Abstract
J Clin Hypertens (Greenwich). 2012; 14:372–382. ©2012 Wiley Periodicals, Inc.
The prevalence of hypertension in children and adolescents is increasing, especially in obese and ethnic children. The adverse long‐term effects of hypertension beginning in youth are known; therefore, it is important to identify young patients who need intervention. Unfortunately, measuring blood pressure (BP) is difficult due to the variety of techniques available and innate biologic variation in BP levels. Ambulatory BP monitoring may overcome some of the challenges clinicians face when attempting to categorize a young patient’s BP levels. In this article, the authors review the use of ambulatory BP monitoring in pediatrics, discuss interpretation of ambulatory BP monitoring, and discuss gaps in knowledge in usage of this technique in the management of pediatric hypertension.
The prevalence of hypertension (HTN) in children and adolescents has traditionally been considered to be 1% to 2%, but recent studies have suggested that it is increasing. Data from blood pressure (BP) screenings conducted in Houston, TX, demonstrated a prevalence of HTN of just over 3%, 1 with an even higher prevalence of 4.5% in obese children. 2 These data are supported by a recent review of BP data in 8‐ to 17‐year‐old children from the National Health and Nutrition Examination Survey (NHANES) and other related population‐based studies conducted in the United States from 1963–2002, 3 which demonstrate an increase in the prevalence of high BP in US children. The prevalence of HTN has reached nearly 4%, and the prevalence of prehypertension has now reached 10%.
It appears that the recent trends in high BP in American children have had a much greater effect on non‐Hispanic blacks and Mexican Americans than on whites, mirroring the increased risk of HTN and cardiovascular (CV) disease seen among minority adults in the United States. 4 This increased prevalence of high BP in the young has been seen elsewhere, including in Canada, 5 Portugal, 6 and Brazil. 7 Thus, it appears that HTN is on the rise in the young, and specific populations of children and adolescents appear to be at increased risk, most notably obese children and ethnic minority children.
Office, Home, or Ambulatory Measurement of BP?
The long‐term consequences of persistent HTN are well‐known, and include CV events as well as development of chronic kidney disease (CKD). Since these outcomes likely have their origins in childhood, it is important to correctly identify children with high BP so that appropriate preventative measures can be instituted. Yet, the clinician who evaluates children and adolescents with high BP faces several challenges, the first of which is to decide what type of BP measurements to rely on for diagnostic purposes.
Office BP readings, typically the first step in identifying children with high BP, have frequently been shown to be subject to error, especially those that are part of routine vital signs determination. 8 The largest source of such error is probably the increasing reliance on automated devices, which are convenient to use but have numerous inherent inaccuracies, especially in young children. 9 A recent study conducted in children with CKD, for example, showed that an automated BP device systematically overestimated both systolic BP (SBP) and diastolic BP (DBP), which could lead to inaccurate classification of BP status. 10 For this reason, it has been recommended that elevated office BP readings using an automated device be repeated by auscultation. 8 , 11 , 12
Additional problems with office BP measurement, even if done by auscultation, include improper rate of deflation, digit preference, accommodation, and the white‐coat effect. Rigorous training is needed to ensure that auscultatory BP is being measured correctly, which can be difficult to sustain over time. 13 But even careful office BP measurement by auscultation may not be able to overcome the white‐coat effect, which can loosely be defined as an increase in BP in the office setting above the BP as measured outside of the office. The white‐coat effect has been shown to be extremely common in children, 14 and in many large pediatric studies, the incidence of white‐coat HTN (WCH) exceeds 40%. 15 , 16
Home or “self‐measured” BP is another option for assessing whether a patient truly has high BP. In adults, self‐measured BP is considered a crucial step in confirming office BP elevation and is recommended prior to instituting antihypertensive treatment. 17 The situation in pediatrics is a bit different, primarily for two reasons: (1) lack of home BP devices that have been validated in the pediatric age group, and (2) lack of widely accepted normative data for home BP in children. The device issue is complex and is beyond the scope of this review. Suffice it to say that although there are a wide variety of BP devices marketed for home use, few manufacturers have pursued validation studies in children and none are mass‐marketed with child‐sized cuffs (frequently smaller cuffs can be purchased for an additional cost after the device itself has been purchased). Since all marketed devices use the oscillometric technique, the concerns raised previously about oscillometric BP measurement 8 , 9 , 10 , 11 , 12 would also apply to home BP readings obtained using currently available devices.
The lack of normative data for home BP in children is another significant drawback to wider application of this technique. Although the European Society of Hypertension recently endorsed use of home BP monitoring in children, 18 it should be noted that the normative data cited in those recommendations were derived from a relatively small population of Greek children, which may not be applicable to children with different ethnic/racial backgrounds. Furthermore, home BP monitoring in children has not shown good correlation with other BP measurement techniques. A 2004 study by Stergiou showed home BP readings in children to be significantly lower than both casual and ambulatory BP measurements. 19 A more recent review by the same authors noted the differences in BP values obtained by office, home, and ambulatory measurement in children and also emphasized the lack of validated devices for home BP measurement in children. 20 Thus, although home BP measurement may provide useful supplemental information on childhood BP, it does not appear to be ready for widespread use in the diagnosis of pediatric HTN.
Given the above issues with both office and home BP measurements in children, ambulatory BP monitoring (ABPM) has emerged as perhaps the most appropriate BP measurement method to establish the diagnosis of HTN. Although some barriers to more widespread use of ABPM in pediatrics remain, 21 it is the only BP measurement technique that eliminates the white‐coat effect, eliminates problems with observer bias and improper technique, and allows assessment of nocturnal BP, which is becoming increasingly recognized as having tremendous prognostic significance in both adults 22 and children. 23 ABPM has also been shown to be cost‐effective in the evaluation of childhood HTN, 16 particularly because of its ability to prevent overdiagnosis of HTN. 24 Thus, while older consensus recommendations for evaluation of childhood HTN were cautious regarding the use of ABPM, 12 more recent guidelines have been enthusiastic regarding routine application of this technique. 18
Conditions in Which ABPM May Prove Useful
Secondary HTN
When HTN is detected in very young children, signs suggesting systemic disease are present, 25 or casual BP is at stage 2 levels, secondary HTN may be present and ABPM should be considered to assist with diagnosis (Table I). In fact, greater nocturnal SBP load and 24‐hour DBP load on ambulatory BP monitoring have been found to be highly specific for a secondary cause for HTN in a US study (Figure 1) 26 and the specificity for blunted nocturnal dipping to predict secondary HTN was near 90% in a study of children from the Czech Republic. 27
Table I.
Conditions Where Use of ABPM May Improve Patient Care
| Condition | Benefit | |
|---|---|---|
| Secondary (coenzyme A, coarctation of the aorta, renal) | ABPM may indicate greater chance for secondary causes | |
| Genetic risk for HTN | Evaluate patients with strong family history of HTN | |
| Williams & Turner syndrome may have stiff arteries | ||
| Neurofibromatosis 1 may have renal artery stenosis | ||
| White‐coat/masked HTN | Can be diagnosed only by ABPM | |
| Prehypertension | Confirm diagnosis | |
| Obesity | Rule out white‐coat and masked HTN especially with concomitant obstructive sleep apnea, polycystic ovary syndrome, or metabolic syndrome | |
| Risk for target organ damage | Can help determine whether imaging is needed | |
| Diabetes | Tight BP control reduces albuminuria | |
| Solid organ transplant | May uncover masked HTN or nighttime HTN | |
| Renal disease | Chronic renal insufficiency/transplant | Tighter 24‐h BP control to delay progression or prevent graft loss |
| Renal scarring | Abnormal ABPM correlates with renal scarring | |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; HTN, hypertension.
Figure 1.
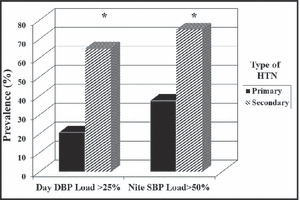
Utility of ambulatory blood pressure monitoring in the diagnosis of secondary hypertension (HTN). Presence of daytime diastolic blood pressure (DBP) load >25% and nocturnal systolic blood pressure (SBP) load >50% had 92% specificity for predicting secondary HTN. 26
ABPM is also useful in conditions where patients are at high risk for HTN. A significant positive correlation was found between the extent of renal scarring related to frequent urinary tract infections and SBP and DBP standard deviation score on ABPM, 28 suggesting that ABPM should be performed in children with this history. Other diseases where ABPM is helpful in diagnosing HTN include Williams syndrome, 29 Cushing syndrome, 30 anorexia nervosa, 31 chronic fatigue syndrome, 32 polycystic ovary syndrome, 33 and repaired coarctation of the aorta. 34 One study examined the effect of stimulants for attention deficit hyperactivity disorder on ABPM parameters. They found that total and waking DBP were 3 mm Hg to 4 mm Hg higher on active treatment, a magnitude that may be clinically relevant in the child with borderline BP levels. 35
ABPM can also be used to examine the effect of lifestyle on CV risk. In adults, higher ABPM levels were found in less active patients even after adjusting for age, body mass index, alcohol intake, and smoking. 36 In adolescents, shorter sleep duration assessed by actigraphy was related to higher 48‐hour and nighttime BP levels. 37 Psychosocial stress may also adversely affect ABPM levels in children. 38 Higher salt intake was associated with nondipper status in youth 39 and caffeine increased ABPM levels in adolescents, with the greatest effect during the daytime when sympathetic nervous system responses dominate BP control. 40
ABPM may also be helpful in evaluation of patients at genetic risk for HTN. 41 Twin studies suggest that heredity has a large impact on ABPM. 42 Therefore, it is not surprising that children with a parental history of HTN have higher BP than those without a history. Interestingly, the genetic influence was not found for office BP. 43 Early life events are also important. Children born to mothers with preeclampsia had significantly higher mean 24‐hour ABPM levels than healthy controls. 44 Impaired fetal growth has also been linked to higher ambulatory SBP at 12 years of age 45 and low birth weight was associated with reduced nocturnal dipping (day‐night BP difference) in children. 46
WCH, where a patient’s office BP level is elevated (≥95th percentile) in the clinic but normal on ABPM (average BP <90th percentile) is a condition that can only be diagnosed with ABPM. It may relate to transient, stress‐induced elevation of BP associated with the medical examination. This phenomenon is especially pronounced in obese patients 47 and in younger patients, with the effect diminishing substantially by age older than 12 years. 48 WCH should be considered in youth with borderline levels of BP since there is a direct correlation between office BP levels and presence of WCH such that the likelihood of WCH decreases as office BP increases. 14 , 49 Some pediatric HTN experts suggest that ABPM to rule out WCH should be performed in children with prehypertension and stage 1 HTN, as those with stage 2 HTN or significantly lower BP (at least 10% <95th percentile) were unlikely to have WCH. 49 The exact prevalence of WCH is not known and varied estimates have been published in the pediatric literature. One study examined male athletes undergoing a pre‐sports physical. The investigators found that 88% of patients with an abnormal BP reading at the screening actually had WCH diagnosed by ABPM. More recent studies from pediatric HTN centers have indicated a prevalence of WCH of 30% 50 to 40%. 16
WCH is an important condition to document because it may not be entirely benign. Adult studies have found that patients with WCH have higher left ventricular mass (LVM), 51 evidence for endothelial dysfunction, 52 and increased carotid intimal‐media thickness (IMT) 53 than normal controls. This target organ damage may account for the increase in adverse CV disease outcomes noted with WCH in adults. 54 Emerging data in children also suggest that young patients with WCH have a tendency towards elevated LVM index, 55 , 56 with one study finding that one third of young patients with WCH had an LVM index >95th percentile for age and sex. 57 A recent study has even documented abnormal reactive hyperemia in the middle cerebral artery 58 and increased carotid IMT in WCH compared with controls. 59
Masked HTN
Masked HTN (normal clinic BP with abnormal ABPM) is a condition that is gaining increasing scrutiny as more clinics are adopting ABPM. Masked HTN should be suspected when other providers report hypertensive BP levels when measured under similar conditions yet resting BP levels are <95th percentile in the clinic. Presence of obesity may also increase the chances of identifying this ABPM pattern. 60 If the clinical presentation (ie left ventricular hypertrophy is present) seems inconsistent with the clinic BP, masked HTN may also be the culprit. The prevalence of masked HTN is not well defined but likely much lower than for WCH. Estimates ranging from 5.7% in healthy children 61 to 9.4% in patients referred to an HTN specialist 55 to 15% in a clinic that predominantly sees patients with secondary HTN 62 to 24% in renal transplant recipients. 63 Similar to WCH, masked HTN is not a benign entity. Masked HTN is associated with increased CV risk 64 and progression of CKD in adults. 65 Masked HTN identifies youth with increased risk to progress to sustained office HTN 61 who are at risk for target organ damage such as elevated LVM. 55 One study found that the prevalence of LVH was equal for adolescents with masked HTN compared with patients with confirmed stage 1 HTN (around 20%). 66 In a study of children with CKD, the prevalence of MH was quite high (38%) and the odds ratio for LVH were equal for masked HTN and confirmed HTN (4.1 vs 4.3). 67 For this reason, many pediatric HTN experts feel that masked HTN needs aggressive management.
Prehypertension
Patients with borderline levels of office BP (within 20% of the 95th percentile) 68 are especially likely to benefit from ABPM. As seen above, these patients may be more likely to have WCH or masked HTN. However, if they are found to be prehypertensive, which had a prevalence of 20.8% in one study of youth referred to an HTN clinic, 50 the treatment options may differ. Furthermore, even with normal mean ABPM values, increased BP variability is associated with target organ damage in adults. 69 A few studies of prehypertensive children also suggest that LVM, 70 , 71 carotid IMT, 71 and arterial stiffness 71 may be higher in prehypertensive youth compared with their normotensive counterparts.
ABPM and Obesity‐Related CV Risk Factors
ABPM is increasingly being used in evaluation of CV risk in young patients with obesity and multiple CV risk factors. Central adiposity has been linked to elevated ABPM means. 72 Young obese patients also demonstrated reduced dipping, 73 which may increase risk for masked HTN. 74 Obesity‐related insulin resistance remained a significant determinant of daytime DBP even after adjustment for level of adiposity. 75 Obstructive sleep apnea (OSA), which is more prevalent in obese insulin‐resistant children, is also linked to higher mean ABPM, 76 daytime BP variability, and reduced nocturnal dipping. 77 Degree of ABPM abnormality is directly correlated with severity of OSA. Children with an apneic‐hypopneic index >5 had significantly higher nocturnal BP levels than less severely affected patients. 78 Children with OSA are also more likely to have a pronounced early morning BP surge. 79 This ABPM pattern is associated with increased risk for adverse CV outcomes in adults. 80
ABPM and Risk for Target Organ Damage
Ambulatory, rather than office BP, relates more strongly to LVM 81 in both hypertensive and normotensive adults. 82 Although one study of children referred to an HTN clinic did not find an association between BP load and LVM, 83 other studies have seen this relationship. 84 , 85 Severity of BP elevation may also be important as BP load relates to LVM 84 and a linear increase in LVM was seen in one study with increasing nighttime SBP. 81 Increased BP variability (higher standard deviation score) is also important as it increased the odds ratio of having increased LVM index by 54%. 85
Vascular damage, a risk factor for stroke, 86 is also associated with ABPM in adults 87 even after adjusting for office BP, 88 suggesting that ABPM provides incremental information. Few data are available in children, but one study found higher carotid IMT in children diagnosed with HTN, with the strongest correlation seen between IMT and daytime SBP index (r=0.47; P=.003). 89 Higher IMT was also found in young obese patients compared with lean controls. This is significant because even though the obese group had a significantly higher mean ABPM compared with the lean, only one quarter were actually hypertensive. 47 Although one group did not find a difference in carotid IMT between lean and obese patients, 90 the obese children did have higher mean ABPM and this was associated with higher carotid stiffness and lower endothelial function measured by brachial flow‐mediated dilation. 90 In healthy adolescents referred for evaluation of BP, arterial stiffness as measured by pulse wave velocity was found to be correlated with SBP, with the correlations for DBP significant only for ABPM but not for office BP (Figure 2). 91
Figure 2.
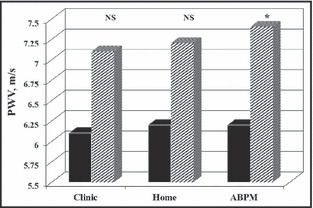
Arterial stiffness by blood pressure (BP) measurement technique. PWV indicates pulse wave velocity; ABPM, ambulatory blood pressure monitoring; NS, not signficicant. 91
ABPM measures are more frequently abnormal in adults with renal damage (elevated albumin excretion) as compared with office BP levels. 92 In children, no relationship between ABPM and creatinine clearance or albumin excretion was found in a study of children with HTN 81 although another study was able to correlate nighttime SBP to creatinine clearance, but only in African American patients. 93 A more recent study found that children with high BP loads had lower glomerular filtration rate (GFR) (79.15 mL/min/1.73 m2 vs 96.78 mL/min/1.73 m2; P<.006) and higher protein excretion (198.29 mg/m2/d vs 118.31 mg/m2/d; P<.036) compared with patients with normal loads. 94
ABPM in High‐Risk Conditions
Many pediatric patients with chronic medical conditions are at increased risk for HTN or HTN‐related target organ damage and would benefit from ABPM. Children with type 1 diabetes mellitus (T1DM) often experience nocturnal, white‐coat, or masked HTN, conditions that can be diagnosed only with ABPM. 95 Poor diabetic control may be a contributing factor as glycated hemoglobin is related to abnormal mean ambulatory SBP 96 and reduced BP dipping. 97 BP control is especially important in young patients with T1DM because nocturnal HTN has been associated with higher carotid IMT 23 while higher DBP load 98 and blunted nocturnal dipping 99 , 100 were found with albuminuria, a sign of early renal damage. Reduced dipping has also been seen in uncomplicated obesity 101 and obesity with type 2 diabetes mellitus. 102
Children with CKD are known to be at high risk for CV disease 103 making close control of BP a high priority. Masked HTN may also be more prevalent in these patients 67 and recent data from the Chronic Kidney Disease in Children (CKiD) study suggests that children with masked HTN have lower GFR at baseline with more rapid decline in GFR during follow‐up as compared with CKD patients who were normotensive or had WCH (Figure 3). 104 The recent Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial has shown that intensified control of HTN according to ABPM results led to decreased progression of CKD. 105 For this reason, many pediatric nephrologists are incorporating ABPM into their clinical practice. 106 , 107
Figure 3.
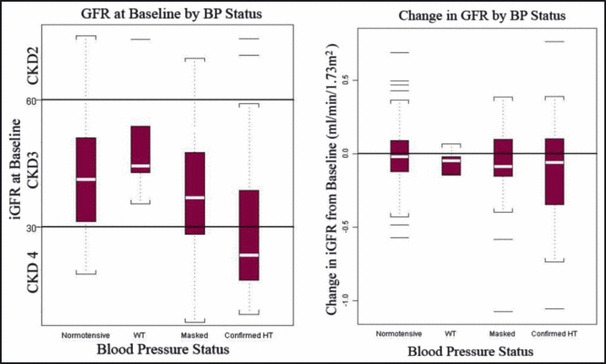
Glomerular filtration rate (GFR) at baseline and change in GFR by blood pressure classification in the Chronic Kidney Disease in Children (CKiD) Study.104 CKD indicates chronic kidney disease; WT, weight; HT, height.
HTN is very common after solid organ transplantation and may relate to the type of immunosuppressive agent used. 108 However, the BP elevation may be missed by casual BP measurement because it often occurs only at night. 109 Furthermore, even with normal office BP, the ABPM may demonstrate poor BP control, 110 which is why use of ABPM frequently leads to changes in BP regimen in these patients. 111 Since ABPM parameters relate to both LVM 112 and carotid IMT 113 in pediatric kidney transplant recipients, ABPM is rapidly becoming an essential feature of routine care of these children.
Measurement of ABPM
Detailed recommendations for performance of ambulatory BP studies in children and adolescents were issued by the American Heart Association (AHA) in 2008, 114 and interested readers should consult that document for specifics on how to measure ambulatory BP in the young. One measurement issue that warrants further consideration is selection of ABPM equipment appropriate for use in children, a crucial step in obtaining valid data. Unfortunately, few devices have undergone rigorous validation testing 115 in children and none has received an unequivocal recommendation from the dabl Educational Trust, an international consortium that reviews validation experiments for BP devices (http://www.dableducational.org/sphygmomanometers/p_devices_3_abpm.html, accessed February 15, 2012). Furthermore, auscultatory and oscillometric devices may not provide equivalent mean values for SBP and DBP. For this reason, most pediatric centers use the oscillometric device with the largest number of normative data available to date. 116
Interpretation of ABPM in Children
Current recommendations for interpretation of ambulatory BP studies in children and adolescents were issued by the AHA in 2008 114 and have been subsequently adopted by other consensus organizations. 18 These recommendations represented the first attempt at standardizing the interpretation of ABPM in pediatrics and have subsequently been applied by many investigators in the field of childhood HTN. While they have proven to be generally useful, we believe that their application over time has demonstrated that some modifications are needed. The following discussion will highlight key points of the AHA recommendations, with suggested modifications noted.
Editing
Recording frequency in BP measurement is often every 20 minutes for daytime measures and every 30 minutes at night. At least one valid reading per hour, including during sleep, is necessary for a study to be considered complete. 114 In general, manual editing of BP readings generated by the ABPM device should be extremely limited, if done at all. The device software should be programmed to edit out invalid readings, generally readings with SBP >240 mm Hg and <70 mm Hg, DBP >140 mm Hg and <40 mm Hg, heart rate >125 beats per minute, and pulse pressure <40 mm Hg but >100 mm Hg with a DBP less than SBP. 117 Consideration can be given to modifying these settings if the device is being used to measure ambulatory BP in very young children. 114 Visual inspection of the raw BP readings for gross outliers may also be considered, but automated editing is preferred. The exception to the above would be manual editing of any resting BP readings taken with the ABPM device in the office. While this practice is important to demonstrate comparability of readings obtained by the device to the patient’s prior BP readings that day, these readings may still be affected by the white‐coat effect and do not truly represent the patient’s ambulatory BP.
Analysis Periods
Typically, three analysis periods are used for analysis of ambulatory BP studies: wake, sleep, and the entire 24 hours. There is some variation in the literature on definition of wake and sleep periods, with some investigators using fixed time periods (eg, 8 am–10 pm for wake and 12 am–6 am for sleep) and others recommending division of the day according to patient‐supplied diary information. 118 The fixed time period approach has the disadvantage of loss of several hours of BP readings in the early morning and late evening and may not correspond to patients’ actual sleep‐wake times, leading to some inaccuracies in the mean BPs and BP loads for the wake and sleep time periods. This approach also does not account for any daytime napping. In fact, a recent study that compared classification of BP status using different definitions of wake and sleep times concluded that the fixed time period approach led to significant misclassification and that use of patient‐supplied time periods was superior. 118 Thus, it is strongly recommended that patients be given diaries to record their sleep‐wake times and that these times be programmed into the analysis software.
Calculations
Once the monitoring period has been divided into sleep‐wake times, the 24‐hour, wake, and sleep mean BPs should be calculated, and the individual readings compared with reference data, to generate the BP load (percent of readings above threshold) for the 24‐hour, wake, and sleep periods.
There are also more advanced variables that can be calculated from ABPM studies, although these have not found widespread clinical use in pediatrics. Morning BP surge is the difference between morning BP and the nadir during sleep. In adults, it is correlated with stroke risk independent of mean and nocturnal ABPM. 80 Short‐term BP variability is estimated by the standard deviation (SD) or coefficient of variance 119 for daytime or nighttime readings and is associated with risk for target organ damage and CV mortality. 80 More advanced analysis of BP variability can also be performed using fast Fourier analyses. One study of young patients with chronic renal insufficiency found that reduced BP variability correlated with GFR and albuminuria. 120 Hyperbaric index is the area under the curve above a preselected BP threshold. It may be a better representation of total CV burden related to HTN in adults. 121 Smoothness index measures optimal 24‐hour BP control. It is the ratio between the effect of treatment on average hourly BP (change in BP pretreatment to post‐treatment) and the SD and may be a better prediction of regression of LVM with treatment. 80 Ambulatory arterial stiffness index is calculated as 1 – regression slope of DBP/SBP from 24‐hour ABPM (Figure 4). Whether it truly represents arterial wall stiffness is controversial, 80 it was elevated in youth with HTN and correlated with arterial stiffness (pulse wave velocity). 122
Figure 4.
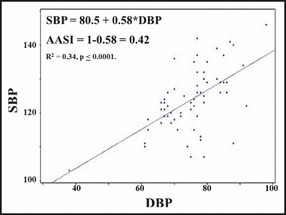
Calculation of ambulatory arterial stiffness index (AASI) (Urbina, unpublished data, 2012). SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Reference Thresholds
Once all the values have been calculated, comparisons to reference standards should be made. At present, the most commonly used pediatric reference data are those originally published by the group in Germany 116 , 123 and updated in the 2008 AHA statement. 114 Separate tables are available for boys and girls by both age and height. Since height is such an important determinant of BP in children, it is preferred to use the tables for height, except in children less than 120 cm tall, in whom it may be necessary to use the tables by age, which do go down to 5 years.
The drawbacks of the German normative data have been recently reviewed. 21 A significant issue for those caring for children of diverse ethnic backgrounds is that the German ambulatory BP data included only Caucasian children. There are some data that indicate the possibility that racial differences may exist in ambulatory BP and that these differences may begin in adolescence. 124 Other issues that have been raised with respect to the German ambulatory BP data include a lack of variability in DBP (Figure 5) and small numbers of shorter children in the database, making the analysis of ambulatory BP studies problematic in children with impaired growth, specifically in children with CKD. It is clear that better normative data are needed. In the meantime, the German data should probably be used in most if not all instances.
Figure 5.
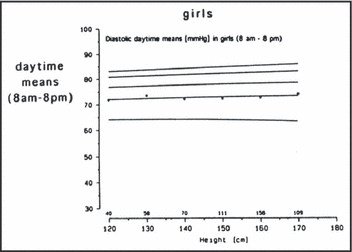
Graph of mean daytime diastolic ambulatory blood pressure for girls according to height. 123 Reprinted with permission from Soergel et al.123
Definition of HTN
Classification of a patient as either normotensive or hypertensive by ABPM requires use of both the office BP reading (preferably obtained by auscultation) and the results of the ambulatory BP study. Building on earlier work by Lurbe and Sorof, 125 the AHA statement proposed criteria for classification of children as being normotensive, white coat hypertensive, prehypertensive, masked hypertensive, and hypertensive. 114 Since publication of the AHA statement, however, the field of pediatric ABPM has continued to advance rapidly and it is clear that the classification scheme in the statement has a number of deficiencies that in our opinion merit some revisions. Chief among these are the following:
-
•
Lack of comment on isolated abnormalities in diastolic ambulatory BP. The classification scheme outlined in the statement suggests that children undergoing ABPM should be classified based on office and ambulatory SBP. Yet, in routine clinical use of ABPM, it is apparent that some children have isolated diastolic HTN. Diastolic HTN on ABPM has also been shown to potentially signal the presence of underlying secondary causes of HTN. 26 Thus, we feel that DBP should also be incorporated into the classification.
-
•
No guidance on classification of patients with isolated nocturnal HTN. As discussed previously, nocturnal HTN has significant prognostic implications in certain patient populations, including those with CKD and diabetes. Further, isolated nocturnal HTN occurs commonly in other clinical situations, particularly in solid organ transplantation. 109 Thus, isolated abnormalities of sleep BP on ABPM should be given the same weight as abnormalities of wake BP.
-
•
The classification scheme in the AHA statement does not provide guidance on how to classify patients with normal office BP and normal mean ambulatory BP but an elevated ambulatory BP load. Should these children be considered normotensive or masked hypertensive? The investigators of the CKiD study have decided to classify these children as having masked HTN, 67 but further study is probably needed to validate this approach. Certainly a patient with prehypertension in the office and elevated load on ABPM should be evaluated more carefully and frequently.
-
•
The AHA statement mistakenly states that the office BP cutpoint for prehypertension is a BP >95th percentile, but the actual definition by the National High Blood Pressure Education Program is that prehypertension is office BP ≥90th percentile and <95th percentile. 12
-
•
Lack of recommendations on use of ambulatory mean arterial pressure (MAP). The majority of devices used to perform ABPM utilize the oscillometric technique, which directly measures MAP and back‐calculates SBP and DBP using manufacturer‐specific software algorithms. The resultant calculated SBP and DBP values have been shown to vary significantly compared with SBP and DBP values obtained by auscultation. It may be more appropriate to utilize MAP to classify the results of ABPM studies since this is the one BP parameter that is measured directly by most devices used to perform ABPM in children. Furthermore, treatment guided by ambulatory MAP has been shown to reduce the rate of progression of CKD in the recently published ESCAPE trial, 105 further highlighting the importance of this ambulatory BP parameter. However, lack of sufficient normative MAP ambulatory BP data makes this recommendation more difficult to implement.
While further study is probably needed to answer some of the above questions, we do feel that the classification scheme in the AHA statement can be simply modified to address some of the more obvious issues, including what to do about DBP, isolated nocturnal BP elevation and the correct identification of children with prehypertension. We have summarized these modifications in Table II.
Table II.
Suggested Revised Schema for Staging of Ambulatory BP Levels in Children
| Classification | Office BP, Percentilea | Mean Ambulatory SBP or DBP, Percentileb,c | SBP or DBP Load, %c |
|---|---|---|---|
| Normal BP | <95th | <95th | <25 |
| White‐coat HTN | >95th | <95th | <25 |
| Masked HTN | <95th | >95th | >25 |
| Pre‐HTN | ≥90th and <95th | <95th | 25–50 |
| Ambulatory HTN | >95th | >95th | 25–50 |
| Severe ambulatory HTN (at risk for end‐organ damage) | >95th | >95th | >50 |
Abbreviations: DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure. aBased on the National High Blood Pressure Education Program Task Force Standards. 12 bBased on ambulatory blood pressure (BP) monitoring values of Soergel and colleagues116 or the smoothed values of Wuhl. 123 cFor either the wake or sleep period of the study or both.
Discussion
ABPM is now well established as a useful clinical tool in the evaluation of the pediatric patient presenting for evaluation of HTN. Due to the high prevalence of WCH, initial ABPM has been estimated to produce a net savings of more than $2.4 million per 1000 children. 16 Furthermore, HTN treatment guided by ABPM was shown to prevent progression of carotid IMT in renal transplant recipients 113 and was associated with regression of carotid IMT and LVM in adolescents with primary HTN. 126 ABPM measures also maintain relative rank (tracking) better than office readings over a 15‐year period 127 and ABPM results have less variability than office BP readings. The ability of ABPM to better approximate a patient’s true BP level may allow reduction of sample size by up to 75% in antihypertensive drug efficacy studies. 128
There are gaps in the knowledge base, however. More validation experiments need to be conducted in children with a variety of ABPM devices and comparisons between auscultatory and oscillometric devices need to be made in terms of utility, ease of use, and normal mean values. Age‐related differences between casual and ambulatory BP measures need to be defined further. 129 Normative data across different ethnicities are also lacking. This is especially important as many studies have demonstrated a different prevalence for HTN depending on which normal cutpoints were employed and whether casual 130 , 131 or ambulatory data 132 were used. Correlations between BP variability and target organ damage in youth with HTN should also be sought and more data on the usefulness of circadian BP control are needed.
Current guidelines for use of ABPM are also insufficient. There is clear evidence for the benefit of performing ABPM in children suspected of being hypertensive but no hard indications are given. 114 The AHA ABPM statement also does not provide clinicians with recommendations on patient management after ABPM has been performed. 114 For instance, there is risk for target organ damage with WCH, masked HTN, and prehypertension (as outlined above), yet no consensus has been reached as to whether these conditions mandate drug therapy to lower BP. Hopefully, the recommendations for use of ABPM in children will be strengthened based on recently published studies. Despite these limitations, ABPM will see increasing use in pediatric patients.
Conclusions
Since intensive BP control only achievable with ABPM has already proven effective in reducing the prevalence of LVH 15 and progression to end‐stage renal disease in children, 105 it is clear that consistent application of ABPM will assist clinicians in improving BP control and reduce the burden of CV risk in children and adolescents with HTN.
References
- 1. McNiece KL, Poffenbarger TS, Turner JL et al. Prevalence of hypertension and pre‐hypertension among adolescents. J Pediatr. 2007;150:640–644, 644 e641. [DOI] [PubMed] [Google Scholar]
- 2. Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school‐aged children. Pediatrics. 2004;113:475–482. [DOI] [PubMed] [Google Scholar]
- 3. Din‐Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 4. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. [DOI] [PubMed] [Google Scholar]
- 5. Salvadori M, Sontrop JM, Garg AX, et al. Elevated blood pressure in relation to overweight and obesity among children in a rural Canadian community. Pediatrics. 2008;122:e821–e827. [DOI] [PubMed] [Google Scholar]
- 6. Maldonado J, Pereira T, Fernandes R, et al. An approach of hypertension prevalence in a sample of 5381 Portuguese children and adolescents. The AVELEIRA registry. “Hypertension in children.” Blood Press. 2011;20:153–157. [DOI] [PubMed] [Google Scholar]
- 7. Polderman J, Gurgel RQ, Barreto‐Filho JA, et al. Blood pressure and BMI in adolescents in Aracaju, Brazil. Public Health Nutr. 2011;14:1064–1070. [DOI] [PubMed] [Google Scholar]
- 8. Podoll A, Grenier M, Croix B, Feig DI. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538–e543. [DOI] [PubMed] [Google Scholar]
- 9. Butani L, Morgenstern BZ. Are pitfalls of oxcillometric blood pressure measurements preventable in children? Pediatr Nephrol. 2003;18:313–318. [DOI] [PubMed] [Google Scholar]
- 10. Flynn JT, Pierce CB, Miller ER III, et al. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434–440, e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McBryde KD. Blood pressure in pediatric chronic kidney disease‐It’s in the ears of the beholder. J Pediatr. 2012;160:363–365. [DOI] [PubMed] [Google Scholar]
- 12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 13. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals From the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 14. Matsuoka S, Kawamura K, Honda M, Awazu M. White coat effect and white coat hypertension in pediatric patients. Pediatr Nephrol. 2002;17:950–953. [DOI] [PubMed] [Google Scholar]
- 15. Seeman T, Dostalek L, Gilik J. Control of hypertension in treated children and its association with target organ damage. Am J Hypertens. 2012;25:389–395. [DOI] [PubMed] [Google Scholar]
- 16. Swartz SJ, Srivaths PR, Croix B, Feig DI. Cost‐effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177–1181. [DOI] [PubMed] [Google Scholar]
- 17. White WB. Ambulatory blood‐pressure monitoring in clinical practice. N Engl J Med. 2003;348:2377–2378. [DOI] [PubMed] [Google Scholar]
- 18. Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. [DOI] [PubMed] [Google Scholar]
- 19. Stergiou GS, Alamara CV, Kalkana CB, et al. Out‐of‐office blood pressure in children and adolescents: disparate findings by using home or ambulatory monitoring. Am J Hypertens. 2004;17:869–875. [DOI] [PubMed] [Google Scholar]
- 20. Stergiou GS, Karpettas N, Kapoyiannis A, et al. Home blood pressure monitoring in children and adolescents: a systematic review. J Hypertens. 2009;27:1941–1947. [DOI] [PubMed] [Google Scholar]
- 21. Flynn JT. Ambulatory blood pressure monitoring in children: imperfect yet essential. Pediatr Nephrol. 2011;26:2089–2094. [DOI] [PubMed] [Google Scholar]
- 22. Fan HQ, Li Y, Thijs L, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. [DOI] [PubMed] [Google Scholar]
- 23. Lee SH, Kim JH, Kang MJ, et al. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flynn JT. What’s new in pediatric hypertension? Curr Hypertens Rep. 2001;3:503–510. [DOI] [PubMed] [Google Scholar]
- 26. Flynn JT. Differentiation between primary and secondary hypertension in children using ambulatory blood pressure monitoring. Pediatrics. 2002;110:89–93. [DOI] [PubMed] [Google Scholar]
- 27. Seeman T, Palyzova D, Dusek J, Janda J. Reduced nocturnal blood pressure dip and sustained nighttime hypertension are specific markers of secondary hypertension. J Pediatr. 2005;147:366–371. [DOI] [PubMed] [Google Scholar]
- 28. Patzer L, Seeman T, Luck C, et al. Day‐ and night‐time blood pressure elevation in children with higher grades of renal scarring. J Pediatr. 2003;142:117–122. [DOI] [PubMed] [Google Scholar]
- 29. Ferrero GB, Biamino E, Sorasio L, et al. Presenting phenotype and clinical evaluation in a cohort of 22 Williams‐Beuren syndrome patients. Eur J Med Genet. 2007;50:327–337. [DOI] [PubMed] [Google Scholar]
- 30. Bassareo PP, Marras AR, Pasqualucci D, Mercuro G. Increased arterial rigidity in children affected by Cushing’s syndrome after successful surgical cure. Cardiol Young. 2010;20:610–614. [DOI] [PubMed] [Google Scholar]
- 31. Oswiecimska J, Ziora K, Adamczyk P, et al. Effects of neuroendocrine changes on results of ambulatory blood pressure monitoring (ABPM) in adolescent girls with anorexia nervosa. Neuro Endocrinol Lett. 2007;28:410–416. [PubMed] [Google Scholar]
- 32. Hurum H, Sulheim D, Thaulow E, Wyller VB. Elevated nocturnal blood pressure and heart rate in adolescent chronic fatigue syndrome. Acta Paediatr. 2011;100:289–292. [DOI] [PubMed] [Google Scholar]
- 33. Luque‐Ramirez M, Alvarez‐Blasco F, Mendieta‐Azcona C, et al. Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2141–2148. [DOI] [PubMed] [Google Scholar]
- 34. Bassareo PP, Marras AR, Manai ME, Mercuro G. The influence of different surgical approaches on arterial rigidity in children after aortic coarctation repair. Pediatr Cardiol. 2009;30:414–418. [DOI] [PubMed] [Google Scholar]
- 35. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24‐h ambulatory blood pressure in children with ADHD: a double‐blind, randomized, cross‐over trial. Pediatr Nephrol. 2006;21:92–95. [DOI] [PubMed] [Google Scholar]
- 36. Palatini P, Graniero GR, Mormino P, et al. Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Circulation. 1994;90:2870–2876. [DOI] [PubMed] [Google Scholar]
- 37. Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meininger JC, Liehr P, Mueller WH, et al. Stress‐induced alterations of blood pressure and 24 h ambulatory blood pressure in adolescents. Blood Press Monit. 1999;4:115–120. [PubMed] [Google Scholar]
- 39. Wilson DK, Sica DA, Miller SB. Ambulatory blood pressure nondipping status in salt‐sensitive and salt‐resistant black adolescents. Am J Hypertens. 1999;12:159–165. [DOI] [PubMed] [Google Scholar]
- 40. Savoca MR, MacKey ML, Evans CD, et al. Association of ambulatory blood pressure and dietary caffeine in adolescents. Am J Hypertens. 2005;18:116–120. [DOI] [PubMed] [Google Scholar]
- 41. Malbora B, Baskin E, Bayrakci US, et al. Ambulatory blood pressure monitoring of healthy schoolchildren with a family history of hypertension. Ren Fail. 2010;32:535–540. [DOI] [PubMed] [Google Scholar]
- 42. Somes GW, Harshfield GA, Alpert BS, et al. Genetic influences on ambulatory blood pressure patterns. The Medical College of Virginia Twin Study. Am J Hypertens. 1995;8:474–478. [DOI] [PubMed] [Google Scholar]
- 43. Alpay H, Ozdemir N, Wuhl E, Topuzoglu A. Ambulatory blood pressure monitoring in healthy children with parental hypertension. Pediatr Nephrol. 2009;24:155–161. [DOI] [PubMed] [Google Scholar]
- 44. Tenhola S, Rahiala E, Halonen P, et al. Maternal preeclampsia predicts elevated blood pressure in 12‐year‐old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res. 2006;59:320–324. [DOI] [PubMed] [Google Scholar]
- 45. Rahiala E, Tenhola S, Vanninen E, et al. Ambulatory blood pressure in 12‐year‐old children born small for gestational age. Hypertension. 2002;39:909–913. [DOI] [PubMed] [Google Scholar]
- 46. Salgado CM, Jardim PCBV, Teles FBG, Nunes MC. Low birth weight as a marker of changes in ambulatory blood pressure monitoring. Arq Bras Cardiol. 2009;92:107–121. [DOI] [PubMed] [Google Scholar]
- 47. Stabouli S, Kotsis V, Papamichael C, et al. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal‐medial thickness. J Pediatr. 2005;147:651–656. [DOI] [PubMed] [Google Scholar]
- 48. Stergiou GS, Rarra VC, Yiannes NG. Changing relationship between home and office blood pressure with increasing age in children: the Arsakeion School study. Am J Hypertens. 2008;21:41–46. [DOI] [PubMed] [Google Scholar]
- 49. Sorof JM, Poffenbarger T, Franco K, Portman R. Evaluation of white coat hypertension in children: importance of the definitions of normal ambulatory blood pressure and the severity of casual hypertension. Am J Hypertens. 2001;14:855–860. [DOI] [PubMed] [Google Scholar]
- 50. Florianczyk T, Werner B. Usefulness of ambulatory blood pressure monitoring in diagnosis of arterial hypertension in children and adolescents. Kardiol Pol. 2008;66:12–17; discussion 18. [PubMed] [Google Scholar]
- 51. Palatini P, Mormino P, Santonastaso M, et al. Target‐organ damage in stage I hypertensive subjects with white coat and sustained hypertension: results from the HARVEST study. Hypertension. 1998;31:57–63. [DOI] [PubMed] [Google Scholar]
- 52. Gomez‐Cerezo J, Rios Blanco JJ, Suarez Garcia I, et al. Noninvasive study of endothelial function in white coat hypertension. Hypertension. 2002;40:304–309. [DOI] [PubMed] [Google Scholar]
- 53. Landray MJ, Sagar G, Murray S, et al. White coat hypertension and carotid atherosclerosis. Blood Press. 1999;8:134–140. [DOI] [PubMed] [Google Scholar]
- 54. Gustavsen PH, Hoegholm A, Bang LE, Kristensen KS. White coat hypertension is a cardiovascular risk factor: a 10‐year follow‐up study. J Hum Hypertens. 2003;17:811–817. [DOI] [PubMed] [Google Scholar]
- 55. Stabouli S, Kotsis V, Toumanidis S, et al. White‐coat and masked hypertension in children: association with target‐organ damage. Pediatr Nephrol. 2005;20:1151–1155. [DOI] [PubMed] [Google Scholar]
- 56. Lande MB, Meagher CC, Fisher SG, et al. Left ventricular mass index in children with white coat hypertension. J Pediatr. 2008;153:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kavey RE, Kveselis DA, Atallah N, Smith FC. White coat hypertension in childhood: evidence for end‐organ effect. J Pediatr. 2007;150:491–497. [DOI] [PubMed] [Google Scholar]
- 58. Pall D, Lengyel S, Komonyi E, et al. Impaired cerebral vasoreactivity in white coat hypertensive adolescents. Eur J Neurol. 2011;18:584–589. [DOI] [PubMed] [Google Scholar]
- 59. Pall D, Juhasz M, Lengyel S, et al. Assessment of target‐organ damage in adolescent white‐coat and sustained hypertensives. J Hypertens. 2010;28:2139–2144. [DOI] [PubMed] [Google Scholar]
- 60. Lurbe E, Invitti C, Torro I, et al. The impact of the degree of obesity on the discrepancies between office and ambulatory blood pressure values in youth. J Hypertens. 2006;24:1557–1564. [DOI] [PubMed] [Google Scholar]
- 61. Lurbe E, Torro I, Alvarez V, et al. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–498. [DOI] [PubMed] [Google Scholar]
- 62. Furusawa EA, Filho UD, Junior DM, Koch VH. Home and ambulatory blood pressure to identify white coat and masked hypertension in the pediatric patient. Am J Hypertens. 2011;24:893–897. [DOI] [PubMed] [Google Scholar]
- 63. Paripovic D, Kostic M, Spasojevic B, et al. Masked hypertension and hidden uncontrolled hypertension after renal transplantation. Pediatr Nephrol. 2010;25:1719–1724. [DOI] [PubMed] [Google Scholar]
- 64. Bjorklund K, Lind L, Zethelius B, et al. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107:1297–1302. [DOI] [PubMed] [Google Scholar]
- 65. Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:406–411. [DOI] [PubMed] [Google Scholar]
- 66. McNiece KL, Gupta‐Malhotra M, Samuels J, et al. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension. 2007;50:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mitsnefes M, Flynn J, Cohn S, et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Graves JW, Althaf MM. Utility of ambulatory blood pressure monitoring in children and adolescents. Pediatr Nephrol. 2006;21:1640–1652. [DOI] [PubMed] [Google Scholar]
- 69. Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep. 2006;8:199–204. [DOI] [PubMed] [Google Scholar]
- 70. Stabouli S, Kotsis V, Rizos Z, et al. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24:1545–1551. [DOI] [PubMed] [Google Scholar]
- 71. Urbina EM, Khoury PR, McCoy C, et al. Cardiac and vascular consequences of pre‐hypertension in youth. J Clin Hypertens (Greenwich). 2011;13:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lurbe E, Alvarez V, Liao Y, et al. The impact of obesity and body fat distribution on ambulatory blood pressure in children and adolescents. Am J Hypertens. 1998;11:418–424. [DOI] [PubMed] [Google Scholar]
- 73. Shatat IF, Freeman KD, Vuguin PM, et al. Relationship between adiponectin and ambulatory blood pressure in obese adolescents. Pediatr Res. 2009;65:691–695. [DOI] [PubMed] [Google Scholar]
- 74. Torok K, Palfi A, Szelenyi Z, Molnar D. Circadian variability of blood pressure in obese children. Nutr Metab Cardiovasc Dis. 2008;18:429–435. [DOI] [PubMed] [Google Scholar]
- 75. Marcovecchio ML, Patricelli L, Zito M, et al. Ambulatory blood pressure monitoring in obese children: role of insulin resistance. J Hypertens. 2006;24:2431–2436. [DOI] [PubMed] [Google Scholar]
- 76. Leung LCK, Ng DK, Lau MW, et al. Twenty‐four‐hour ambulatory BP in snoring children with obstructive sleep apnea syndrome. Chest. 2006;130:1009–1017. [DOI] [PubMed] [Google Scholar]
- 77. Amin RS, Carroll JL, Jeffries JL, et al. Twenty‐four‐hour ambulatory blood pressure in children with sleep‐disordered breathing. Am J Respir Crit Care Med. 2004;169:950–956. [DOI] [PubMed] [Google Scholar]
- 78. Li AM, Au CT, Sung RY, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63:803–809. [DOI] [PubMed] [Google Scholar]
- 79. Amin R, Somers VK, McConnell K, et al. Activity‐adjusted 24‐hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. [DOI] [PubMed] [Google Scholar]
- 80. Head GA, McGrath BP, Mihailidou AS, et al. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J Hypertens. 2012;30:253–266. [DOI] [PubMed] [Google Scholar]
- 81. Belsha C, Wells T, McNiece K, et al. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. 1998;11(4 Pt 1): 410–417. [DOI] [PubMed] [Google Scholar]
- 82. Verdecchia P. White‐coat hypertension in adults and children. Blood Press Monit. 1999;4:175–179. [PubMed] [Google Scholar]
- 83. Brady TM, Fivush B, Flynn JT, Parekh R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152:73–78. [DOI] [PubMed] [Google Scholar]
- 84. Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. [DOI] [PubMed] [Google Scholar]
- 85. Richey PA, Disessa TG, Hastings MC, et al. Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr. 2008;152:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. O’Leary DH, Polak JF, Kronmal RA, et al. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 87. Lekakis JP, Zakopoulos NA, Protogerou AD, et al. Arterial stiffness assessed by pulse wave analysis in essential hypertension: relation to 24‐h blood pressure profile. Int J Cardiol. 2005;102:391–395. [DOI] [PubMed] [Google Scholar]
- 88. Kamarck TW, Polk DE, Sutton‐Tyrrell K, Muldoon MF. The incremental value of ambulatory blood pressure persists after controlling for methodological confounds: associations with carotid atherosclerosis in a healthy sample. J Hypertens. 2002;20:1535–1541. [DOI] [PubMed] [Google Scholar]
- 89. Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40–44. [DOI] [PubMed] [Google Scholar]
- 90. Aggoun Y, Farpour‐Lambert NJ, Marchand LM, et al. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29:792–799. [DOI] [PubMed] [Google Scholar]
- 91. Stergiou GS, Giovas PP, Kollias A, et al. Relationship of home blood pressure with target‐organ damage in children and adolescents. Hypertens Res. 2011;34:640–644. [DOI] [PubMed] [Google Scholar]
- 92. Palatini P, Mormino P, Santonastaso M, et al. Ambulatory blood pressure predicts end‐organ damage only in subjects with reproducible recordings. HARVEST Study Investigators. Hypertension and Ambulatory Recording Venetia Study. J Hypertens. 1999;17:465–473. [DOI] [PubMed] [Google Scholar]
- 93. Harshfield G, Pulliam D, Alpert B. Ambulatory blood pressure and renal function in healthy children and adolescents. Am J Hypertens. 1994;7:282–285. [DOI] [PubMed] [Google Scholar]
- 94. Lubrano R, Travasso E, Raggi C, et al. Blood pressure load, proteinuria and renal function in pre‐hypertensive children. Pediatr Nephrol. 2009;24:823–831. [DOI] [PubMed] [Google Scholar]
- 95. Sulakova T, Janda J, Cerna J, et al. Arterial HTN in children with T1DM‐‐frequent and not easy to diagnose. Pediatr Diabetes. 2009;10:441–448. [DOI] [PubMed] [Google Scholar]
- 96. Chatterjee M, Speiser PW, Pellizzarri M, et al. Poor glycemic control is associated with abnormal changes in 24‐hour ambulatory blood pressure in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol. 2009;22:1061–1067. [DOI] [PubMed] [Google Scholar]
- 97. Dost A, Klinkert C, Kapellen T, et al. Arterial hypertension determined by ambulatory blood pressure profiles: contribution to microalbuminuria risk in a multicenter investigation in 2,105 children and adolescents with type 1 diabetes. Diabetes Care. 2008;31:720–725. [DOI] [PubMed] [Google Scholar]
- 98. Darcan S, Goksen D, Mir S, et al. Alterations of blood pressure in type 1 diabetic children and adolescents. Pediatr Nephrol. 2006;21:672–676. [DOI] [PubMed] [Google Scholar]
- 99. Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- 100. Horoz OO, Yuksel B, Bayazit AK, et al. Ambulatory blood pressure monitoring and serum nitric oxide concentration in type 1 diabetic children. Endocr J. 2009;56:477–485. [DOI] [PubMed] [Google Scholar]
- 101. Shatat IF, Flynn JT. Relationships between renin, aldosterone, and 24‐hour ambulatory blood pressure in obese adolescents. Pediatr Res. 2011;69:336–340. [DOI] [PubMed] [Google Scholar]
- 102. Ettinger LM, Freeman K, DiMartino‐Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147:67–73. [DOI] [PubMed] [Google Scholar]
- 103. Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood‐onset chronic renal failure. Circulation. 2002;106:100–105. [DOI] [PubMed] [Google Scholar]
- 104. Samuels JACS, Bell C, Poffenbarger T, et al. Ambulatory hypertension by ABPM is associated with declining iGFR in children with chronic kidney disease: preliminary findings from the Chronic Kidney Disease in Children (CKiD) study (abstract). E-PAS. 2008;68:5355. [Google Scholar]
- 105. Wuhl E, Trivelli A, Picca S, et al. Strict blood‐pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. [DOI] [PubMed] [Google Scholar]
- 106. Flynn JT. Impact of ambulatory blood pressure monitoring on the management of hypertension in children. Blood Press Monit. 2000;5:211–216. [DOI] [PubMed] [Google Scholar]
- 107. Woroniecki RP, Flynn JT. How are hypertensive children evaluated and managed? A survey of North American pediatric nephrologists Pediatr Nephrol. 2005;20:791–797. [DOI] [PubMed] [Google Scholar]
- 108. Roche SL, Kaufmann J, Dipchand AI, Kantor PF. Hypertension after pediatric heart transplantation is primarily associated with immunosuppressive regimen. J Heart Lung Transplant. 2008;27:501–507. [DOI] [PubMed] [Google Scholar]
- 109. McGlothan KR, Wyatt RJ, Ault BH, et al. Predominance of nocturnal hypertension in pediatric renal allograft recipients. Pediatr Transplant. 2006;10:558–564. [DOI] [PubMed] [Google Scholar]
- 110. Ferraris JR, Ghezzi L, Waisman G, Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant. 2007;11:24–30. [DOI] [PubMed] [Google Scholar]
- 111. Krmar RT, Berg UB, Krmar RT, Berg UB. Blood pressure control in hypertensive pediatric renal transplants: role of repeated ABPM following transplantation. Am J Hypertens. 2008;21:1093–1099. [DOI] [PubMed] [Google Scholar]
- 112. Basiratnia M, Esteghamati M, Ajami GH, et al. Blood pressure profile in renal transplant recipients and its relation to diastolic function: tissue Doppler echocardiographic study. Pediatr Nephrol. 2011;26:449–457. [DOI] [PubMed] [Google Scholar]
- 113. Balzano R, Lindblad YT, Vavilis G, et al. Use of annual ABPM, and repeated carotid scan and echocardiography to monitor cardiovascular health over nine yr in pediatric and young adult renal transplant recipients. Pediatr Transplant. 2011;15:635–641. [DOI] [PubMed] [Google Scholar]
- 114. Urbina E, Alpert B, Flynn J, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research. Hypertension. 2008;52:433–451. [DOI] [PubMed] [Google Scholar]
- 115. Jones DP, Richey PA, Alpert BS. Validation of the AM5600 ambulatory blood pressure monitor in children and adolescents. Blood Press Monit. 2008;13:349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wuhl E, Witte K, Soergel M, et al. Distribution of 24‐h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 117. Winnicki M, Canali C, Mormino P, Palatini P. Ambulatory blood pressure monitoring editing criteria: is standardization needed? Hypertension and Ambulatory Recording Venetia Study (HARVEST) Group, Italy. Am J Hypertens. 1997;10:419–427. [PubMed] [Google Scholar]
- 118. Jones HE, Sinha MD. The definition of daytime and nighttime influences the interpretation of ABPM in children. Pediatr Nephrol. 2011;26:775–781. [DOI] [PubMed] [Google Scholar]
- 119. J. C. S. Joint Working Group . Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring. Circ J. 2012;76:508–519. [DOI] [PubMed] [Google Scholar]
- 120. Wuhl E, Hadtstein C, Mehls O, Schaefer F. Ultradian but not circadian blood pressure rhythms correlate with renal dysfunction in children with chronic renal failure. J Am Soc Nephrol. 2005;16:746–754. [DOI] [PubMed] [Google Scholar]
- 121. Hermida RC, Fernandez JR, Mojon A, Ayala DE. Reproducibility of the hyperbaric index as a measure of blood pressure excess. Hypertension. 2000;35:118–125. [DOI] [PubMed] [Google Scholar]
- 122. Simonetti GD, Von VRO, Wuhl E, Mohaupt MG. Ambulatory arterial stiffness index is increased in hypertensive childhood disease. Pediatr Res. 2008;64:303–307. [DOI] [PubMed] [Google Scholar]
- 123. Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty‐four‐hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. [DOI] [PubMed] [Google Scholar]
- 124. Vaughan CJ, Murphy MB. The use of ambulatory blood pressure monitoring in the evaluation of racial differences in blood pressure. J Cardiovasc Risk. 1994;1:132–135. [DOI] [PubMed] [Google Scholar]
- 125. Lurbe E, Sorof JM, Daniels SR. Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 2004;144:7–16. [DOI] [PubMed] [Google Scholar]
- 126. Litwin M, Niemirska A, Sladowska‐Kozlowska J, et al. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr Nephrol. 2010;25:2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Z, Snieder H, Harshfield GA, et al. A 15‐year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gimpel C, Wuhl E, Arbeiter K, et al. Superior consistency of ambulatory blood pressure monitoring in children: implications for clinical trials. J Hypertens. 2009;27:1568–1574. [DOI] [PubMed] [Google Scholar]
- 129. Stergiou GS, Karpettas N, Panagiotakos DB, Vazeou A. Comparison of office, ambulatory and home blood pressure in children and adolescents on the basis of normalcy tables. J Hum Hypertens. 2011;25:218–223. [DOI] [PubMed] [Google Scholar]
- 130. Diaz LN, Garin EH. Comparison of ambulatory blood pressure and Task Force criteria to identify pediatric hypertension. Pediatr Nephrol. 2007;22:554–558. [DOI] [PubMed] [Google Scholar]
- 131. Kennedy S, Mackie F, Craig E, Kainer G. The choice of threshold limits for pediatrics ambulatory blood pressure monitoring influences clinical decisions. Blood Press Monit. 2006;11:119–123. [DOI] [PubMed] [Google Scholar]
- 132. Jones DP, Richey PA, Alpert BS. Comparison of ambulatory blood pressure reference standards in children evaluated for hypertension. Blood Press Monit. 2009;14:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


