Secondary causes for hypertension other than renal disease were ruled out, including sleep apnea. Despite being prescribed 6 antihypertensive drugs from different classes (including an angiotensin receptor blocker, calcium channel blocker, β‐blocker, centrally acting sympatholytic, vasodilator, and α1‐blocker), 24‐hour ambulatory BP monitoring (ABPM) (Spacelab No. 90207, Redmond, WA) assessed between hemodialysis (starting the day following hemodialysis and ending in adequate time for the beginning of the next dialysis) showed an increased systolic and diastolic BP of 172/100 mm Hg (daytime: 172/100 mm Hg, nighttime: 170/98 mm Hg). Several interventions of dialysis modalities such as decreasing dialysate sodium concentration, dietary efforts to reduce interdialytic weight gain, and/or reduction of estimated dry weight had not lead to sustained improvement of BP control. Antihypertensive medications were titrated to maximum tolerated dose, and yet BP remained substantially above target levels.
The study protocol was approved by the local ethics committee and the study is being performed according to the Declaration of Helsinki and good clinical practice guidelines. Written informed consent was obtained from the patient before study entry.
The femoral artery was accessed with standard endovascular technique. A Symplicity catheter (Medtronic Ardian Inc, Palo Alto, CA) was advanced in each renal artery by angiography and renal denervation was performed in the standard manner. Renal angiography showed one main renal artery with adequate length (≥20 mm) on both sides. However, the diameter of the right renal artery was only 2.6 mm (Figure) and of the left renal artery only 3.3 mm, thereby failing the anatomical eligibility criteria of the Symplicity trials. 5 , 6 After positioning a 6F‐guided catheter in the ostium of the renal artery, the catheter was advanced into the right and then left renal artery. Six right‐sided (55 s, 80 s, 47 s, 49, 20 s, and 72 s) and 4 left‐sided (91 s, 77 s, 76 s, 120 s) radiofrequency ablations, controlled and regulated by a pre‐programmed Symplicity generator, were applied in a spiral pattern separated by about 0.5 cm. These ablation durations aborted spontaneously and automatically prior to the default period of 2 minutes due to catheter tip temperatures achieving preprogrammed limits. In all instances, the catheter was repositioned and treatment was reported. Arterial spasms were treated with repeat administration of 0.2 mg of nitroglycerine and angiographic evaluation after renal denervation showed no major irregularities. As per protocol, 5000 IE of heparin was administered intravenously to maintain an activated clotting time of >250 seconds. Diffuse visceral pain, limited to the duration of active radiofrequency ablation, was managed with anxiolytics and narcotics. Neither access nor arterial uncontrollable adverse events occurred during the procedure or were identified in the 6 months following.
Figure FIGURE.
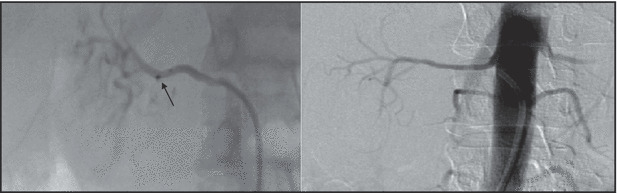
Left panel: Angiography of the right renal artery (diameter 2.6 mm) with positioned Symplicity catheter (tip marked with arrow; Medtronic Ardian Inc, Palo Alto, CA) at the first point of radiofrequency ablation. Right panel: Angiography of the right renal artery after renal denervation.
Twenty‐four‐hour ABPM (Spacelab No. 90207) performed between hemodialysis (starting the day following hemodialysis and ending in adequate time for the beginning of the next dialysis) showed a remarkable BP reduction to 144/84 mm Hg (daytime: 139/82 mm Hg, nighttime: 154/87 mm Hg) after 3 months, which was further reduced to 134/70 mm Hg (daytime: 134/72 mm Hg, nighttime: 136/66 mm Hg) after 6 months, although neither antihypertensive medication nor hemodialytic parameters were changed.
Discussion
Renal denervation has been shown to be an effective, durable, and safe intervention in patients with treatment‐resistant hypertension without any significant changes in renal function. 5 , 6 No published data to date demonstrate the safety and efficacy of intravascular renal denervation for BP control or CV morbidity in patients with ESRD on hemodialysis. This strategy is linked to the assumptions that the native nonfunctioning kidney continues to participate in BP control and CV morbidity and mortality. In 2002, Zoccali and colleagues 9 demonstrated that sympathetic nerve overactivity, as assessed by plasma norepinephrine concentration, was predictive of survival and CV events in patients with ESRD. In the same year, a study reported that after renal transplantation patients with excellent graft function and no clinical signs of uremia still had elevated muscle sympathetic nerve activity assessed by microneurography at levels of chronic hemodialysis patients. These results imply that the diseased kidneys might be the source of a still‐elevated activity level of the central nervous system. The results further imply that renal somatic afferent chemoreceptors or mechanoreceptors may be hyperactive in ESRD. Indeed, bilateral nephrectomy in renal transplant recipients resulted in a normalization of muscle sympathetic nerve activity, which did not differ from a healthy control group, suggesting that sympathetic activation is mediated by afferent signals from the native kidney to the central sympathetic system. 2 Laboratory animal studies have revealed that afferent sensory signals of sensory nerves are sympathoinhibitory and that renal damage may diminish this inhibitory effect, leading to enhanced sympathetic activity. 10 While therapeutic nephrectomy has been considered for patients with malignant hypertension on dialysis, the inherent morbidity and mortality of bilateral retroperitoneal procedures has limited its adoption. Thus, the potential for a brief, intra‐arterial procedure to denervate the native nonfunctioning kidney offers promise to alter the CV prognosis in hypertension.
In contrast to a recently reported case, 7 we used the Symplicity system for the renal denervation procedure. This system is the only currently approved device for renal denervation, using the preprogrammed radiofrequency algorithm. We did not observe any uncontrollable adverse event during the procedure or during the short‐term follow‐up of 6 months. A second case report of renal denervation also used the Symplicity system but no data about the diameter of the renal arteries or applied energy time were given. 8 In our patient, with small‐diameter renal artery and likely low renal blood flow, only 1 of 10 ablation procedures completed the programmed 120‐second treatment duration. The small diameter of the renal arteries may have reduced endothelial cooling due to blood flow, with the generator automatically terminating treatment to avoid excessive temperature rise. Nonetheless, the procedure appears to have been completed without adverse vascular events and resulted in a substantial clinical benefit of a 38/30‐mm Hg reduction of mean 24‐hour ABPM.
Conclusions
As a first proof‐of‐concept case we could demonstrate that renal denervation with the Symplicty catheter seems to be safe and effective BP treatment in a treatment‐resistant hypertensive patient with ESRD. Larger prospective studies are underway examining the safety and benefits in a larger cohort of ESRD patients on hemodialysis. Additional studies are examining the effect of native kidney denervation in ESRD patients with a transplanted kidney. Denervation of the native nonfunctioning kidney’s neural contribution to central sympathetic drive could reduce the excessive CV morbidity and mortality seen in ESRD patients.
Acknowledgments and disclosures: We gratefully acknowledge the expert technical assistance of Ulrike Höfer and the assistant personnel of the angiography laboratory. There is no source of funding. Conflict of interest: A.S. has received lectures fees from Medtronic Inc. P.A.S. was an employee of Medtronic Inc. R.E.S. has received grants, performed lectures, and received consultancy fees from Medtronic Inc.
References
- 1. Schlaich MP, Sobotka PA, Krum H, et al. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54:1195–1201. [DOI] [PubMed] [Google Scholar]
- 2. Hausberg M, Kosch M, Harmelink P, et al. Sympathetic nerve activity in end‐stage renal disease. Circulation. 2002;106:1974–1979. [DOI] [PubMed] [Google Scholar]
- 3. Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. [DOI] [PubMed] [Google Scholar]
- 4. Rafiq K, Noma T, Fujisawa Y, et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krum H, Schlaich M, Whitbourn R, et al. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 6. Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension (the Symplicity HTN‐2 trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 7. Prochnau D, Lauten A, Busch M, et al. Catheter‐based radiofrequency ablation therapy of the renal sympathetic‐nerve system for drug resistant hypertension in a patient with end‐stage renal disease. Int J Cardiol. 2012;154:e29–e30. [DOI] [PubMed] [Google Scholar]
- 8. Di Daniele N, De Francesco M, Violo L, et al. Renal sympathetic nerve ablation for the treatment of difficult‐to‐control or refractory hypertension in a haemodialysis patient. Nephrol Dial Transplant. 2012;27:1689–1690. [DOI] [PubMed] [Google Scholar]
- 9. Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end‐stage renal disease. Circulation. 2002;105:1354–1359. [DOI] [PubMed] [Google Scholar]
- 10. Ditting T, Freisinger W, Siegel K, et al. Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension. 2012;59:467–476. [DOI] [PubMed] [Google Scholar]


