Abstract
The authors hypothesized that carvedilol controlled‐release plus lisinopril combination therapy (C+L) would increase endothelial function and decrease oxidative stress to a greater extent than hydrochlorothiazide plus lisinopril combination therapy (H+L) in obese patients with hypertension. Twenty‐five abdominally obese patients (aged 54.4±7.3 years; 14 women) with hypertension/prehypertension were enrolled in a 7‐month (two 3‐month treatment periods separated by a 1‐month washout), randomized, double‐blind, controlled, crossover clinical trial comparing C+L vs H+L. Endothelial function, measured by digital reactive hyperemic index (RHI), circulating oxidized low‐density lipoprotein (oxLDL), 8‐isoprostane, and asymmetric dimethylarginine (ADMA) were obtained at baseline, post‐period 1, post‐washout, and post‐period 2. Analyses were adjusted for baseline measurements by analysis of covariance, with robust variance estimation for confidence intervals and P values. C+L treatment compared to H+L treatment significantly improved RHI (0.74, 95% confidence interval, 0.31–1.19, P =.001). This difference persisted after adjustment for the change in systolic blood pressure. No significant treatment differences were observed for oxLDL, 8‐isoprostane, or ADMA. These data provide evidence that independent of blood pressure–lowering, C+L therapy improves endothelial function to a greater extent than H+L therapy. Levels of oxidative stress were not significantly different between treatments, suggesting that other mechanisms may be responsible for the improvement in endothelial function.
Obesity and hypertension often coexist and each condition is independently associated with endothelial dysfunction. Endothelial dysfunction is thought to be one of the earliest detectable signs of atherosclerosis. The presence of endothelial dysfunction independently predicts the development of atherosclerosis and future cardiovascular events. 1 , 2 , 3 Healthy vascular endothelial cells control arterial tone by producing vasodilating factors such as nitric oxide, a free radical that signals underlying smooth muscle cells to relax. 4 Nitric oxide helps to prevent atherosclerosis by interfering with monocyte adhesion to the arterial wall, inhibiting smooth muscle cell proliferation and decreasing platelet aggregation. 5 , 6 , 7 Impaired bioavailability of nitric oxide (decreased production and/or increased inactivation) often manifests as endothelial dysfunction. 8 In the context of hypertension and obesity, oxidative stress has been implicated as a primary cause of reduced nitric oxide bioavailability and impaired endothelial function. 9 , 10
When considering antihypertensive medication for obese individuals, agents that have the potential to improve endothelial function may be desirable. Multiple studies have shown that angiotensin‐converting enzyme (ACE) inhibitors improve endothelial function, 11 , 12 , 13 probably by increasing bradykinin in the arterial wall 14 and decreasing oxidative stress. 15 Traditionally, β‐adrenergic receptor blockers (β‐blockers) have not been favored as first‐line antihypertensive agents in obese patients, especially in those with type 2 diabetes or prediabetes since first‐ and second‐generation agents have been shown to worsen glycemic control. 16 , 17 However, evidence is accumulating that third‐generation β‐blockers such as carvedilol and nebivolol do not negatively affect glucose metabolism. 18 , 19 Moreover, studies have reported beneficial effects of these medications on endothelial function, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 with reduction of oxidative stress as a purported mechanism. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36
Since third‐generation β‐blockers and ACE inhibitors independently reduce oxidative stress and augment endothelial function, it is possible that when used together as combination antihypertensive therapy, these drugs may act in a complimentary fashion to improve vascular health. Therefore, we conducted a randomized, controlled, crossover (all participants received both therapies) clinical trial and hypothesized that carvedilol controlled‐release plus lisinopril combination therapy (C+L) would increase endothelial function and decrease oxidative stress to a greater extent than hydrochlorothiazide plus lisinopril combination therapy (H+L) in obese patients with hypertension/prehypertension. We chose to compare these specific pairs of medications because they are commonly used antihypertensive medication combinations. Hydrochlorothiazide was selected as the main comparator (in essence, this was the case because lisinopril was used in both arms) because of its neutral vascular effects. 11 , 23
Methods
Patient Population
Twenty‐five hypertensive/prehypertensive patients (systolic blood pressure [SBP] ≥130 mm Hg and/or diastolic blood pressure [DBP] ≥85 mm Hg or currently taking antihypertensive medication) with abdominal obesity (waist circumference ≥102 cm for men and ≥88 cm for women) were enrolled. The blood pressure criteria were based on values corresponding with the hypertension component of the metabolic syndrome. 37 Patients were excluded if they were not on a stable (≥1 month) cardiovascular medication regimen, currently (<1 month) using antihypertensive medications, had contraindications for β‐blocker or ACE inhibitor therapy, or had a history of myocardial infarction, angina, or heart failure. Patients were recruited from local medical clinics and through advertisements. The study protocol was approved by an institutional review board and consent was obtained from all participants.
Study Design
We performed a 7‐month, randomized, double‐blind, active‐control, crossover (participants received both combination therapies, one in each period) clinical trial. Patients were treated for 3 months each with C+L and H+L in a randomized order. Study periods were separated by a 1‐month washout period during which all antihypertensive therapy was discontinued. Measurements of study variables were made at baseline (immediately prior to randomization), month 3 (post‐period 1), month 4 (post‐washout), and month 7 (post‐period 2). All testing was performed in the morning after patients had been fasting for at least 12 hours.
Antihypertensive Treatment Protocol
Patients who were taking antihypertensive medication(s) at the time of screening underwent a 1‐month washout period prior to randomization during which all antihypertensive therapy was discontinued. C+L combination therapy was initiated at 20 mg and 10 mg (low dose), respectively. Patients returned 1 week later and doses of carvedilol CR and lisinopril were increased to 40 mg and 20 mg (high dose), respectively, if SBP was ≥130 mm Hg or DBP was ≥85 mm Hg. H+L combination therapy was initiated at 12.5 mg and 10 mg (low dose), respectively. Patients returned 1 week later and doses of hydrochlorothiazide and lisinopril were increased to 25 mg and 20 mg (high dose), respectively, if SBP was ≥130 mm Hg or DBP was ≥85 mm Hg. Patients and investigators/study staff members were blinded to the treatment assignments. Study medications were withheld on the mornings of all study visits.
Measurement of Clinical Variables
Height and weight were obtained using a standard stadiometer and electronic scale, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was obtained at end‐expiration and measured midway between the base of the rib cage and the superior iliac crest. Sitting blood pressure measurements were obtained manually on the same arm using the same cuff size and equipment after the patient had been resting quietly for 10 minutes. The final 2 of 3 consecutive measurements, separated by 3 minutes, were averaged and used for analysis. Fasting lipid profile, glucose, and insulin assays were conducted with standard procedures by Quest Diagnostics (Minneapolis, MN). Homeostasis model assessment of insulin resistance (HOMA‐IR), a surrogate measure of insulin resistance, was calculated using previously described methods. 38
Oxidative Stress Biomarkers
Blood plasma for biomarker analysis was stored at −80°C until the end of the study, at which time all samples were assayed together. Circulating oxidized low‐density lipoprotein (oxLDL) (Mercodia, Inc, Winston‐Salem, NC), 8‐isoprostane (Cayman Chemical Company, Ann Arbor, MI), and asymmetric dimethylarginine (ADMA) (ALPCO, Salem, NH) were measured in duplicate by enzyme‐linked immunosorbent assay in the University of Minnesota Cytokine Reference Laboratory (CLIA licensed).
Endothelial Function Assessment
Endothelial function was measured noninvasively by digital reactive hyperemia (EndoPAT 2000; Itamar Medical, Caesarea, Israel). Digital reactive hyperemia is nitric oxide–dependent, 39 associated with coronary artery blood flow 40 and multiple cardiovascular risk factors, 41 and independently predicts future cardiovascular events. 3 Following 10 minutes of quiet rest in the supine position, finger probes were placed on the index fingers of both hands to measure baseline and reactive hyperemic pulse amplitude. Inside the probes, a uniform pressure (10 mm Hg < DBP) was placed on the fingers, which allowed for the detection of small pulse volume changes throughout the cardiac cycle. Following the collection of 5 minutes of baseline data, a blood pressure cuff on the upper arm was inflated to a suprasystolic level for 5 minutes. Following cuff release, the change in pulse amplitude during reactive hyperemia was measured for 5 minutes. The ratio of the hyperemic to the baseline pulse amplitude (corrected for the same ratio on the control finger) was calculated and expressed as the reactive hyperemic index (RHI). Endothelium‐independent hyperemic index (EIHI) was quantified by calculating the ratio of the hyperemic to the baseline (immediately pre‐nitroglycerin) pulse amplitude in the control finger (arm not previously occluded for RHI measurement) following the administration of 0.4 mg sublingual nitroglycerin.
Statistical Analysis
Baseline characteristics were tabulated with respect to randomized order of C+L and H+L. Outcomes were evaluated at baseline, end of period 1, end of washout, and end of period 2. Treatment effects for period 1 used the difference from baseline while effects for period 2 were based on the difference from the end of washout. SBP was selected a priori as a variable that may influence results and was adjusted for in secondary analyses. Generalized estimating equations were used with exchangeable working correlation structure and robust variance estimation was used for confidence intervals and P values. All statistical analyses were performed using R v2.9.2 with the “gee” library v4.13–14 to account for correlated responses.
Results
Twenty‐five abdominally obese patients (age, 54.4±7.3 years; 14 women; waist circumference, 123.7±15.7 cm) with hypertension/prehypertension (SBP, 138±13 mm Hg; DBP, 85±11 mm Hg) were enrolled. None of the patients had type 1or type 2 diabetes mellitus. Two of the 25 patients who were randomized did not have any follow‐up, and one was evaluated after the first period only. Across both first and second periods, 12 of 23 (52%) required the high dose of C+L, while 9 of 22 (41%) required the high dose of H+L. There were no significant differences in any of the variables at baseline by treatment order group (Table I). One patient had a missing value for the primary outcome of RHI at the end of washout.
Table I.
Baseline Characteristics
| Covariate | Overall | C+L/H+L Group | H+L/C+L Group |
|---|---|---|---|
| (N=23) | (n=9) | (n=14) | |
| Age, y | 54.0 (7.5) | 52.7 (9.21) | 54.9 (6.39) |
| Sex (male) | 10 (43.5%) | 3 (33.3%) | 7 (50.0%) |
| Race (white) | 23 (100.0%) | 9 (100.0%) | 14 (100.0%) |
| BMI, kg/m2 | 38.2 (7.53) | 38.5 (6.89) | 38.0 (8.17) |
| SBP, mm Hg | 136 (12.1) | 135 (8.7) | 137 (14.0) |
| DBP, mm Hg | 83.9 (10.3) | 84.9 (7.1) | 83.3 (12.2) |
| Heart rate, beats per min | 71.3 (9.12) | 74.3 (10.8) | 69.3 (7.64) |
| Total cholesterol, mg/dL | 183 (37.5) | 199 (34.8) | 172 (36.6) |
| LDL cholesterol, mg/dL | 104 (33.1) | 116 (35.2) | 96.4 (30.4) |
| HDL cholesterol, mg/dL | 51.0 (12.1) | 58.2 (10.5) | 46.4 (11.0) |
| Triglycerides, mg/dL | 138 (41.5) | 123 (21.9) | 148 (48.5) |
| Glucose, mg/dL | 97.5 (11.2) | 92.4 (8.63) | 101 (11.7) |
| Insulin, mU/La | 8.39 (4.54) | 7.0 (6.06) | 9.27 (3.29) |
| HOMA‐IRa | 2.05 (1.22) | 1.69 (1.64) | 2.28 (0.88) |
| RHIb | 2.12 (0.59) | 2.11 (0.53) | 2.13 (0.64) |
| EIHI | 3.09 (3.1) | 4.17 (4.0) | 2.39 (2.26) |
| oxLDL, U/L | 48.3 (11.7) | 52.3 (7.59) | 45.7 (13.4) |
| 8‐Isoprostane, pg/mL | 7.9 (11.5) | 6.81 (7.67) | 8.61 (13.6) |
| ADMA, μmol/L | 0.46 (0.06) | 0.45 (0.06) | 0.46 (0.05) |
Abbreviations: ADMA, asymmetric dimethylarginine; BMI, body mass index; DBP, diastolic blood pressure; EIHI, endothelium‐independent hyperemic index; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; oxLDL, oxidized low‐density lipoprotein; RHI, reactive hyperemic index; SBP, systolic blood pressure. Data are shown as mean (standard deviation) and No. (%) where indicated. aFive patients missing data. bOne patient missing the value at the end of washout only.
The trajectories of patients’ RHI values from baseline across period 1, washout, and period 2 are displayed in the Figure. At the conclusion of the washout period, the changes observed over period 1 appeared to have persisted somewhat, suggesting a potential carry‐over effect and raising the possibility that the data from period 2 may be challenging to interpret. Therefore, a follow‐up analysis examining the data from the first period only (data with no concerns about reliability) was conducted with adjustment for baseline measurements. There was one patient with a missing value at the end of washout but not the end of period 2.
Figure FIGURE.
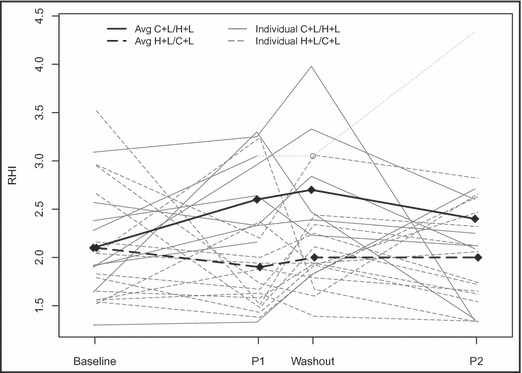
Change in RHI by period according to treatment order grouping. Open circle represents the patient who was missing data from washout and the measurement from the end of period 1 carried forward.
Compared with H+L, C+L was found to have significantly higher RHI of 0.67 (95% confidence interval [CI], 0.17–1.16) after adjusting for period and baseline SBP (P=.008). Due to the missing data at the end of washout, the experience for 1 of the 9 patients randomized to C+L/H+L only contributed information from the first period. This patient’s value at the end of period 2 was the largest observed RHI at any time point in the study. When that patient’s RHI at the end of the first period was carried forward and used to calculate the change over period 2, the treatment effect was attenuated to 0.56 (95% CI, 0.05–1.07) although it remained statistically significant (P=.032).
As mentioned previously, evaluation of treatment differences in RHI (adjusting for baseline RHI and SBP) at the end of washout suggested a significant difference between the two treatment groups of 0.72 (95% CI, 0.26–1.17) as period 2 began (P=.002). As such, a follow‐up analysis was performed after removing the potentially unreliable data from the second period with adjustment for baseline measurements of RHI and SBP. The results were similar, with a significant difference of 0.75 (95% CI, 0.31–1.19) with P<.001.
Since blood pressure can directly influence endothelial function and we observed a nonstatistically significant difference (P=.20) in SBP by period in favor of H+L, a secondary analysis, adjusting for the change in SBP, was also conducted. Although attenuated, a meaningful effect persisted of 0.62 (95% CI, 0.12–1.11) with P=.015.
There were no significant differences by treatment for BMI, SBP, DBP, heart rate, total cholesterol, LDL cholesterol, high‐density lipoprotein cholesterol, triglycerides, glucose, insulin, HOMA‐IR, EIHI, oxLDL, 8‐isoprostane, or ADMA (Table II).
Table II.
Changes From Baseline Across Period 1, Washout, and Period 2
| Covariate | Period 1 | Washout | Period 2 | Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| SBP, mm Hg | |||||
| C+L/H+L | −6.44 (8.44) | −1.62 (9.98) | −21.1 (15.4) | −5.17 (−13.00 to 2.65) | .195 |
| H+L/C+L | −15.4 (14.5) | 2.07 (11.8) | −16.4 (14.4) | ||
| Total cholesterol, mg/dL | |||||
| C+L/H+L | −7.44 (21.0) | −5.12 (23.3) | −8.12 (23.9) | −0.57 (−11.63 to 10.48) | .919 |
| H+L/C+L | 0.57 (24.1) | 0.64 (24.9) | 8.43 (24.0) | ||
| LDL cholesterol, mg/dL | |||||
| C+L/H+L | −9.67 (20.0) | −5.88 (21.2) | −7.25 (25.3) | 4.58 (−4.73 to 13.88) | .335 |
| H+L/C+L | 1.64 (19.3) | 5.57 (26.7) | 7.79 (19.5) | ||
| HDL cholesterol, mg/dL | |||||
| C+L/H+L | −1.0 (9.22) | 0.5 (7.5) | 0.0 (9.04) | 0.23 (−3.27 to 3.73) | .899 |
| H+L/C+L | −0.79 (4.68) | −1.14 (5.46) | −1.71 (5.01) | ||
| Triglycerides, mg/dL | |||||
| C+L/H+L | 2.89 (51.4) | 0.62 (33.1) | −4.25 (47.6) | −8.18 (−31.46 to 15.10) | .491 |
| H+L/C+L | −0.93 (41.9) | 3.79 (64.8) | 11.7 (52.9) | ||
| HOMA‐IR | |||||
| C+L/H+L | −0.11 (1.77) | 0.89 (3.53) | 0.17 (1.42) | −0.09 (−1.22 to 1.04) | .875 |
| H+L/C+L | 0.67 (0.81) | −0.01 (0.87) | 0.0 (0.82) | ||
| RHI | |||||
| C+L/H+L | 0.48 (0.59) | 0.61 (0.59) | 0.22 (1.18) | −0.70 (−1.20 to −0.20) | .006 |
| H+L/C+L | −0.27 (0.83) | −0.11 (0.55) | −0.13 (0.65) | ||
| EIHI | |||||
| C+L/H+L | −0.99 (2.18) | −0.71 (3.02) | 0.01 (1.57) | 1.01 (−0.32 to 2.34) | .138 |
| H+L/C+L | −0.09 (1.13) | 0.04 (0.8) | −0.38 (1.13) | ||
| Oxidized LDL, U/L | |||||
| C+L/H+L | 0.72 (16.6) | 0.33 (16.4) | −2.79 (19.7) | 1.03 (−6.85 to 8.90) | .798 |
| H+L/C+L | 4.59 (12.7) | 7.47 (15.9) | 6.38 (12.4) | ||
| 8‐isoprostane, pg/mL | |||||
| C+L/H+L | 0.78 (9.25) | 5.09 (4.78) | 4.1 (9.86) | −0.67 (−6.62 to 5.27) | .825 |
| H+L/C+L | 1.44 (14.7) | −2.64 (15.1) | −0.93 (11.3) | ||
| ADMA, umol/L | |||||
| C+L/H+L | 0.01 (0.07) | −0.02 (0.07) | −0.03 (0.06) | −0.01 (−0.05 to 0.02) | .476 |
| H+L/C+L | 0.0 (0.03) | 0.01 (0.05) | 0.01 (0.06) | ||
Abbreviations: ADMA, asymmetric dimethylarginine; CI, confidence interval; C+L, carvedilol controlled‐release plus lisinopril combination therapy; EIHI, endothelium‐independent hyperemic index; HDL, high‐density lipoprotein; H+L, hydrochlorothiazide plus lisinopril combination therapy; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; RHI, reactive hyperemic index; SBP, systolic blood pressure.
Discussion
Our findings are in line with previous studies that have reported beneficial effects of third‐generation β‐blockers and ACE inhibitors on endothelial function. 11 , 12 , 13 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 In a previous study in patients with type 2 diabetes mellitus, we demonstrated that compared with metoprolol, carvedilol significantly improved brachial artery flow‐mediated dilation, a measure of conduit artery endothelial function. 20 The current study extends these observations by showing that C+L combination therapy improves endothelial function in resistance arteries (digital reactive hyperemia), suggesting a systemic vascular effect in an obese hypertensive/prehypertensive population. Endothelial improvements were observed with C+L despite the fact that this drug combination lowered blood pressure to a lesser degree compared with H+L and that as a group, average baseline blood pressure was relatively low in this patient population (136/84 mm Hg).
The current data suggest that reduction in level of systemic oxidative stress is not the mechanism responsible for improvement in endothelial function with C+L. This finding is in agreement with our earlier study, which did not show reductions of oxidative stress with carvedilol therapy in patients with type 2 diabetes mellitus. 20 Since our measures of oxidative stress were primarily systemic (blood plasma) and not specific to the vascular wall, it is still possible that carvedilol acts directly on the arterial wall to reduce the oxidative burden. Alternatively, other mechanisms, such as decreased peripheral vascular resistance, may be at least partially responsible for improvements in endothelial function with carvedilol. Unlike other β‐blockers, which generally do not reduce peripheral vascular resistance, carvedilol possesses α1‐adrenergic receptor–blocking effects 42 , 43 favoring increased nitric oxide bioavailability and peripheral arterial vasodilation.
Improvements in endothelial function with C+L therapy persisted somewhat after withdrawal in the washout period. The study was designed on the premise that any beneficial effects as a result of treatment would disappear within 1 month after discontinuation of therapy. Interestingly, however, it appears that the improvement in endothelial function with C+L may be durable up to at least 1 month after withdrawal of therapy. This finding suggests that the beneficial effects of C+L on the vasculature may involve changes in structural and/or mechanical effects of the arteries since it persisted longer than expected after the drugs were out of the system. Although noteworthy in a scientific sense, the clinical relevance of this finding may be somewhat limited since most patients use antihypertensive medications indefinitely.
Limitations
Study limitations included the fact that 2 participants dropped out of the trial following randomization, one did not complete period 2 and one did not have a useable RHI evaluation at the end of washout. In addition, we did not obtain information on the dietary and physical activity patterns of the participants.
Conclusions
Results of this study provide evidence that combination antihypertensive therapy with carvedilol CR and lisinopril significantly improves resistance artery endothelial function in abdominally obese individuals with hypertension/prehypertension. From a clinical perspective, treatment of hypertension with third‐generation β‐blockers and ACE inhibitors may be a preferred first‐line strategy since accumulating evidence suggests that these agents are associated with beneficial vascular effects. Future research in this area should attempt to clarify whether reduction in oxidative stress (measuring biomarkers specific to arterial oxidative stress) with carvedilol and other third‐generation β‐blockers is the primary mechanism of endothelial function improvement and continue to examine which antihypertensive medications offer the most beneficial effect on the health of the vasculature since preventing cardiovascular morbidity and mortality is the ultimate goal of therapy.
Acknowledgments and disclosures: Funding was provided by an investigator‐initiated grant (awarded to A.S.K.) from GlaxoSmithKline Pharmaceuticals, plc. Itamar Medical, Inc, provided assistance in the analysis of endothelium‐independent hyperemic index data. We are grateful to the participants who donated their time to this study. Dr Kelly has received research grant support from GlaxoSmithKline and Amylin/Eli Lilly and is a consultant (clinical trial advisory board) for Novo Nordisk. Dr Gonzalez‐Campoy receives research grant support from Novartis, Pfizer, Sanofi‐Aventis, Novo Nordisk, Leptos, Takeda, Amylin, Astra‐Zeneca, Boehringer Ingelheim, and Ipsen; is a member of the speakers’ bureau for Merck, Pfizer, Boehringer Ingelheim, Forest, and GlaxoSmithKline; and is a consultant for Merck, Pfizer, Leptos, and Roche. Dr Katz is a member of the speaker’s bureau for Eli Lilly, Novo Nordisk, and Amylin Pharmaceuticals. Dr Bank is a member of the speakers’ bureau for Forest Pharmaceuticals and receives research grant support from GlaxoSmithKline and Amylin/Eli Lilly. Dr Rudser, Ms Metzig, and Ms Thalin have no disclosures.
References
- 1. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 2. Halcox JP, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intima‐media thickness. Circulation. 2009;119:1005–1012. [DOI] [PubMed] [Google Scholar]
- 3. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 4. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. [DOI] [PubMed] [Google Scholar]
- 5. Bath PM, Hassall DG, Gladwin AM, et al. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb. 1991;11:254–260. [DOI] [PubMed] [Google Scholar]
- 6. Garg UC, Hassid A. Nitric oxide‐generating vasodilators and 8‐bromo‐cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium‐derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ross R. The pathogenesis of atherosclerosis – an update. N Engl J Med. 1986;314:488–500. [DOI] [PubMed] [Google Scholar]
- 9. Dobrian AD, Davies MJ, Schriver SD, et al. Oxidative stress in a rat model of obesity‐induced hypertension. Hypertension. 2001;2:554–560. [DOI] [PubMed] [Google Scholar]
- 10. Silver AE, Beske SD, Christou DD, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase‐p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. [DOI] [PubMed] [Google Scholar]
- 11. Higashi Y, Sasaki S, Nakagawa K, et al. A comparison of angiotensin‐converting enzyme inhibitors, calcium antagonists, beta‐blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35:284–291. [DOI] [PubMed] [Google Scholar]
- 12. Mancini GB, Henry GC, Macaya C, et al. Angiotensin‐converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–265. [DOI] [PubMed] [Google Scholar]
- 13. Nagamia S, Pandian A, Cheema F, et al. The role of quinapril in the presence of a weight loss regimen: endothelial function and markers of obesity in patients with the metabolic syndrome. Prev Cardiol. 2007;10:204–209. [DOI] [PubMed] [Google Scholar]
- 14. Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin‐converting enzyme inhibition increases human vascular tissue‐type plasminogen activator release through endogenous bradykinin. Circulation. 2003;107:579–585. [DOI] [PubMed] [Google Scholar]
- 15. Khan BV, Sola S, Lauten WB, et al. Quinapril, an ACE inhibitor, reduces markers of oxidative stress in the metabolic syndrome. Diabetes Care. 2004;27:1712–1715. [DOI] [PubMed] [Google Scholar]
- 16. Gress TW, Nieto FJ, Shahar E, et al. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–912. [DOI] [PubMed] [Google Scholar]
- 17. Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065–1070. [DOI] [PubMed] [Google Scholar]
- 18. Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt AC, Graf C, Brixius K, Scholze J. Blood pressure‐lowering effect of nebivolol in hypertensive patients with type 2 diabetes mellitus: the YESTONO study. Clin Drug Investig. 2007;27:841–849. [DOI] [PubMed] [Google Scholar]
- 20. Bank AJ, Kelly AS, Thelen AM, et al. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Hypertens. 2007;20:777–783. [DOI] [PubMed] [Google Scholar]
- 21. Broeders MA, Doevendans PA, Bekkers BC, et al. Nebivolol: a third‐generation beta‐blocker that augments vascular nitric oxide release: endothelial beta(2)‐adrenergic receptor‐mediated nitric oxide production. Circulation. 2000;102:677–684. [DOI] [PubMed] [Google Scholar]
- 22. Kalinowski L, Dobrucki LW, Szczepanska‐Konkel M, et al. Third‐generation beta‐blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–2752. [DOI] [PubMed] [Google Scholar]
- 23. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104:511–514. [DOI] [PubMed] [Google Scholar]
- 24. Jawa A, Nachimuthu S, Pendergrass M, et al. Beta‐blockers have a beneficial effect upon endothelial function and microalbuminuria in African‐American subjects with diabetes and hypertension. J Diabetes Complications. 2008;22:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korkmaz H, Karaca I, Koc M, et al. Early effects of treatment with nebivolol and quinapril on endothelial function in patients with hypertension. Endothelium. 2008;15:149–155. [DOI] [PubMed] [Google Scholar]
- 26. Lekakis JP, Protogerou A, Papamichael C, et al. Effect of nebivolol and atenolol on brachial artery flow‐mediated vasodilation in patients with coronary artery disease. Cardiovasc Drugs Ther. 2005;19:277–281. [DOI] [PubMed] [Google Scholar]
- 27. Matsuda Y, Akita H, Terashima M, et al. Carvedilol improves endothelium‐dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753–759. [DOI] [PubMed] [Google Scholar]
- 28. Pasini AF, Garbin U, Stranieri C, et al. Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257. [DOI] [PubMed] [Google Scholar]
- 29. Fahlbusch SA, Tsikas D, Mehls C, et al. Effects of carvedilol on oxidative stress in human endothelial cells and healthy volunteers. Eur J Clin Pharmacol. 2004;60:83–88. [DOI] [PubMed] [Google Scholar]
- 30. Fratta PA, Garbin U, Nava MC, et al. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J Hypertens. 2005;23:589–596. [DOI] [PubMed] [Google Scholar]
- 31. Lopez BL, Christopher TA, Yue TL, et al. Carvedilol, a new beta‐adrenoreceptor blocker antihypertensive drug, protects against free‐radical‐induced endothelial dysfunction. Pharmacology. 1995;51:165–173. [DOI] [PubMed] [Google Scholar]
- 32. Lysko PG, Webb CL, Gu JL, et al. A comparison of carvedilol and metoprolol antioxidant activities in vitro. J Cardiovasc Pharmacol. 2000;36:277–281. [DOI] [PubMed] [Google Scholar]
- 33. Mollnau H, Schulz E, Daiber A, et al. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol. 2003;23:615–621. [DOI] [PubMed] [Google Scholar]
- 34. Oelze M, Daiber A, Brandes RP, et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II‐treated rats. Hypertension. 2006;48:677–684. [DOI] [PubMed] [Google Scholar]
- 35. Yasunari K, Maeda K, Nakamura M, et al. Effects of carvedilol on oxidative stress in polymorphonuclear and mononuclear cells in patients with essential hypertension. Am J Med. 2004;116:460–465. [DOI] [PubMed] [Google Scholar]
- 36. Yue TL, McKenna PJ, Gu JL, et al. Carvedilol, a new vasodilating beta adrenoceptor blocker antihypertensive drug, protects endothelial cells from damage initiated by xanthine‐xanthine oxidase and neutrophils. Cardiovasc Res. 1994;28:400–406. [DOI] [PubMed] [Google Scholar]
- 37. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 38. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 39. Nohria A, Gerhard‐Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. [DOI] [PubMed] [Google Scholar]
- 40. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 41. Hamburg NM, Keyes MJ, Larson MG, et al. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bristow MR, Larrabee P, Muller‐Beckmann B, et al. Effects of carvedilol on adrenergic receptor pharmacology in human ventricular myocardium and lymphocytes. Clin Investig. 1992;70(Suppl 1):S105–S113. [DOI] [PubMed] [Google Scholar]
- 43. Nichols AJ, Gellai M, Ruffolo RR Jr. Studies on the mechanism of arterial vasodilation produced by the novel antihypertensive agent, carvedilol. Fundam Clin Pharmacol. 1991;5:25–38. [DOI] [PubMed] [Google Scholar]


