Abstract
J Clin Hypertens (Greenwich). 2011;13:557–562. ©2011 Wiley Periodicals, Inc.
Failure of blood pressure (BP) to decline appropriately overnight (nondipping) is associated with increased risk. This may be due to inappropriately raised supine central BP and this study’s first aim was to examine this hypothesis. Secondly, aortic stiffness, central hemodynamics, and left ventricular (LV) mass were measured as other possible mechanisms of higher risk. Brachial and central BP (supine and seated), aortic stiffness, central hemodynamics, and LV dimensions were measured in 95 patients with hypertension (mean age 62±8 standard deviation). Central hemodynamics were recorded by combined radial tonometry and 3‐dimensional echocardiography. Seated brachial and central systolic BP (SBP) were similar between dippers (n=52) and nondippers (n=43). However, nondippers had higher supine brachial (132±14 mm Hg vs 126±11 mm Hg; P=.029) and central (121±15 mm Hg vs 115±11 mm Hg; P=.024) SBP. Aortic stiffness was not different between groups (P=.76), but LV mass index (33.0±6.2 vs 29.4±7.2 g/m2.7; P=.019), stroke volume index (30.2±6.2 mL/m2 vs 27.4±6.0 mL/m2; P=.040), and LV stroke work (3246±815 mm Hg/mL/m2 vs 2778±615 mm Hg/mL/m2; P=.005) were all higher in nondippers. Dipper status independently predicted LV mass index (β=3.61; P=.001). Nondippers have higher supine brachial and central SBP, significantly different central hemodynamics, and elevated LV mass index compared with dippers. These cardiovascular anomalies possibly contribute to increased mortality risk.
In normotensive and hypertensive individuals, the failure of nighttime blood pressure (BP) to decline >10% compared with daytime BP (nondipping) is associated with increased target organ damage 1 and risk of cardiovascular and all‐cause mortality. 2 , 3 , 4 Nondipping is a relatively common condition that occurs in 25% of patients with hypertension, but with greater prevalence in certain patient populations such as those with diabetes. 5 The mechanisms underlying the increased risk associated with nondipping are incompletely understood. Some data indicate that nondippers may have higher supine stroke volume 6 and increased large artery stiffness. 7 , 8 This hemodynamic milieu, involving delivery of increased stroke volume into a noncompliant proximal aorta, may reasonably expect to result in an increase in supine brachial BP (the hallmark of nondipping) and central BP. The first aim of this study was to determine the postural (supine and seated) differences in brachial and central BP between patients with a dipping and nondipping BP profile. We hypothesized that nondippers would have higher supine brachial and central BP. Secondly, in addition to BP, we sought to determine other factors that potentially contribute to increased risk in nondippers. This was assessed from arterial stiffness, ventricular‐vascular interaction, and target organ damage defined by left ventricular (LV) mass.
Methods
Patients and Protocol
Study participants comprised 95 consecutive patients younger than 75 years with uncomplicated essential hypertension recruited by media advertisement in south‐east Queensland, Australia (a population comprising approximately 3 million). Initial diagnosis of hypertension was made by each participant’s general practitioner according to their usual practice. Exclusionary criteria included uncontrolled clinic BP (≥180/100 mm Hg), known secondary forms of hypertension, and a history of cardiovascular disease or renal disease, as ascertained by medical records or self‐reporting. Each participant underwent assessment during a single clinic visit and were studied while taking their regular antihypertensive medications. Brachial BP and central BP were recorded in the seated and then the supine positions. Regional arterial stiffness was measured only in the supine position because this is not typically measured (and the methodology therefore less well‐validated) while seated. Blood biochemistry (via standard hospital pathology procedures), echocardiography, and 24‐hour ambulatory BP (ABP) were also performed on the same day. The investigation conformed with the principles outlined in the Declaration of Helsinki and all participants provided informed consent.
ABP and Dipper Status
Each patient underwent 24‐hour ABP using a validated device (TM2430; A&D Mercury, A&D Medical, Thebarton, South Australia, Australia) 9 with readings obtained every 30 minutes during the day (6 am–10 pm) and every 60 minutes during the night (10 pm–6 am). The device was fitted at the hospital by a trained nonclinician. The nighttime to daytime systolic BP (SBP) ratio was used to determine nocturnal BP classification for each participant. Individuals whose nocturnal SBP was ≤10% lower than daytime SBP were classified as nondippers, while those with a nocturnal decline in SBP >10% of daytime values were termed dippers. 1
Brachial and Central BP
Brachial SBP and diastolic BP (DBP) was measured by automatic device (Omron HEM‐907; OMRON Europe B.V. (OMCE), Hoofddorp, The Netherlands) in each patient after 5 minutes of rest in each of the seated and supine positions. The average of the two readings taken 1 minute apart were used for statistical analysis. Immediately after measurement of brachial BP in each posture, central BP was estimated by the average of duplicate recordings obtained by radial applanation tonometry (SphygmoCor 8.1; AtCor Medical, Sydney, NSW, Australia). The SphygmoCor software uses a highly reproducible 10 generalized transfer function that is validated under hemodynamic perturbations. 11 The average brachial SBP and DBP readings obtained during each posture were used to calibrate the radial pressure waveforms. Pulse pressure amplification was defined as the ratio of the peripheral pulse pressure to the central pulse pressure. End‐systolic pressure (ESP) was determined from the nadir of the dichrotic notch on the central pressure waveform. All BP and arterial stiffness measures were recorded by nonclinician technical staff appropriately trained with the methods.
Arterial Stiffness
Supine and seated systemic arterial stiffness was estimated from augmentation index (by radial tonometry), which was defined as the augmentation pressure (P2 − P1) as a percentage of central pulse pressure. Measures of regional arterial stiffness were recorded by aortic and brachial pulse wave velocity. Duplicate measures were obtained in the supine position via electrocardiography‐gated sequential applanation tonometry (SphygmoCor 8.1; AtCor Medical) as previously described. 12 Both aortic stiffness 13 and augmentation index 14 are independent predictors of cardiovascular mortality.
Echocardiography and Ventricular‐Vascular Coupling
Real‐time 3‐dimensional echocardiography was recorded from an apical window over 4 cardiac cycles with a matrix array transducer (X4 transducer; Philips ie33, Andover, MA). LV mass and volumes were measured offline by an experienced operator blinded to each patient’s dipper status using dedicated software (4D analysis; Tomtec Gmbh, Unterschlessheim, Germany) as previously described. 15 Cardiac dimensions were assessed in accordance with the American Society of Echocardiography guidelines. 16 Stroke volume was calculated as end‐diastolic volume–end‐systolic volume indexed to body surface area (mL/m2). Cardiac output was defined as the product of stroke volume index and heart rate (mL/m2/min). Peripheral vascular resistance was determined from mean arterial pressure/cardiac output index ×1000, expressed as peripheral resistance units. 17 Net arterial load faced by the left ventricle was assessed by arterial elastance (EA=ESP/stroke volume index) and LV performance index (ELV) was calculated by ESP/end‐systolic volume index (mm Hg/mL/m2). Arterial and LV interaction was assessed by the coupling ratio EA/ELV. LV stroke work was calculated by ESP×stroke volume index (mm Hg/mL/m2). 18 Complete imaging data were not available in 12 patients (n=5 nondippers).
Statistical Analysis
Analysis was performed with SPSS for Windows software version 16.0 (SPSS Inc, Chicago, IL) and significance was determined as P<.05. Differences between groups were assessed by independent t tests (for continuous variables) and chi‐square analysis with Yates continuity correction (for categorical variables). Within group data was assessed by paired t tests. Pearson correlations were used to assess relationships between variables. Comparison of r values was analysed by Fisher Z transformation. Multiple regression analysis by the backward method was used for predictors of LV mass index. Intercorrelations among predictor variables were tested for multicollinearity, with tolerance values <0.10 indicating collinearity.
Results
Participant Characteristics
Study population characteristics and differences between dippers and nondippers are shown in Table I. There were 43 (45%) participants identified as nondippers. There were no differences between dippers and nondippers with respect to age, body mass index, sex, 24‐hour ABP, daytime ABP, blood biochemistry, or medication prescription. As expected, nighttime BP was significantly higher in the nondippers.
Table I.
Patient Characteristics
| Variable | All (N=95) | Dippers (n=52) | Nondippers (n=43) | P Value |
|---|---|---|---|---|
| Age, y | 62±8 | 62±6 | 62±9 | .879 |
| Body mass index, kg/m2 | 30±5 | 30±5 | 29±5 | .254 |
| Sex, % men | 52 | 52 | 51 | 1.00 |
| 24‐H ambulatory SBP, mm Hg | 131±11 | 129±8 | 133±13 | .114 |
| 24‐H ambulatory DBP, mm Hg | 77±7 | 76±6 | 78±8 | .234 |
| Daytime SBP, mm Hg | 134±11 | 134±9 | 134±14 | .786 |
| Daytime DBP, mm Hg | 79±7 | 79±7 | 79±8 | .971 |
| Nighttime SBP, mm Hg | 119±14 | 112±9 | 128±14 | <.001 |
| Nighttime DBP, mm Hg | 69±9 | 65±7 | 74±9 | <.001 |
| Plasma glucose, mmol/L | 5.7±1.1 | 5.7±1.1 | 5.6±1.0 | .429 |
| Triglycerides, mmol/L | 1.7±1.1 | 1.7±1.3 | 1.6±0.9 | .583 |
| HDL cholesterol, mmol/L | 1.5±0.6 | 1.5±0.7 | 1.3±0.5 | .098 |
| LDL cholesterol, mmol/L | 3.0±0.9 | 3.0±0.8 | 3.1±1.0 | .805 |
| ACE inhibitor, % | 31 | 35 | 28 | .632 |
| AR blocker, % | 61 | 64 | 58 | .750 |
| Calcium channel blocker, % | 18 | 21 | 14 | .521 |
| β‐Blocker, % | 13 | 10 | 16 | .507 |
| Diuretic, % | 22 | 21 | 23 | 1.00 |
| α‐Blocker, % | 1 | 2 | 0 | 1.00 |
Abbreviations: ACE, angiotensin‐converting enzyme; AR, angiotensin receptor; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Data are expressed as mean±standard deviation. P values are for dippers compared with nondippers.
Brachial and Central BP
Table II shows the group differences for supine and seated BP. In the seated position, there were no significant differences in brachial BP or central BP between dippers and nondippers (P>.05 for all). However, in the supine position, nondippers had significantly higher brachial SBP and central SBP. Furthermore, the change in brachial SBP (2.7±9.35 mm Hg vs −2.44 mm Hg ±10.32 mm Hg; P=.013) and central SBP (3.64±8.90 mm Hg vs −0.52±9.66 mm Hg; P=.033) from the seated to supine positions was significantly higher in the nondippers compared with the dippers. Supine, but not seated, brachial SBP was significantly correlated with nighttime SBP (Figure). Moreover, the change in brachial SBP from the seated to supine positions was significantly associated with nighttime SBP (r=0.23; P=.023), but not daytime SBP (r=0.07; P=.486) or 24‐hour ambulatory SBP (r=0.11; P=.284). The change in heart rate from the seated to supine positions was not significantly different (P=.561) between dippers (−5±5 beats per minute) and nondippers (−5±5 beats per minute). Mean arterial pressure dropped significantly in the dippers (−2.4±6.3 mm Hg; P=.008) but not the nondippers (0.2±6.3 mm Hg; P=.808) from the seated to supine positions (between‐group P=.044).
Table II.
Supine and Seated Blood Pressure Between Dippers and Nondippers
| Variable | Dippers (n=52) | Nondippers (n=43) | P Value | |
|---|---|---|---|---|
| Brachial SBP, mm Hg | Seated | 129±14 | 129±14 | .847 |
| Supine | 126±11 | 132±14 | .029 | |
| Brachial DBP, mm Hg | Seated | 77±8 | 78±10 | .923 |
| Supine | 74±8 | 76±8 | .273 | |
| Brachial pulse pressure, mm Hg | Seated | 52±12 | 52±10 | .842 |
| Supine | 53±11 | 56±11 | .084 | |
| Central SBP, mm Hg | Seated | 116±13 | 118±114 | .489 |
| Supine | 115±11 | 121±15 | .024 | |
| Central pulse, mm Hg | Seated | 37±11 | 39±10 | .389 |
| Supine | 40±11 | 45±11 | .061 | |
| Pulse pressure amplification, ratio | Seated | 1.41±0.20 | 1.35±0.16 | .115 |
| Supine | 1.33±0.17 | 1.29±0.14 | .180 | |
| Heart rate, beats per min | Seated | 71±10 | 69±10 | .298 |
| Supine | 66±8 | 64±9 | .405 | |
| Mean arterial pressure, mm Hg | Seated | 94±9 | 95±10 | .733 |
| Supine | 92±8 | 95±10 | .075 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure. Data are presented as mean±standard deviation. P values are for dippers compared with nondippers.
Figure FIGURE.
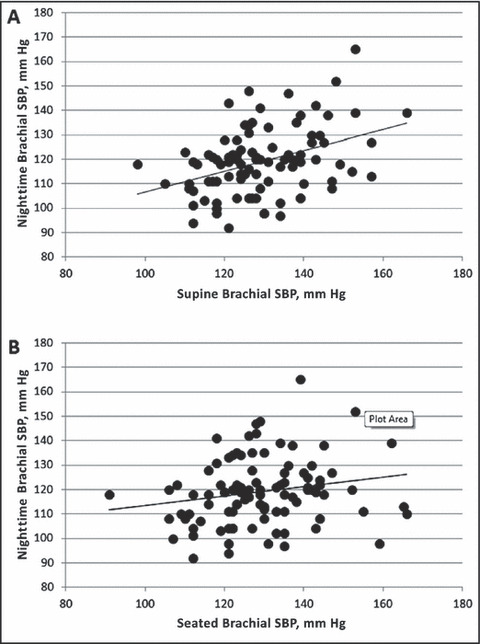
Relationship between nighttime systolic blood pressure (SBP), supine brachial SBP (panel A: r=0.39; P<.001), and seated brachial SBP (panel B: r=0.19; P=.06). The slope of the relationship with nighttime SBP is significantly stronger with supine brachial SBP compared with seated brachial SBP (Z=2.11; P=.038).
Arterial Stiffness
There was no significant difference between groups for aortic (dippers, 9.4±2.0 m/s; nondippers, 9.5±2.6 m/s; P=.763) or brachial pulse wave velocity (dippers, 8.1±1.1 m/s; nondippers, 8.2±1.0 m/s; P=.631). Similarly, augmentation index was not significantly different between groups in the seated (dippers, 20±12%; nondippers, 23%±10%; P=.165) or supine (dippers, 24%±11%; nondippers, 26%±10%; P=.367) positions.
Echocardioigraphy and Ventricular‐Vascular Coupling
As shown in Table III, nondippers had significantly higher LV mass index, stroke volume index, and LV stroke work, but no significant difference for other measures relating to ventricular‐vascular interaction. Univariate correlates of LV mass index were body mass index (r=0.40; P<.001), ELV (r=−0.40; P<.001), nighttime SBP (r=0.31; P=.004), EA (r=−0.28; P=.01), and dipper status (0=dipper, 1=nondipper; r=0.24; P=.03). There were no significant associations between LV mass index and office brachial or central BP (P>.05 for all). A multiple regression model for predictors of LV mass index was constructed in which the above significant univariate correlates were included as independent variables. Significant independent predictors were body mass index, unadjusted β (95% confidence interval)=0.56 (0.34–0.77), P<.001; ELV,β=−1.23 (−1.76 to −0.69), P<.001, and dipper status, β=3.61 (1.46–5.76), P=.001). Model‐adjusted R 2 was 0.39 and P<.001.
Table III.
Cardiac Size and Ventricular‐Vascular Coupling Between Dippers and Nondippers
| Variable | Dippers (n=45) | Nondippers (n=38) | P Value |
|---|---|---|---|
| LV mass index, g/m2.7 | 29.4±7.2 | 33.0±6.2 | .019 |
| LV end‐systolic volume, mL | 17.2±4.7 | 17.9±4.2 | .488 |
| LV end‐diastolic volume, mL | 44.6±9.3 | 48.1±9.5 | .096 |
| Stroke volume index, mL/m2 | 27.4±6.0 | 30.2±6.2 | .040 |
| Cardiac output index, mL/m2/min | 1810±423 | 1942±455 | .179 |
| LV stroke work, mm Hg/mL/m2 | 2778±615 | 3246±815 | .005 |
| Arterial elastance index, EA; mm Hg/mL/m2 | 4.00±1.01 | 3.70±0.85 | .157 |
| LV performance index, ELV; mm Hg/mL/m2 | 6.51±1.89 | 6.41±2.12 | .820 |
| Peripheral vascular resistance, peripheral resistance units | 54.0±14 | 51.4±12.6 | .384 |
| EA/ELV coupling ratio | 0.64±0.17 | 0.60±0.12 | .212 |
Abbreviation: LV, left ventricular. Data are presented as mean±standard deviation. P values are for dippers compared with nondippers.
Stroke work index was significantly correlated with nighttime SBP (r=0.348, P=.002). There was a significant correlation between stroke work index and supine brachial SBP (r=0.514; P<.001), which was a significantly stronger association (Z=2.08; P=.041) than the correlation between stroke work index and seated brachial SBP (r=0.320; P=.004).
Discussion
There are several novel findings of this study. Firstly, despite similar seated office BP and no difference in aortic stiffness, nondippers had significantly higher supine brachial SBP compared with dippers. Secondly, the supine brachial SBP abnormality was accompanied by significantly higher central SBP in the supine, but not seated, position. This is an observation that, together with raised LV mass index, may help to explain (at least in part) the increased cardiovascular risk associated with nondipping. Finally, stroke volume and cardiac stroke work were higher in nondippers, with the latter being highly correlated with supine SBP, thus providing a possible hemodynamic explanation for the raised supine BP level in these patients.
Mechanisms of Abnormal Supine BP
The characteristic feature of nondipping is a significantly elevated nighttime (supine) BP relative to daytime BP. This abnormally raised supine brachial BP was also evident in the office after a few minutes of supine rest. Acute cardiovascular responses to postural change (within seconds) involve complex neural and vascular interactions. However, after <3 minutes of stabilization in the supine position, under normal circumstances, mean arterial pressure should be lower than in the seated position as a result of peripheral vascular vasodilation and reduced heart rate. Further to this, pulse pressure should increase because DBP falls while SBP remains relatively unchanged. 19 These hemodynamic alterations are initiated by posture‐induced stimulation of cardiopulmonary low‐pressure and arterial (carotid and aortic) high‐pressure sensors. 20
In this current study, patients who were dippers followed a normal hemodynamic response to postural change as described above. Conversely, when the nondippers moved into the supine position, brachial and central SBP increased, while mean arterial pressure failed to change, despite a drop in heart rate. Our data potentially explain these anomalies by an increase in stroke volume and LV stroke work, which, in the absence of differences in other functional parameters related to brachial or central BP (ie, aortic stiffness, EA, peripheral vascular resistance, heart rate) would generate a greater rise in both brachial and central SBP. This chronic elevation of central BP in the supine state would reasonably be expected to contribute toward increases in LV mass 21 , 22 (as was observed in nondippers) and additional risk related to cardiovascular mortality. 14 , 23
Our findings lend support to those of Takakuwa and colleagues, 6 who reported that nocturnal cardiac output and stroke volume were significantly higher in nondippers. On the other hand, in general disagreement with two other studies, 7 , 8 we found that neither central (EA and aortic), peripheral (brachial), nor systemic (augmentation index) arterial stiffness measures were abnormally elevated in nondippers. An important difference of our study was that dippers and nondippers had similar BP at the time arterial stiffness measures were acquired. Thus, our data are unlikely to be confounded by between‐group BP variations. 24 Different measuring techniques for arterial stiffness, as well as classifications of nocturnal BP and racial variation between studies could also account for discrepancies. The mechanisms underlying abnormal hemodynamics related to nondipping may be multifactorial, including disordered supine natriuresis, 25 volume expansion, 6 or neurohormonal irregularity with raised norepinephrine, 26 to name a few, and further studies are required to tease out direct causes of nondipping.
Limitations
This was a selected population of patients receiving treatment for hypertension. Thus, it is unknown whether these findings are broadly applicable and more research with greater numbers of patients is required. Although 24‐hour ABP is regarded as the “gold standard” method for determining BP control, some studies have reported weak reproducibility of the method. 27 Therefore, since we did not perform multiple 24‐hour ABP recordings, it may be possible that patients in the current study were incorrectly assigned to either the dipper or nondipper groups. Furthermore, our protocol of 60‐minute intervals between nighttime BP readings was made for the benefit of patient comfort, so as to lessen the possibility of sleep disturbance that may raise nighttime BP values. Nonetheless, this protocol may have also contributed to incorrect assignment of dipper status.
Conclusions
This study found that nondippers had significantly raised brachial and central SBP while in the supine position, but not while seated. Central hemodynamics related to ventricular‐vascular interaction were also altered and LV mass index was significantly raised compared with dippers. Irregular supine hemodynamics were related to LV stroke work and, in the long‐term, higher central BP may contribute to adverse cardiac remodeling. Together, these unfavorable changes may help to explain the extra cardiovascular risk associated with nondipping.
Acknowledgments and disclosures:
This study was supported by a National Health and Medical Research Council of Australia project grant and Career Development Award (reference 569669 and 569519, both to JS). Dr Sharman has research collaborations with AtCor Medical.
References
- 1. Routledge FS, McFetridge‐Durdle JA, Dean CR. Night‐time blood pressure patterns and target organ damage: a review. Can J Cardiol. 2007;23:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben‐Dov IZ, Kark JD, Ben‐Ishay D, et al. Predictors of all‐cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. [DOI] [PubMed] [Google Scholar]
- 3. Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. [DOI] [PubMed] [Google Scholar]
- 4. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 5. Pickering TG, Kario K. Nocturnal non‐dipping: what does it augur? Curr Opin Nephrol Hypertens. 2001;10:611–616. [DOI] [PubMed] [Google Scholar]
- 6. Takakuwa H, Ise T, Kato T, et al. Diurnal variation of hemodynamic indices in non‐dipper hypertensive patients. Hypertens Res. 2001;24:195–201. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Staessen JA, Lu L, et al. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study Hypertension. 2007;50:333–339. [DOI] [PubMed] [Google Scholar]
- 8. Tsioufis C, Stefanadis C, Antoniadis D, et al. Absence of any significant effects of circadian blood pressure variations on carotid artery elastic properties in essential hypertensive subjects. J Hum Hypertens. 2000;14:813–818. [DOI] [PubMed] [Google Scholar]
- 9. Palatini P, Frigo G, Bertolo O, et al. Validation of the A&D TM‐2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects’ characteristics. Blood Press Monit. 1998;3:255–260. [PubMed] [Google Scholar]
- 10. Holland DJ, Sacre JW, McFarlane SJ, et al. Pulse wave analysis is a reproducible technique for measuring central blood pressure during hemodynamic perturbations induced by exercise. Am J Hypertens. 2008;21:1100–1106. [DOI] [PubMed] [Google Scholar]
- 11. Sharman JE, Lim R, Qasem AM, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47:1203–1208. [DOI] [PubMed] [Google Scholar]
- 12. Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. [DOI] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 14. Vlachopoulos C, Aznaouridis K, O’Rourke MF, et al. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;15:1865–1871. [DOI] [PubMed] [Google Scholar]
- 15. Jenkins C, Bricknell K, Hanekom L, et al. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time threedimensional echocardiography. J Am Coll Cardiol. 2004;44:878–886. [DOI] [PubMed] [Google Scholar]
- 16. Park SH, Shub C, Nobrega TP, et al. Two‐dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: correlation with autopsy and m‐mode echocardiography. J Am Soc Echocardiogr. 1996;9:119–128. [DOI] [PubMed] [Google Scholar]
- 17. Nichols WW, O’Rourke MF. Mcdonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London, UK: Hodder Arnold; 2005. [Google Scholar]
- 18. Chantler PD, Lakatta EG, Najjar SS. Arterial‐ventricular coupling: Mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pump B, Kamo T, Gabrielsen A, et al. Mechanisms of hypotensive effects of a posture change from seated to supine in humans. Acta Physiol Scand. 2001;171:405–412. [DOI] [PubMed] [Google Scholar]
- 20. Pump B, Christensen NJ, Videbak R, et al. Left atrial distension and antiorthostatic decrease in arterial pressure and heart rate in humans. Am J Physiol. 1997;273:H2632–H2638. [DOI] [PubMed] [Google Scholar]
- 21. Saba PS, Roman MJ, Pini R, et al. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. J Am Coll Cardiol. 1993;22:1873–1880. [DOI] [PubMed] [Google Scholar]
- 22. Sharman JE, Fang ZY, Haluska B, et al. Left ventricular mass in patients with type 2 diabetes mellitus is independently associated with central but not peripheral pulse pressure. Diabetes Care. 2005;28:937–939. [DOI] [PubMed] [Google Scholar]
- 23. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 24. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 25. Uzu T, Takeji M, Yamauchi A, et al. Circadian rhythm and postural change in natriuresis in non‐dipper type of essential hypertension. J Hum Hypertens. 2001;15:323–327. [DOI] [PubMed] [Google Scholar]
- 26. Kario K, Mitsuhashi T, Shimada K. Neurohumoral characteristics of older hypertensive patients with abnormal nocturnal blood pressure dipping. Am J Hypertens. 2002;15:531–537. [DOI] [PubMed] [Google Scholar]
- 27. Musso NR, Lotti G. Reproducibility of ambulatory blood pressure monitoring. Blood Press Monit. 1996;1:105–109. [PubMed] [Google Scholar]


