Abstract
J Clin Hypertens (Greenwich). 2012;14:191–197. ©2012 Wiley Periodicals, Inc.
This retrospective study assessed the efficacy of a recently described, mechanism‐based algorithm for treating resistant hypertension. Charts of consecutive patients seen for resistant hypertension were reviewed. Algorithm‐based intervention was limited to either or both of just 2 options: (1) strengthening of the diuretic regimen, usually with addition of spironolactone; and (2) treatment with the combination of an α‐ + nonmetabolized β‐blocker. Of 27 patients, 24 (89%) achieved control, including 13 (54%) in whom the diuretic regimen was strengthened, 6 (25%) in whom α‐/β‐blockade was instituted, and 5 (21%) who received both interventions. The most frequent medication adjustments were addition of a potassium‐sparing diuretic in 16 (67%), doxazosin in 9 (37.5%), and replacing a metabolized with a nonmetabolized β‐blocker in 6 (25%). The authors conclude that treatment based on this algorithm can both simplify and improve the management of resistant hypertension and merits further evaluation in prospective studies.
Although many effective antihypertensive drugs are currently available, resistant hypertension, often defined as hypertension that is not controlled on a regimen of at least 3 drugs, including a diuretic, is still seen in perhaps 9% to 18% of treated hypertensive patients. 1 , 2 Reviews and treatment guidelines uniformly offer only general recommendations that are largely limited to reducing sodium intake, increasing dosage (particularly of the diuretic), and adding drugs, particularly spironolactone. 3 , 4 They do not provide specific guidance to clinicians regarding drug selection. Controlled treatment trials document that various add‐on drugs lower blood pressure (BP) but do not identify any as superior, or identify which patient is most or least likely to respond to which drug, again leaving clinicians with many choices and no guidance. Reflecting this, many physicians regularly add drug after drug without particular rationale and with limited success, unnecessary costs, and side effects. A simplified, physiologically rational and effective treatment approach, one which offers physicians logical drug selection guidance, could be of considerable value in managing resistant hypertension.
Toward this goal, a treatment algorithm was recently described based on addressing any or all of 3 mechanisms prominently involved in BP regulation: sodium/volume, the renin‐angiotensin system (RAS), and the sympathetic nervous system (SNS). 5 Of relevance, most antihypertensive drugs address one or more of these mechanisms.
The algorithm (Figure) assumes that patients are already on a regimen that includes at least 1 drug that targets sodium/volume (by definition, patients must be taking a diuretic), and one that targets the RAS (an angiotensin‐converting enzyme [ACE] inhibitor), angiotensin receptor blocker (ARB), direct renin inhibitor [DRI] or β‐blocker). 5 When adding drugs to this baseline regimen, the algorithm simplifies the decision tree by focusing on one or both of just 2 treatment options: (1) address sodium/volume by strengthening the diuretic regimen, often with the addition of a potassium‐sparing agent; and/or (2) address the sympathetically mediated component with combined α‐ + β‐blockade. The latter option was further refined by selecting β‐blockers whose β‐blocking effect is not greatly affected by first‐pass hepatic metabolism. 6 , 7 , 8 , 9 Thus betaxolol, bisoprolol, atenolol, or nebivolol were preferred over propranolol, metoprolol, carvedilol, and labetalol in order to eliminate the problem of nonresponse due to poor bioavailability. 6 , 7 , 8 , 9
Figure FIGURE.
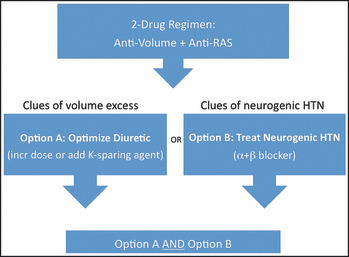
Mechanism‐based algorithm for treating resistant hypertension. RAS indicates renin‐angiotensin system; HTN, hypertension.
In recent years, this algorithm has been used for management of patients who presented to the investigator (SJM) with resistant hypertension. This report is based on a retrospective review of the BP outcome of those patients; their treatment was not part of a controlled trial. The chart review was approved by the institutional review board.
Methods
The charts of all new patients seen for management of resistant hypertension by the investigator (SJM) at the Hypertension Center of the New York Presbyterian Hospital – Weill Cornell Medical Center between January 1, 2008, and March 31, 2010, were reviewed.
Patients between the age of 21 and 80 years were included. Patients were considered to have resistant hypertension at the initial visit if they were taking ≥3 drugs, at least one of which was a diuretic, with initial office BP >140/90 mm Hg or home BP >135/85 mm Hg. Patients who reported home readings <135/85 mm Hg were excluded regardless of their office readings. For patients with diabetes or chronic kidney disease, the latter defined arbitrarily in this study as an average serum creatinine ≥2.0 mg/dL in men and ≥1.5 mg/dL in women, resistant hypertension was defined as office BP >130/80 mm Hg, with home readings >125/75 mm Hg if monitored. Office BP values were averages of at least 3 readings taken after at least 5 minutes of rest. Home BP readings were not standardized.
Of the 48 patients who were initially identified, 9 were subsequently excluded—2 who were considered to have white coat hypertension and 7 who were found to have secondary hypertension (3 had primary hyperaldosteronism and 4 had renovascular hypertension)—leaving 39 patients. Three additional patients were excluded because their BP normalized without intervention, and 1 was excluded because concurrent treatment by an outside physician varied from the algorithm, leaving 35 patients. Finally, 8 patients who did not achieve BP control but had ≤2 follow‐up visits before completion of algorithm‐guided treatment were considered lost to follow‐up and were excluded. Data from the remaining 27 patients are reported.
The algorithm (Figure) presents a decision tree limited to 2 therapeutic interventions: (1) strengthening of the diuretic regimen, usually with addition of a potassium‐sparing agent; and (2) treatment with the combination of an α‐ and a β‐blocker. All patients received one or both of these 2 interventions, with the choice of intervention based on clinical judgment, employing the clinical clues described previously (Table I). 5 Strengthening of the diuretic regimen usually consisted of adding a potassium‐sparing diuretic to a previously prescribed thiazide or loop diuretic. In some cases, the dose of a thiazide or loop diuretic was increased or one was substituted for the other. Institution of α‐ + β‐blockade consisted of one of the following: (1) in patients already taking a β‐blocker, an α‐blocker was added; (2) in patients already taking an α‐blocker, a β‐blocker was added; (3) both an α‐ and β‐blocker were added; or (4) a β‐blocker whose bioavailability is substantially affected by first‐pass hepatic metabolism was replaced by one whose bioavailability is not affected. Patients who were taking carvedilol or labetalol were not considered to be taking an α‐blocker because of their variable bioavailability and the lower potency of α‐ as opposed to β‐blockade. 6 , 7
Table I.
Clinical Clues Helpful in Drug Selection in the Management of Resistant Hypertension
| Clinical and biochemical clues suggesting the need for a more potent diuretic regimen | Clinical circumstances suggesting the presence of neurogenic hypertension |
|---|---|
| High sodium intake | Conditions associated with both blood pressure elevation and increased sympathetic tone |
| Size of patient | Acute stroke |
| Presence of edema | Sleep apnea |
| Low plasma renin activity | Alcoholism |
| Blood urea nitrogen, creatinine, and uric acid levelsunchanged by current diuretic | Paroxysmal hypertension |
| Chronic renal disease | Clinical situations suggestive of neurogenic hypertension |
| Hypertension refractory to drug combinations that target sodium/volume and the renin‐angiotensin system | |
| Absence of clinical and biochemical clues of volume excess | |
| Labile or paroxysmal hypertension | |
| Unexplained severe hypertension | |
| Hypertension with sinus tachycardia | |
| Psychological factors |
Treatment outcomes were categorized as either controlled or uncontrolled hypertension. Hypertension was considered controlled if office BP readings at one or both of the final 2 visits was ≤140/90 mm Hg or if home readings were reported as ≤135/85 mm Hg. For patients with diabetes or chronic kidney disease, the cutoffs were ≤130/80 mm Hg and ≤125/75 mm Hg, respectively. Outcomes were also assessed in subgroups differentiated by baseline systolic BP (SBP) (140–159 mm Hg vs >160 mm Hg). The drug regimens at the initial and final visits were compared. The findings are presented as descriptive data.
Results
The clinical characteristics of the 27 patients are presented in Table II. Seventeen had elevation of both office and home SBP, 5 had elevated office SBP without monitoring of home BP, and 5 had elevated home SBP with normal office SBP. At the initial visit, the mean office and home BP values were 150/89 mm Hg and 160/87 mm Hg, respectively. The initial office SBP was ≥160 mm Hg (≥150 mm Hg in patients with chronic kidney disease [CKD] or diabetes mellitus [DM]) in 10 of the 27 patients (Table III). Fourteen patients (52%) reported a history of adverse reactions to antihypertensive agents, with reactions to, on average, 2.2 drugs each.
Table II.
Clinical Characteristics (N=27)
| Demographics and medical history | |
| Age, y | 60.9±11.6 |
| Men, % | 63.0 |
| Race (white/black/other) | 21/5/1 |
| BMI | 29.4±5.5 |
| Overweight or obese, No. (%) | 21 (78) |
| History of clinical dyslipidemia, No. (%) | 4 (14.8) |
| History of clinically apparent CAD, No. (%) | 3 (11.1) |
| DM, No. (%) | 2 (7.4) |
| CKD, No. (%) | 2 (7.4) |
| Hypertension history | |
| Duration | 16.7±10.7 |
| Highest recorded BP | 203±22/116±28 |
| History of intolerance to ≥1 BP drug, No. (%) | 14 (51.9) |
| Initial office BP | 150±16/89±16 |
| Initial office SBP ≥160 mm Hg, No. (%) | 10/27 (37.0) |
| Initial home BP (n=22) | 160±16/87±13 |
Abbreviations: BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; SBP, systolic blood pressure.
Table III.
Initial and Final Office Blood Pressure
| Patient group | Initial Blood Pressure | Final Blood Pressure |
|---|---|---|
| All patients (n=27) | 149.6±15.7 | 125.9±14.4 |
| 89.4±15.6 | 80.2±9.7 | |
| Initial systolic BP ≥160 (n=10) | 166.7±7.1 | 129.8±15.8 |
| 99.1±18.5 | 83.7±12.3 |
The final office and home BP readings were 125.9±14.4/80.2±9.7 mm Hg and 134.8±12.9/80.2±11.6 mm Hg, respectively, representing a fall of 22.7/9.2 mm Hg and 25.0/6.9 mm Hg for each. Hypertension control was achieved in 24 patients (88.9%), including 16 of 17 patients (94%) with initial SBP <160 mm Hg (<150 mm Hg if CKD/DM) and 8 of 10 (80%) with an initial SBP ≥160 mm Hg (≥150 mm Hg in DM/CKD). Of the 24 patients who achieved BP control, 19 (79.2%) achieved the target BP by the first follow‐up visit. The mean number of revisits was 4.3.
Pharmacologic Interventions
At the initial visit, patients were taking, on average, 4.1±1.4 drugs, and at the final visit, 4.4±1.4 drugs. Patients with initial SBP ≥160 mm Hg were taking more drugs than those with initial SBP 140 mm Hg to 159 mm Hg.
The frequency of use of each drug class at the initial and final visits is shown in Table IV. At the initial visit, 27 of 27 patients were taking a diuretic (by definition), 21 of 27 a β‐blocker, 11 of 27 a calcium channel blocker (CCB), and 24 of 27 an ACE inhibitor, ARB, and/or DRI, including 7 who were taking drugs from 2 of the latter 3 drug classes. Only 2 were taking a potassium‐sparing agent. Seven were taking an α‐blocker (doxazosin, 5; prazosin, 2). Eleven were taking combined α‐/β‐blockade, including 5 who were taking labetalol or carvedilol, and 6 who were taking a separate α‐ and β‐blocker. Seven were taking clonidine, and 5, a vasodilator (hydralazine, 2; minoxidil, 2; and both, 1).
Table IV.
Preintervention and Postintervention Antihypertensive Drug Regimens
| Overall (N=27) | Initial SBP ≥160 mm Hg (n=10) | |||
|---|---|---|---|---|
| Drug | Preinter‐vention | Postinter‐vention | Preinter‐vention | Postinter‐vention |
| Thiazide or loop diuretic | 26 | 27 | 10 | 10 |
| K+‐sparing diuretic | 2 | 18 | 1 | 5 |
| CCB | 11 | 7 | 7 | 6 |
| ACE inhibitor, ARB, or DRI | 24 | 23 | 8 | 9 |
| Vasodilator | 5 | 1 | 2 | 1 |
| Central α‐agonist | 7 | 6 | 3 | 3 |
| β‐Blocker | 21 | 19 | 8 | 8 |
| Metabolizeda | 16 | 5 | 5 | 3 |
| Nonmetabolizedb | 6 | 14 | 3 | 5 |
| α‐Blocker | 7 | 16 | 1 | 5 |
| Drugs, No. | 4.1±1.3 | 4.4±1.3 | 4.6±2.0 | 5.1±1.6 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DRI, direct renin inhibitor; SBP, systolic blood pressure. aCarvedilol, labetalol, metoprolol. bAtenolol, betaxolol, bisoprolol, nebivolol.
As shown in Table IV, the 2 drug classes most frequently added were potassium‐sparing diuretics and α‐blockers. The number taking a potassium‐sparing diuretic increased from 2 (7.4%) to 15 (55.6%), and the number taking an α‐blocker increased from 7 (25.9%) to 16 (59.3%). In contrast, there was no increase in the proportion taking a CCB, ARB, ACE inhibitor, DRI, or central α‐agonist, with a trend toward less frequent use of CCBs and ACE inhibitors. Vasodilators were stopped in 4 of the 5 patients who were taking one at the initial visit, including both of the patients who were taking minoxidil. In no case was a DRI, vasodilator, or central α‐agonist added, and combination ACE inhibitor/ARB therapy was not instituted in any.
Table V displays the interventions in the 24 patients in whom BP control was achieved. The diuretic regimen was modified in 13 of 24 (54.1%), an α‐ + β‐blocker regimen was instituted or modified in 6 of 24 (25%), and both interventions were employed in 5 of 24 patients (20.8%). Thus, in successfully treated patients, option A (diuretic) was employed in 18 of 24 patients (75%) and option B (α‐ + β‐blocker) in 11 of 24 patients (45.8%).
Table V.
BP Outcomes and Treatment Options Employed
| All Patients | Patients With Initial SBP ≥160 mm Hg | |||
|---|---|---|---|---|
| Outcome | N=27 | Percent | N=10 | Percent |
| BP controlled | 24 | 88.9 | 8 | 80 |
| Option A only: strengthen diuretic | 13 | 54.1 | 5 | 62.5 |
| Option B only: α‐blocker+nonmetabolized β‐blocker | 6 | 25.0 | 1 | 12.5 |
| Both option A+option B | 5 | 20.8 | 2 | 25.0 |
| Total | ||||
| Option A employed | 18 | 7 | ||
| Option B employed | 11 | 3 | ||
| BP not controlled | 3 | 11.1 | 2 | 20.0 |
Abbreviations: BP, blood pressure; SBP, systolic blood pressure.
Among the 18 patients whose diuretic regimen was modified, a loop or thiazide diuretic was added or strengthened in 10 and a potassium‐sparing diuretic was added in 13 (spironolactone, 8; amiloride, 5). Thiazide or loop diuretic dose was increased in 4 patients, a thiazide diuretic was added to a K‐sparing agent in 1, a thiazide diuretic was replaced by a loop diuretic in 3 patients, and a loop diuretic by a thiazide diuretic in 2 patients. At the final visit, 15 of the 24 patients were taking a K‐sparing diuretic.
Among the 11 patients in whom an α‐ + β‐blocker regimen was instituted or modified, an α‐blocker was added to a previously prescribed β‐blocker in 5 patients, both an α‐ and β‐blocker were added in 1 patient, a β‐blocker dose was increased in 1 patient, and a β‐blocker whose bioavailability is vulnerable to first‐pass hepatic metabolism was replaced with one whose bioavailablity is less affected in 6 patients. Among the 8 patients whose β‐blocker regimen was modified, heart rate decreased from 72.9±11.6 beats per minute to 65.3±9.1 beats per minute (P=.1415).
The BP did not reach target in 3 patients. In 1, it was lowered from 170/100 mm Hg to 140/90 mm Hg (and subsequently normalized after extended follow‐up). In another, it fell from 180/126 mm Hg to 135/95 mm Hg. The third patient, whose final BP was 155/100 mm Hg, had possible primary hyperaldosteronism, but was lost to follow‐up before a definitive diagnosis could be made.
Discussion
The purpose of this study was to retrospectively assess the efficacy of a mechanism‐based algorithm with the potential to both simplify and improve the management of resistant hypertension. In this study, resistant hypertension was controlled in 88.9% of patients, including 80% in the subgroup with more severe hypertension. Further, among the 3 patients who did not achieve control, BP was greatly improved in 2, and 1 possibly had primary hyperaldosteronism.
A key feature of the algorithm is the needed simplification of the decision tree, which was narrowed down to 2 easy‐to‐remember options. The observed effectiveness of this approach encourages its further study as an alternative to the common practice of adding drug after drug without rationale, and often without success. Finally, the algorithm also introduces a new model for studying the management of resistant hypertension, focusing on a comprehensive approach rather than on the partial effectiveness of a single add‐on drug.
The algorithm is conveniently limited to 2 treatment options: (1) strengthening of the diuretic regimen, achieved in most cases by adding a potassium‐sparing diuretic, usually spironolactone; and (2) treating with the combination of an α‐ and a β‐blocker, a combination whose use has received little attention in the management of resistant hypertension. An interesting observation is that the drugs featured in the algorithm, spironolactone, which targets the sodium/volume mechanism, and doxazosin, which targets sympathetically mediated BP elevation, fit hand in glove with what was missing from the drug regimens at the initial visit. Only 7% of patients were taking a potassium‐sparing diuretic, and only 26% were taking an α‐blocker, after excluding 5 who were taking labetalol or carvedilol, as discussed below. These low proportions reproduce almost precisely the drug pattern seen in the Symplicity HTN‐2 renal nerve ablation trial for patients with resistant hypertension, where, prior to intervention, only 17% were taking an aldosterone antagonist and 26% were taking an α‐blocker (Table VI). 10 At the final visit in the current study, 56% were taking a potassium‐sparing diuretic and 59% were taking an α‐blocker.
Table VI.
Preintervention Medications: This Study and Symplicity HTN‐2 Study
| Drug Class | Patients, % | |
|---|---|---|
| This Study | HTN‐2 Study | |
| Diuretic | 100 | 90 |
| ACE inhibitor or ARB | 81 | 95 |
| DRI | 19 | 17 |
| β‐Blocker | 78 | 76 |
| CCB | 41 | 81 |
| Aldosterone antagonist | 7 | 17 |
| Vasodilators | 19 | 16 |
| α‐Blockers | 26 | 26 |
| Centrally acting sympatholytics | 26 | 52 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DRI, direct renin inhibitor.
The two treatment options were not selected in randomized fashion. They were selected based on clinical judgment using the clues of volume excess and of neurogenic hypertension, as previously suggested (Table I). 5 It was not the intention of the study to determine which of the two options was effective in a larger proportion of patients. The study instead was intended to determine whether these 2 very different treatment options, separately or together, could bring resistant hypertension under control in most cases.
Another important finding was that adding or strengthening the dosage of other agents, such as vasodilators, central α‐agonists, CCBs, ACE inhibitors, ARBs, or a DRI, was not employed and was not needed. This is consistent with studies that demonstrated the limited antihypertensive effect of increasing dosage of ACE inhibitors such as enalapril or quinapril above 20 mg/d, or of combining an ACE inhibitor with an ARB. 11 , 12 , 13 The results support the strategy of adding treatment directed at mechanisms other than the RAS in patients already taking an acceptable dose of a drug that targets the RAS. The results suggest that problematic drugs such as clonidine, whose use is frequently associated with undesirable side effects, and minoxidil, whose use is not without danger, are usually not necessary in the management of resistant hypertension.
Option A: Strengthening of the Diuretic Regimen
The effectiveness of strengthening the diuretic regimen in the management of resistant hypertension, particularly through addition of spironolactone or eplerenone, has been previously reported. 14 , 15 , 16 The ineffectiveness of the most commonly prescribed dosage of hydrochlorothiazide (HCTZ), 25 mg, has also been reported. 17 In this study, although all patients were taking a diuretic at the initial visit, only 9 (33.3%) were on a regimen stronger than 25 mg of HCTZ. It is often forgotten that in the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), it was 25 mg of chlorthalidone, not HCTZ, that was as effective as other antihypertensive agents. 18 Given the high sodium intake common today and the widespread use of 25 mg of HCTZ rather than the more potent chlorthalidone, strengthening the diuretic regimen is clearly an important component in managing resistant hypertension. In this study, the diuretic regimen was usually strengthened by adding a potassium‐sparing agent rather than by increasing the dose of HCTZ or replacing it with chlorthalidone. This approach may be preferred both because it reduces the risk of hypokalemia and because of the growing body of evidence of the effectiveness of spironolactone in managing resistant hypertension.
Although strengthening the diuretic regimen has consistently been shown to be effective in treating resistant hypertension, it achieves BP control in only about half of cases. This indicates that in many patients, resistant hypertension is sustained by mechanisms other than sodium/volume. In these patients, strengthening the diuretic regimen will often be ineffective, and could even be harmful. Therefore, although rarely mentioned, simply placing all patients with resistant hypertension on a stronger diuretic regimen is not an optimal strategy. In this context, utilization of clues of volume excess should be considered in deciding whether to strengthen the diuretic regimen. 5
Option B: Combined α‐ + β‐Blockade
This is the first study to focus on the effectiveness of combined α‐ + β‐blockade specifically in the management of resistant hypertension. The results suggest that a significant proportion of patients with resistant hypertension may have sympathetically mediated neurogenic hypertension and that α‐/β‐blockade may be an important component in the armamentarium of resistant hypertension management.
Two important factors in treating neurogenic hypertension are the use of both α‐ and β‐blockade rather than β‐blockade alone and consideration of the important effects of first‐pass hepatic metabolism on achieving β‐blockade. Neither β‐blockade alone nor α‐blockade alone reduces sympathetically mediated BP reactivity. 19 , 20 In contrast, the combination of the 2 does, and therefore would seem relevant to the treatment of neurogenically mediated resistant hypertension. 19 , 20 The considerable antihypertensive effect of α‐/β‐blockade in treating essential hypertension has also been demonstrated. 21 , 22 , 23
The other important issue, unreliable bioavailability of many β‐blockers, has also received minimal attention. The bioavailability of β‐blockers that are subject to first‐pass hepatic metabolism, including propranolol, metoprolol, carvedilol, and labetalol, among others, varies considerably from patient to patient, and ultrarapid metabolizers and extensive metabolizers often achieve ineffective blood levels. 6 , 7 , 8 , 9 , 24 , 25 , 26 Further, one might expect extensive metabolizers to be overrepresented among patients with resistant hypertension. Unfortunately, the issue of hepatic metabolism of β‐blockers is given little attention despite its undisputed effect on drug levels.
In this study, in patients in whom the absence of a slowed heart rate suggested that β‐blockade had not been achieved, β‐blockers subject to first‐pass hepatic metabolism were replaced with β‐blockers whose effect is not governed by first‐pass metabolism. These included betaxolol, bisoprolol, and pindolol, as well as nebivolol, which is metabolized but whose β‐blocking effect is maintained in a major metabolite. 8 , 27 In this group, a clinically significant fall in heart rate was seen, suggesting that the change in β‐blocker did increase the β‐blocking effect.
The unreliable bioavailability of the α‐/β‐blockers labetalol and carvedilol might explain the glaring paradox of why they don’t lower BP more than β‐blocker monotherapy. 7 , 28 In contrast, α‐ and β‐blockade achieved by using separate drugs that are not vulnerable to first‐pass metabolism lower BP much more. 7 , 21 , 22 , 23 , 28 Because of their unreliable bioavailablity, labetalol and carvedilol should not be assumed to have provided β‐blockade, and especially α‐blockade, particularly in patients whose heart rate has not slowed. Prescription of a β‐blocker that is not subject to first‐pass hepatic metabolism, along with an α‐blocker such as doxazosin is more reliable in providing both α‐ and β‐blockade, lowers BP much more, and also provides the advantage of allowing separate titration of each effect. 7 , 21 , 22 , 23 For this reason, in this study, the regimen of the 5 patients who were taking labetalol or carvedilol at entry was changed to separate α‐ and β‐blockers.
The use of α‐blockers had been discouraged by the ALLHAT study, which found that initial therapy with doxazosin was less effective than chlorthalidone in reducing BP and preventing cardiovascular events. 29 However, as an add‐on drug, particularly in combination with a β‐blocker or an ACE inhibitor, doxazosin has a considerable antihypertensive effect. 21 , 22 , 23 Its use in treating hypertension resistant to other drug classes is logical and should not be discouraged.
Study Strengths
The study population was typical of the population with resistant hypertension with respect to the longstanding history of hypertension (mean of 17 years) and the high proportion with comorbidities including obesity, hyperlipidemia, cerebrovascular or coronary artery disease, diabetes, and chronic renal disease, in whom achievement of target BP is an important concern.
Importantly, this is the first study to examine an approach that was both comprehensive and simple, with the goal of achieving BP control in most patients with resistant hypertension. The approach did achieve that, with nearly 90% achieving control with intervention limited to just two options. The importance of a comprehensive approach that greatly simplifies treatment options cannot be overstated.
Limitations
There are several important limitations. First, this was a retrospective study. Outcomes were not compared with “standard” therapy, although to date, there is no standard approach to the treatment of resistant hypertension. Second, as in other retrospective chart review studies, neither office nor home BP measurements were standardized. Third, as would be expected in the typical outpatient setting, there were 8 dropouts. In this study, to avoid the problem of treatment failures being excluded as “dropouts,” categorization as a dropout was defined strictly as those with ≤2 revisits. All patients with at least 3 follow‐up visits were included.
In this study, CKD was defined by creatinine level, rather than by estimated glomerular filtration rate (eGFR). Although the use of a creatinine cutoff is clearly arbitrary, the use of the eGFR is also problematic. 30 Regardless, the presence or absence of renal disease had little impact on the results and is mentioned only as a descriptor of the study population.
Defining resistant hypertension is always challenging. Previous clinical series relied solely on office BP, with the likelihood that some patients had white coat hypertension rather than truly uncontrolled hypertension. In this study, the accepted definition of resistant hypertension, 3 drugs including a diuretic, was employed, and patients who reported their home BP as normal were excluded. Also, without doubt, in any study of resistant hypertension, BP levels will decline without intervention in some patients upon revisit. Mitigating this is the long history of elevated readings prior to referral. Further, patients whose SBP exceeded 160 mm Hg at the initial visit also did well. Clearly, a control group is needed to enhance reliability of findings.
Conclusions
In a retrospective study, a recently described simplified treatment algorithm brought resistant hypertension under control in 89% of patients. The main advantages of the algorithm are a simplified, mechanistic approach that reduces treatment choices to either or both of just 2 treatment options: (1) strengthen the diuretic regimen, usually by adding a potassium‐sparing agent; and/or (2) treat a neurogenic component with combined α‐/β‐blockade. The ease of use of this mechanism‐based algorithm makes it feasible for widespread use and more widespread success in treating resistant hypertension. Larger, prospective, controlled trials are needed.
Conflict of Interest: There are no conflicts of interest to disclose.
References
- 1. Epstein M. Resistant hypertension: prevalence and evolving concepts. J Clin Hypertens (Greenwich). 2007;9:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 4. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 5. Mann SJ. Drug therapy for resistant hypertension: simplifying the approach. J Clin Hypertens. 2011;13:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richards DA, Prichard BN. Clinical pharmacology of labetalol. Br J Clin Pharmacol. 1979;8:89S–93S. [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan T. Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin Pharmacokinet. 1994;26:335–346. [DOI] [PubMed] [Google Scholar]
- 8. Frishman W. Clinical pharmacology of the new beta‐adrenergic blocking drugs. Part 1. Pharmacodynamic and pharmacokinetic properties. Amer Heart J. 1979;97:663–670. [DOI] [PubMed] [Google Scholar]
- 9. McNeil JJ, Louis WJ. Clinical pharmacokinetics of labetalol. Clin Pharmacokinet. 1984;9:157–167. [DOI] [PubMed] [Google Scholar]
- 10. Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 11. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 12. Salvetti A, Arzilli F. Chronic dose‐response curve of enalapril in essential hypertensives. An Italian multicenter study. Am J Hypertens. 1989;2:352–354. [DOI] [PubMed] [Google Scholar]
- 13. Canter D, Frank GJ, Knapp LE, et al. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. J Hum Hypertens. 1994;8:155–162. [PubMed] [Google Scholar]
- 14. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low‐dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. [DOI] [PubMed] [Google Scholar]
- 15. Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. [DOI] [PubMed] [Google Scholar]
- 16. Setaro JF, Black HR. Refractory hypertension. N Engl J Med. 1992;327:543–547. [DOI] [PubMed] [Google Scholar]
- 17. Messerli FH, Makani H, Benjo A, et al. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta‐analysis of randomized trials. J Am Coll Cardiol. 2011;57:590–600. [DOI] [PubMed] [Google Scholar]
- 18. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 19. Julius S. The blood pressure seeking properties of the central nervous system. J Hypertens. 1988;6:177–185. [DOI] [PubMed] [Google Scholar]
- 20. Mann SJ. Neurogenic essential hypertension revisited: the case for increased clinical and research attention. Am J Hypertens. 2003;16:881–888. [DOI] [PubMed] [Google Scholar]
- 21. Mann SJ, Gerber LM. Low‐dose alpha/beta blockade in the treatment of essential hypertension. Am J Hypertens. 2001;14 (6 Pt 1):553–558. [DOI] [PubMed] [Google Scholar]
- 22. Searle M, Dathan R, Dean S, et al. Doxazosin in combination with atenolol in essential hypertension: a double‐blind placebo‐controlled multicentre trial. Eur J Clin Pharmacol. 1990;39:299–300. [DOI] [PubMed] [Google Scholar]
- 23. Holtzman JL, Kaihlanen PM, Rider JA, et al. Concomitant administration of terazosin and atenolol for the treatment of essential hypertension. Arch Intern Med. 1988;148:539–543. [PubMed] [Google Scholar]
- 24. Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- 25. Kirchheiner J, Heesch C, Bauer S, et al. Impact of the ultrarapid metabolizer genotype of cytochrome P450 2D6 on metoprolol pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2004;76:302–312. [DOI] [PubMed] [Google Scholar]
- 26. Lennard MS, Silas JH, Freestone S, et al. Oxidation phenotype – a major determinant of metoprolol metabolism and response. N Engl J Med. 1982;307:1558–1560. [DOI] [PubMed] [Google Scholar]
- 27. Lefebvre J, Poirier L, Poirier P, et al. The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension. Br J Clin Pharmacol. 2007;63:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prichard BN, Richards DA. Comparison of labetalol with other anti‐hypertensive drugs. Br J Clin Pharmacol. 1982;13:41S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ALLHAT Collaborative Research Group . Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 30. Mann SJ. Pitfalls in diagnosing chronic kidney disease from eGFR (letter). Arch Intern Med. 2009;169:1168–1169. [DOI] [PubMed] [Google Scholar]


