Abstract
J Clin Hypertens (Greenwich). 2012;14:158–164. ©2012 Wiley Periodicals, Inc.
The prevalence of hypertension in the United States has grown dramatically in recent years. Thiazide diuretics have played a major role in the rising rate of blood pressure (BP) control. Accompanying this has been the appearance of adverse drug events, including hospitalizations associated with thiazide‐associated hyponatremia (HTAH). Hyponatremia is a common yet often overlooked side effect of this drug class. Identification of HTAH risk factors may aid in creating strategies to prevent hospitalizations. This is a retrospective, case‐controlled study of 10,805 patients (1802 cases, 9003 controls) examining HTAH risk factors within a group‐model integrated‐care organization. Multivariate analysis revealed that age (odds ratio [OR], 1.75; 95% confidence interval [CI], 1.58–1.93), angiotensin‐converting enzyme (ACE) inhibitor use (OR, 1.53; 95% CI, 1.16–2.00), and hypokalemia (OR, 40.94; 95% CI, 26.46–66.33) were most associated with HTAH. Urinary tract infection (UTI), type 2 diabetes, hyperlipidemia, and gastroesophageal reflux disease (GERD) were also found to be HTAH risk factors. Potassium supplements (OR, 0.60; 95% CI, 0.44–0.83) and weight (OR, 0.91; 95% CI, 0.88–0.93) had protective effects. A predictive model was developed to determine overall HTAH risk given the presence of individual risk factors. Age, weight, hypokalemia, GERD, type 2 diabetes, UTI, and ACE inhibitor use independently correlated with an increased risk of HTAH. This model may be applied in clinical practice to guide thiazide prescribing.
The worldwide prevalence of hypertension was estimated at 26% of the population in the year 2000, or approximately 1 billion people. 1 National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2004 demonstrated that within the United States, hypertension prevalence, awareness, and control have continued to increase. 2 National guidelines place thiazides as first‐line agents in the treatment of hypertension, primarily because of their efficacy in reducing cardiovascular complications in major outcome trials such as the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) 3 and Systolic Hypertension in the Elderly Program (SHEP). 4
Side effects of thiazides, such as hypokalemia, hyperuricemia, and hyperglycemia, are well known. Hyponatremia, in contrast, often goes unrecognized despite being the most common electrolyte disorder in clinical practice. 5 To understand how thiazide diuretics cause hyponatremia, a brief explanation of plasma sodium and volume regulation by the kidney is warranted. Glomerular tubular balance is the negative feedback mechanism that regulates serum sodium and plasma volume. In short, a drop in intravascular volume causes a drop in glomerular filtration rate (GFR). Decreased GFR translates to less sodium being filtered at the glomerulus. Sodium and chloride are reabsorbed in the loop of Henle, causing less sodium and chloride ions to reach the distal tubule. The juxtaglomerular complex detects this drop in sodium and chloride ions and responds by releasing renin. Through a series of steps, renin ultimately triggers the production of angiotensin II, which has a variety of effects, including: (1) triggering the release of aldosterone from the adrenal cortex, causing increased sodium and water reabsorption in the distal tubule and collecting duct; (2) stimulating the release of antidiuretic hormone (ADH) from the posterior pituitary, which, in turn, causes water retention at the distal tubule and collecting duct; (3) stimulation of thirst and increased water intake; and (4) vasoconstriction of the efferent arteriole, maintaining GFR and filtration pressure at the glomerulus. The end result is that intravascular volume is maintained. 6 Thiazide diuretics work by inhibiting the sodium/chloride co‐transporters in the distal tubule. Sodium and chloride reabsorption are thus blocked and these ions are excreted in the urine. This sodium loss is independent of the angiotensin II–mediated aldosterone, ADH, and thirst stimulation that occurs from thiazide‐induced volume depletion. 7 Thiazides are also hypothesized to upregulate aquaporin‐2 channels in the medullary collecting duct, increasing water permeability and water reabsorption, an effect independent of ADH‐mediated water retention. 8 The net result of the above‐described mechanisms is water retention and sodium excretion that alters the sodium/water balance, resulting in hyponatremia.
The onset of thiazide‐associated hyponatremia has been reported to be rapid and unpredictable. 9 Although once thought to appear within 2 weeks of initiation, 10 two large case‐controlled studies have shown that hyponatremia due to thiazides may develop after as long as 12 years of thiazide use. 9 , 11 Findings of previous studies have pointed to potential risk factors for thiazide‐associated hyponatremia. In a population of patients aged 65 and older using thiazides upon hospital admission, the incidence of hyponatremia was reported as 11% to 33%. 12 , 13 Another study cites that hyponatremia with thiazides is a common occurrence in women 70 years and older. 14 Concomitant use of loop and potassium‐sparing diuretics, as well as the presence of psychiatric illnesses that increase thirst or water intake, have also been reported to increase the risk of developing thiazide‐associated hyponatremia. 15 As hypertension awareness and control have grown, and with convincing evidence that thiazides not only control BP but lead to positive health outcomes, 3 thiazide utilization will only continue to increase. Despite these benefits, greater use of thiazides may also result in larger absolute numbers of hospitalizations associated with thiazide‐associated hyponatremia (HTAH). Thus, a predictive model incorporating risk factors for HTAH may offer significant clinical value.
This report is a retrospective, case‐controlled study to determine specific patient risk factors for HTAH within a large, group model–integrated care organization, and to construct a model to predict HTAH risk in the clinical setting. Knowledge of risk factors and an accompanying predictive model would assist clinicians in preventing hospitalizations from this adverse drug event.
Methods
Patients
A total of 10,805 patients were identified from the electronic medical record (EMR) of a large, nonprofit, group model–integrated care organization from 2001 to 2010 using International Statistical Classification of Diseases and Related Health Problems–Ninth Revision (ICD‐9) codes. Cases were defined as patients hospitalized with a primary or secondary diagnosis of hyponatremia while concurrently treated with a thiazide diuretic. Cases must have been health plan members for at least 12 months prior to hospitalization. Patients met the criteria for HTAH if admitted to the hospital with a diagnosis of hyponatremia (ICD‐9 code 276.1); a serum sodium level <135 m Eq/L; and a history of current thiazide use, defined as a prescription for a thiazide diuretic dispensed within 12 months of hospital admission. Because the health plan has a pharmacy benefit, thiazide prescriptions are able to be tracked in the health plan’s EMR. Patients with the following known causes of hyponatremia were excluded: adrenal insufficiency; hypothyroidism; kidney disease, including salt‐wasting nephropathy, chronic kidney disease, and acute renal failure; liver disease, including cirrhosis and hepatomegaly; congestive heart failure; ascites; and syndrome of inappropriate antidiuretic hormone secretion (SIADH), or causes of SIADH (ie, tumors, central nervous system disorders, and pulmonary disorders). Patients taking medications known to induce SIADH were also excluded (eg, desmopressin, oxytocin, cyclophosphamide, vincristine, vinblastine, and bromocriptine).
Control patients did not develop hyponatremia while treated with a thiazide. Five controls were matched to each case based on the date of the earliest thiazide prescription. Controls must have been health plan members for at least 12 months prior to the hospitalization date of the matching case. Data abstracted for both cases and controls included age, sex, weight, ethnicity, use of thiazide diuretics and thiazide combinations, concurrent medications, and intercurrent conditions. Data collection and abstraction of patient information for this study were approved by the Kaiser Permanente institutional review board.
Statistical Analysis
Data were analyzed with SAS version 9.2 (SAS Institute, Cary, NC). Based on data from Chow and colleagues, 9 the largest case‐control study on thiazide‐associated hyponatremia to date, each 10‐year increase in age conferred an approximate 2‐fold increased risk of hyponatremia from a thiazide (hazard ratio [HR], 2.14; 95% confidence interval [CI], 1.59–2.88; P<.0001). This is equivalent to an 8% increased risk per 1‐year age increase. Utilizing this figure, it was determined that 4596 patients were required to provide a 90% power with an α level of 0.05 to detect an 8% difference in the risk of HTAH per 1‐year increase in age. Univariate comparisons between cases and controls were calculated utilizing the analysis of variance F test for continuous variables, and the chi‐square test for discrete variables. Multiple logistic regression was used to determine risk factors for HTAH. It should be noted that only 6048 patients were included in the multivariate analysis; 4757 were excluded because these patients did not have weight data available in the EMR. With weight potentially being an important covariate in determining the risk of HTAH, only patients with weight data were used when conducting the logistic regression. Covariates were selected based on previous literature on thiazide‐associated hyponatremia. ORs and 95% CIs of risk factors for cases and controls were calculated and compared, with a P value of <.05 considered statistically significant.
A predictive model was constructed with potential risk variables for HTAH, with two thirds of the study sample used to build the model, and the remaining one third to validate the model. A score system was created to determine whether a particular patient was at a low, intermediate, or high risk of HTAH. Similar to the multivariate analysis, only the 6048 patients with weight data were used to build the predictive model, as weight is likely a key predictive variable in assessing overall HTAH risk.
Results
A total of 10,805 patients, 1802 cases and 9003 controls, were abstracted and analyzed from the EMR of the integrated care plan. Characteristics of cases and controls are shown in Table I. Univariate analysis revealed that cases were older than controls (71.1±12.4 years vs 60.5±12.1 years, P<.0001), weighed less (74.0±21.7 kg vs 88.2±22.6 kg, P<.0001), and were composed of a greater percentage of women (70.8% vs 57.3%, P<.0001). With the exception of African Americans, a higher percentage of each ethnicity was present in the cases group. Hydrochlorothiazide and thiazide combinations accounted for the majority of thiazide use, with no difference found between cases and controls in drug usage for each category. A higher percentage of cases used angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), potassium supplements, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants (TCA) than controls (P<.0001). No significant difference was found in nonsteroidal anti‐inflammatory drug (NSAID) and potassium‐sparing diuretic usage in both groups. The 5 most common intercurrent conditions present in the cases group were identified (by ICD‐9 codes) and abstracted for the data analysis and included hyperlipidemia, hypokalemia, gastroesophageal reflux disease (GERD), type 2 diabetes, and urinary tract infection (UTI). More cases than controls had each of these 5 intercurrent conditions (P<.0001).
Table I.
Characteristics of Cases and Controls
| Cases (n=1802) | Controls (n=9003) | P Values | |
|---|---|---|---|
| Age, mean (SD) y | 71.1 (12.4) | 60.5 (12.1) | <.0001 |
| Body weight, mean (SD), kg | 74.0 (21.7) | 88.2 (22.6) | <.0001 |
| BMI, mean (SD), kg/m2 | 27.4 (6.5) | 31.1 (6.6) | <.0001 |
| Female sex, No. (%) | 1275 (70.8) | 5155 (57.3) | <.0001 |
| Ethnicity, No. (%) | |||
| Caucasian | 950 (52.7) | 3263 (36.2) | <.0001 |
| Black | 194 (10.8) | 1497 (16.6) | <.0001 |
| Hispanic | 375 (20.8) | 1498 (16.6) | <.0001 |
| Asian/Pacific Islander | 232 (12.9) | 631 (7.0) | <.0001 |
| Other/unknown | 51 (2.8) | 2114 (23.5) | <.0001 |
| Type of thiazide diuretic, No. (%) | |||
| Chlorthalidone | 22 (1.2) | 27 (0.3) | <.0001 |
| Hydrochlorothiazide | 1135 (63.0) | 5464 (60.7) | .0719 |
| Metolazone | 5 (0.3) | 11 (0.1) | .1673 |
| Thiazide combinations | 769 (42.7) | 3851 (42.8) | .9584 |
| Concurrent medications, No. (%) | |||
| ACE inhibitors | 1070 (59.4) | 4427 (49.2) | <.0001 |
| ARBs | 201 (11.2) | 657 (7.3) | <.0001 |
| NSAIDs | 611 (33.9) | 2903 (32.2) | .1771 |
| Potassium‐sparing diuretics | 363 (20.1) | 1898 (21.1) | .3917 |
| Potassium supplements | 290 (16.1) | 1256 (14.0) | .0202 |
| SSRIs | 215 (11.9) | 691 (7.7) | <.0001 |
| Tricyclic antidepressants | 159 (8.8) | 439 (4.9) | <.0001 |
| Intercurrent conditions, No. (%) | |||
| Hyperlipidemia | 848 (47.1) | 2727 (30.3) | <.0001 |
| Hypokalemia | 520 (28.9) | 94 (1.0) | <.0001 |
| GERD | 435 (24.1) | 670 (7.4) | <.0001 |
| Type 2 diabetes | 429 (23.8) | 1180 (13.1) | <.0001 |
| UTI | 429 (23.8) | 390 (4.3) | <.0001 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BMI, body mass index; GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; SD, standard deviation; SSRI, selective serotonin reuptake inhibitors; UTI, urinary tract infection.
A comparison of characteristics between the population containing weight data (n=6048) vs the group lacking weight data (n=4757) is provided in Table II. Both groups are relatively similar across most characteristics, suggesting that limiting the multivariate analysis and predictive model to the 6048 patients with weight data would not substantially alter the final outcome of these individual models.
Table II.
Comparison of Baseline Characteristics for Patient Populations With and Without Weight Measurement
| Patients With Weight Value (n=6048) | Patients Without Weight Value (n=4757) | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 61.9 (12.7) | 62.7 (12.8) | .0013 |
| Body weight, mean (SD), kg | 86.8 (23.0) | N/A | N/A |
| BMI, mean (SD), kg/m2 | 30.4 (6.8) | N/A | N/A |
| Female sex, No. (%) | 3501 (57.9) | 2929 (61.6) | .0001 |
| Type of thiazide diuretic, No. (%) | |||
| Chlorthalidone | 36 (0.6) | 13 (0.3) | .0134 |
| Hydrochlorothiazide | 3372 (55.8) | 3227 (67.8) | <.0001 |
| Metolazone | 6 (0.1) | 10 (0.2) | .1363 |
| Thiazide combinations | 2947 (48.7) | 1673 (35.2) | <.0001 |
| Concurrent medications, No. (%) | |||
| ACE inhibitors | 3461 (57.2) | 2036 (42.8) | <.0001 |
| ARBs | 547 (9) | 311 (6.5) | <.0001 |
| NSAIDs | 1988 (32.9) | 1526 (32.1) | .3834 |
| Potassium‐sparing diuretics | 985 (16.3) | 1276 (26.8) | <.0001 |
| Potassium supplements | 801 (13.2) | 745 (15.7) | .0004 |
| SSRIs | 457 (7.6) | 449 (9.4) | .0005 |
| Tricyclic antidepressants | 331 (5.5) | 267 (5.6) | .7522 |
| Intercurrent conditions, No. (%) | |||
| Hyperlipidemia | 2403 (39.7) | 1172 (24.6) | <.0001 |
| Hypokalemia | 363 (6) | 251 (5.3) | .1058 |
| GERD | 735 (12.2) | 370 (7.8) | <.0001 |
| Type 2 diabetes | 913 (15.1) | 696 (14.6) | .5005 |
| UTI | 452 (7.5) | 367 (7.7) | .6379 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; BMI, body mass index; GERD, gastroesophageal reflux disease; N/A, not applicable; NSAIDs, nonsteroidal anti‐inflammatory drugs; SD, standard deviation; SSRI, selective serotonin reuptake inhibitors; UTI, urinary tract infection.
Multivariate analysis (Figure) demonstrated that each 10‐year increase in age conferred a 75% increased risk of HTAH (OR, 1.75; 95% CI, 1.58–1.93). An incremental increase in weight of 5 kg resulted in a 9% decreased risk of HTAH (OR, 0.91; 95% CI, 0.88–0.93). Sex did not emerge as an HTAH risk factor. Patients taking ACE inhibitors had a higher risk of developing HTAH (OR, 1.53; 95% CI, 1.16–2.00), whereas potassium supplements provided a protective effect (OR, 0.60; 95% CI, 0.44–0.83).
Figure FIGURE.
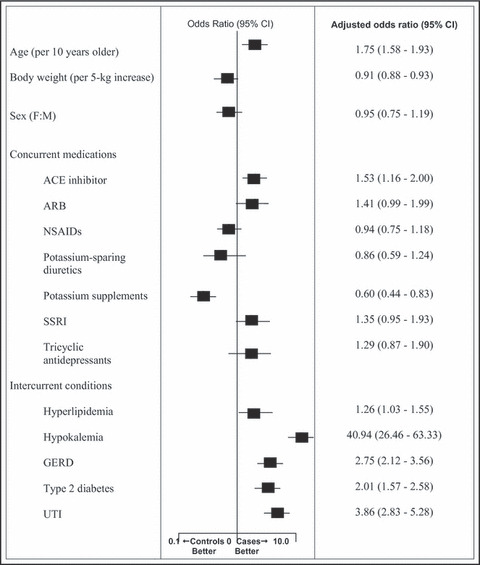
Independent factors related to hospitalizations associated with thiazide‐associated hyponatremia. CI indicates confidence interval; F:M, female:male; ACE, angiotensin‐converting enzyme; ARBs, angiotensin receptor blockers; GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; SSRI, selective serotonin reuptake inhibitors; UTI, urinary tract infection.
Each of the 5 most common intercurrent conditions identified in the cases group proved to be risk factors for HTAH. Notably, patients with hypokalemia had a 40‐fold higher risk (OR, 40.94; 95% CI, 26.46–63.33). Patients with UTI had a nearly 4‐fold higher risk of HTAH (OR, 3.86; 95% CI, 2.83–5.28). GERD, type 2 diabetes, and hyperlipidemia were all associated with an increased HTAH risk as well.
The predictive model for HTAH was constructed using two thirds of cases and controls as the estimation group and the remaining one third as a validation group. Seventeen variables from the previous multivariate analysis were used to build the predictive model. Using the best subset selection method, the model with the fewest number of variables but highest C statistics was created. The model with the highest diagnostic value consisted of 7 variables that independently correlated with HTAH risk: age, weight, hypokalemia, GERD, type 2 diabetes, UTI, and use of ACE inhibitors. A simple score system was developed to determine a patient’s risk of HTAH. The formula is as follows: HTAH risk score = 2.4 + (0.06*age) − (0.01*weight) + (3.64*hypokalemia) + (1.05*GERD) + (0.84*diabetes) + (1.37*UTI) + (0.62*ACE inhibitor) in which age is in years, weight in pounds, and a value of “1” is applied if the corresponding condition or ACE inhibitor therapy is present and “0” if the corresponding disease or ACE inhibitor therapy is absent.
The final score is rounded to the nearest whole integer. The relationship of score to overall HTAH risk is shown in Table III. A risk score of ≤4 corresponds to a low risk of HTAH, a score of 5 to 7 indicates an intermediate risk, and scores ≥8 correlate with a high HTAH risk. In the validation group, when applying a score of ≤4870 of 2333 (37.3%), patients were predicted to be of low HTAH risk. Of these 870 patients, 840 did not develop HTAH, giving the model a 96.6% negative predictive value. When applying a score of ≥8195 of 2333 (8.4%), patients were predicted to have a high risk for HTAH. A total of 145 of these 195 high‐risk patients actually developed HTAH, giving a positive predictive value of 74.4%. The diagnostic accuracy of the score system for HTAH in the validation group is displayed in Table IV.
Table III.
Hospitalizations Associated With Thiazide‐Associated Hyponatremia (HTAH) Score System and Risk Stratification
| HTAH Score | HTAH Risk |
|---|---|
| ≤4 | Low |
| 5–7 | Intermediate |
| ≥8 | High |
Table IV.
Diagnostic Accuracy of the Score System in the Validation Group
| HTAH Score | Sensitivity | Specificity | PPV | NPV | Interpretation |
|---|---|---|---|---|---|
| ≤4 | 0.91 | 0.42 | 0.22 | 0.97 | Low risk of HTAH predicted with 97% certainty |
| ≥8 | 0.42 | 0.97 | 0.74 | 0.91 | High risk of HTAH predicted with 74% certainty |
Abbreviations: HTAH, hospitalizations associated with thiazide‐associated hyponatremia; NPV, negative predictive value; PPV, positive predictive value.
Discussion
The widespread use of thiazide diuretics as first‐line antihypertensive agents has been instrumental in the rising rates of BP control in the United States. Accompanying increased thiazide use has been larger absolute numbers, but not necessarily a greater incidence of HTAH, an adverse drug event often not identified by clinicians. 14 The current report is the largest case‐controlled study to date evaluating HTAH. Our study showed that advanced age correlated with an increased risk of HTAH, whereas an incremental increase in weight provided a protective effect. No significant correlation was found between sex and HTAH. These findings are consistent with previous literature on this topic, including the investigation by Chow and colleagues, 9 the largest published study on thiazide‐induced hyponatremia prior to the current report (439 patients).
The use of ACE inhibitors resulted in a 53% higher risk of HTAH. Several case reports have reported hyponatremia associated with ACE inhibitor therapy, both in patients with normal and with impaired renal function. 16 , 17 This drug‐related adverse event is thought to occur from ACE inhibitor–mediated effects on the renin‐angiotensin‐aldosterone system. ACE inhibitors reduce production of angiotensin II via inhibition of ACE. Through a complex negative feedback loop, renin production is increased, leading to subsequent increases in angiotensin II. Angiotensin II then triggers the release of ADH, which, in turn, causes water retention. In addition, angiotensin directly stimulates the thirst center, leading to polydipsia and dilution of plasma sodium. ACE inhibitors also inhibit tubular sodium reabsorption and aldosterone secretion, further contributing to reducing sodium levels. Additionally, through a reduction in peripheral vascular resistance and greater renal blood flow, renin‐angiotensin‐aldosterone system activation, and ultimately sodium reabsorption, is impaired. 18 Another proposed mechanism is that ACE inhibitors delay bradykinin degradation, inducing an increase in ADH secretion, which subsequently causes a dilution of serum sodium. 7
In contrast to ACE inhibitors, potassium supplements provided a protective effect for HTAH, with patients taking these drugs being 40% less likely to be hospitalized for thiazide‐associated hyponatremia. Chow and colleagues 9 showed an inverse relationship between potassium levels and thiazide‐induced hyponatremia. This finding may explain why usage of potassium supplements conferred a protective effect for HTAH. Use of NSAIDs and potassium‐sparing diuretics did not correlate with an increased risk of HTAH. Chow and associates 9 reported a similar result for NSAIDs. Given the protective effect of potassium supplements, it would appear that potassium‐sparing diuretics would have the same effect. The lack of correlation between these agents and HTAH suggest that this protective effect may be blunted by their sodium‐wasting effects. TCAs and SSRIs, psychotropic agents often associated with hyponatremia, were also not associated with HTAH risk in our study. This is contradictory to accepted knowledge that SSRIs and TCAs induce hyponatremia by causing SIADH. 19 Considering that our study excluded patients with SIADH or causes of SIADH, it follows that TCAs and SSRIs did not correlate with HTAH in our analysis.
We discovered that patients with hypokalemia had a 40‐fold higher risk of developing HTAH, consistent with Chow and colleagues, 9 although the magnitude of risk in our study is dramatically higher. The role of plasma potassium in the occurrence of HTAH merits future study. The effects of factors such as urinalysis, urine‐specific gravity, urine pH, serum creatinine, plasma magnesium, bicarbonate, and glucose should be analyzed when examining the potassium/HTAH relationship.
We also found that patients with UTI had a nearly 4‐fold increased risk of HTAH. A proposed mechanism for this occurence may be a combination of urinary frequency and increased fluid intake. UTIs are quite prevalent in the elderly, 20 a population already at a higher risk for HTAH.
Low serum sodium is often found in the presence of hyperlipidemia and is labeled as pseudohyponatremia because no loss of sodium or increase in free water actually occurs in these patients. Sodium may appear low on laboratory findings due to the space‐occupying effects of lipids in the blood. 21 Thus, the association of hyperlipidemia and hyponatremia in this study is likely a laboratory test artifact and not of clinical significance.
Patients with type 2 diabetes were twice as likely to develop HTAH in our study, a finding that differs from Chow and colleagues, 9 who found no correlation with this condition. The association of diabetes with HTAH in our study can be explained through the same mechanism as for hyperlipidemia. Uncontrolled diabetes is often accompanied by hypertriglyceridemia, which, as described previously, often leads to pseudohyponatremia. 22 It is plausible that a large percentage of patients with type 2 diabetes in our study had elevated triglycerides and subsequent pseudohyponatremia on hospital admission due to this phenomenon. GERD also correlated with a higher incidence of HTAH. Given the limited research and published literature on this association, further study is warranted for this risk factor.
Creation of the predictive model and score system for HTAH demonstrated that for patients treated with thiazide diuretics, age, weight, concurrent therapy with ACE inhibitors, and the presence of the comorbidities hypokalemia, GERD, type 2 diabetes, and UTI all independently correlate with HTAH risk. This model, and subsequent risk stratification based on score, if confirmed, may be applied in clinical practice to predict HTAH risk for patients being treated with thiazides or in patients where initiation of a thiazide is being considered. The model identifies with a nearly 97% certainty (Table IV) patients at a low risk for HTAH, and thus may be useful in confirming the suitability of thiazide therapy in these patients.
Study Strengths
Our report represents a significant addition to previous literature on this topic and has several strengths. First, we performed a power analysis, which showed that our study was sufficiently powered to detect a difference between subgroups, the first study on this subject to do so. Second, we set forth extensive exclusion criteria, eliminating potential confounding conditions and medications and thus determining HTAH risk factors with greater certainty.
Study Limitations
This study has some limitations. Although several key risk factors for HTAH were evaluated in this study, some factors could not be abstracted from the EMR. The most relevant of these factors are medications not entered into a patient’s profile, such as over‐the‐counter medicines, dietary and herbal supplements, and medications obtained from non‐plan pharmacies. Another limitation is the fact that only patients hospitalized with hyponatremia were included as cases in this trial. Outpatients with hyponatremia, as well as asymptomatic patients and those with less severe forms of the condition were not evaluated in this study. Also, given that ICD‐9 codes were used to identify cases, improper coding may have resulted in patients with unrecognized HTAH. Additionally, the onset of thiazide‐associated hyponatremia, as well as the effect of thiazide dose were not examined, two areas worthy of future investigation. The purpose of this study was to simply assess HTAH risk factors, regardless of the dose used or when the thiazide was initiated. Limitations to the use of the predictive model and score system are the fact that it was not applied to patients meeting the extensive exclusion criteria set forth in the beginning of the study. The risk of HTAH in such patients may not be accurately determined using our predictive model and score system.
Conclusions
Advanced age, use of ACE inhibitors, and the presence of hypokalemia, UTI, type 2 diabetes, GERD, or hyperlipidemia were all associated with a greater risk of HTAH. Conversely, incremental increases in weight and the use of potassium supplements conferred a protective effect. Clinicians should take these risk factors into consideration when evaluating treatment with a thiazide. Our predictive model, if independently confirmed, may serve as a means of determining the risk of HTAH in a patient currently taking thiazides, or prior to thiazide initiation, and may ultimately lead to decreased hospitalizations. Closer monitoring, along with the development of strategies and guidelines to manage this condition, are warranted to prevent the occurrence of HTAH in the future.
Acknowledgments
Disclosures and acknowledgments: The authors report no specific funding in relation to this research and have no conflicts of interest to disclose. We would like to thank Qiaowu Li, biostatistician from Kaiser Permanente Southern California Research and Evaluation for conducting the statistical analysis for this paper. We would also like to thank Kaiser Permanente Consultation and Facilitation Service for their guidance and feedback.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:1. [DOI] [PubMed] [Google Scholar]
- 3. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 4. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 5. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;7(suppl 1):S30. [DOI] [PubMed] [Google Scholar]
- 6. Stewart P. Physiology of the kidney, World Anaesthesia Online, Issue 9 (1998). Article 6: [http://www.nda.ox.ac.uk/wfsa/html/u09/u09_016.htm]. Accessed October 17, 2011.
- 7. Liamis G, Haralampos M, Elisaf M. A review of drug‐induced hyponatremia. Am J Kidney Dis. 2008;52:144–153. [DOI] [PubMed] [Google Scholar]
- 8. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium‐induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin‐2, Na‐Cl co‐transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15:2836–2843. [DOI] [PubMed] [Google Scholar]
- 9. Chow KM, Szeto CC, Wong TYH, et al. Risk factors for thiazide‐induced hyponatremia. QJ Med. 2003;96:911–917. [DOI] [PubMed] [Google Scholar]
- 10. Stern RH, Silver SM, Spital A. Hyponatremia. In: Seldin DW, Giebisch G, eds. The Kidney: Physiology and Pathophysiology, 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:1220. [Google Scholar]
- 11. Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens. 2002;16:631–635. [DOI] [PubMed] [Google Scholar]
- 12. Gross P, Ketteler M, Hausman C, et al. Role of diuretics, hormonal derangements, and clinical setting of hyponatremia in medical patients. Klin Wochenschr. 1988;66:662–669. [DOI] [PubMed] [Google Scholar]
- 13. Byatt CM, Millard PH, Levin GE. Diuretics and electrolyte disturbances in 1000 consecutive geriatric admissions. J R Soc Med. 1990;83:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann SJ. The silent epidemic of thiazide‐induced hyponatremia. J Clin Hypertens (Greenwich). 2008;10(6):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adrieff AI, Llache F, Massery SG. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore). 1976;55:121–129. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez‐Martinez H, Gaspard JJ, Espino DV. Hyponatremia due to enalapril in an elderly patient–a case report. Arch Fam Med. 1993;2(7):791–793. [DOI] [PubMed] [Google Scholar]
- 17. Collier JG, Webb DJ. Severe thiazide‐induced hyponatremia during treatment with enalapril. Postgrad Med J. 1987;63:1105–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerber J, Nies AS. Inhibitors of the renin‐angiotensin system. In: Goodman LS, Gilman A, eds. The Pharmacological Basis of Therapeutics, 8th edn. New York, NY: Pergamon Press Inc.; 1990:756–762. [Google Scholar]
- 19. Smith JM. Clinical implications of treating depressed older adults with SSRI‐possible risk of hyponatremia. J Gerontol Nurs. 2010;36(4):22–27. [DOI] [PubMed] [Google Scholar]
- 20. Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self‐reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. [DOI] [PubMed] [Google Scholar]
- 21. Faye S, Payne RB. Rapid measurement of serum water to assess pseudohyponatremia. Clin Chem. 1986;32(6):983–986. [PubMed] [Google Scholar]
- 22. Lai MY, Lin CC, Chung LL, et al. Milky plasma, diabetes, and severe hyponatremia. Kidney Int. 2009;75:996. [DOI] [PubMed] [Google Scholar]


