Abstract
J Clin Hypertens (Greenwich). 2012; 14:407–414. ©2012 Wiley Periodicals, Inc.
Important questions concerning the comparative effectiveness of angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) remain unanswered, including whether they are equally effective in reducing clinical end points and in which populations. An incident cohort of adult patients 18 years and older prescribed an ACE inhibitor or ARB between 2001 and 2008 was identified from Geisinger Clinic, a large community‐based set of medical practices that uses a common electronic health record. Propensity score matching was used to balance the groups on baseline factors. The authors examined differences in mortality and new‐onset coronary disease, chronic kidney disease, stroke, and diabetes for different patient subgroups based on sex and age. A total of 25,035 hypertensive patients newly prescribed an ACE inhibitor or ARB were identified. No differences were found in risk of death, coronary disease, chronic kidney disease, or stroke between those prescribed ACE inhibitors and those prescribed ARBs. Patients prescribed ARBs had a greater rate of new‐onset diabetes (hazard ratio [HR], 1.28; confidence interval [CI], 1.08–1.52), and this was especially true for women (HR, 1.93; CI, 1.22–3.07). Within a large medical‐practice based population, there was no evidence of differential effectiveness between ACE inhibitors and ARBs for most outcomes, with diabetes being the notable exception.
Hypertension is the most common primary diagnosis from office visits in the United States and affects one‐third of adults older than 20 years. 1 , 2 Randomized trials have shown that both angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are effective in reducing blood pressure (BP) in patients with hypertension. Three recent meta‐analyses have compared ACE inhibitors and ARBs, one before 3 and two after 4 , 5 publication of The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). 6 The earlier meta‐analysis found no differences in biochemical parameters (including glucose control) but acknowledged that there were too few cardiovascular (CV) outcomes to allow specific comment. The first of the latter meta‐analyses, which included 31,632 patients treated with an ARB compared with 18,292 patients treated with an ACE inhibitor found an 8% reduction in stroke favoring ARBs and no differences in myocardial infarction (MI), CV death, or death from any cause. 4 ACE inhibitors and ARBs are sometimes prescribed to control the progression of chronic kidney disease (CKD) or diabetes and have been shown to reduce the risk of new‐onset diabetes. 6 The most recent meta‐analysis found that both ACE inhibitors and ARBs reduced the risk of new‐onset diabetes compared with placebo, but the size of the effect was similar. 5 ACE inhibitors and ARBs are very similar in their magnitude of proteinuria reduction, but recent trials such as ONTARGET 6 and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) 7 have failed to link antiproteinuric effects to CV benefit. Matchar and colleagues 3 noted that evidence for the comparative effectiveness of ACE inhibitors and ARBs is lacking for patient subgroups and longer‐term effects.
We sought to examine the comparative effectiveness of ACE inhibitors vs ARBs in reducing rates of death, new‐onset diabetes, stroke, CKD, and coronary artery disease (CAD), both overall and among subgroups of patients defined by age and sex. While randomized trials have provided evidence about the comparative effectiveness of ACE inhibitors and ARBs, our observational study reflects the use of these agents in routine clinical practice, and the large sample size allows for looking at longer‐term effects and subgroup analyses.
Methods
We conducted a propensity score–matched inception cohort study of adult hypertensive patients receiving an ACE inhibitor or ARB using data from the electronic health record (EHR) of Geisinger Clinic. Geisinger Clinic provides care to patients in a 31‐county region of Central and Northeastern Pennsylvania. The base population in the Geisinger Clinic service area is stable, with an out‐migration rate of only 4% over 5 years. Forty‐six percent of the patient population is older than 45 years and 21% are older than 65. The population is 41% male and 95% Caucasian. Geisinger uses the EPICare EHR (Epic, Verona, WI) in all inpatient and outpatient practice sites. Geisinger’s EPICare system requires that each medication prescription be linked to an International Statistical Classification of Diseases and Related Health Problems–Ninth Revision (ICD‐9) coded diagnosis.
Adult (18 years and older) patients were considered hypertensive if they met at least one of the following study inclusion criteria between January 1, 2001, and December 31, 2008: (1) Ever had ≥2 outpatient encounters with a hypertension diagnosis (ICD‐9 401.*−404.*); (2) ever had ≥2 serial elevated BPs (systolic ≥140 mm Hg or diastolic ≥90 mm Hg); (3) ever had ≥1 medication ordered with an associated ICD‐9 code 401.*−404.*; or (4) ever had a hypertension diagnosis appear on the problem list. The medication orders for these patients were queried to identify the subset ever prescribed an ACE inhibitor (benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril) or ARB (candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, valsartan). We then excluded patients in whom the definition of hypertension was met more than 1 week after the first outpatient prescription for ACE inhibitor and/or ARB. In order to create an incident cohort of new users of ACE inhibitors or ARBs, the study sample was limited to patients who were active in the Geisinger Health System for at least 6 months prior to the first ACE inhibitor or ARB order (ie, the “index order” and “index date”). Patients with both an ACE inhibitor and ARB prescription on the index date were excluded.
Baseline variables are listed in Table I. For disease variables, we required either 2 outpatient encounter diagnoses or 1 medication order with the ICD‐9 code(s) of interest or where the disease appeared on the problem list for that visit. We counted the patients as having the disease if it was observed at any time prior to the index date. Whenever 2 encounter diagnoses were used, the earliest of the 2 dates were assigned as the date of diagnosis. For BP, body mass index, pulse, and laboratory values, we used the most recent value prior to (or on) the index date. Laboratory values are frequently unobserved in EHR data, simply because they were never ordered during the observation period. However, whether a laboratory report was ordered might itself be informative. We, therefore, created an indicator variable that a given laboratory was observed (eg, “low‐density lipoprotein observed” in Table I) and a variable that is the value of the laboratory test itself, if observed, and equal to 0 otherwise (eg, “low‐density lipoprotein value” in Table I). This results in no variables with missing values, and is valid if the values from laboratory reports that would have occurred if, contrary to fact, they had been ordered, do not affect the treatment decision or the outcome. We emphasize that the indicator of a laboratory profile being ordered and the resulting laboratory value, but not the result of any laboratory that was not ordered, is the same information that the patient and physician would have at the time of a treatment decision.
Table I.
Propensity Score Matching
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| ARB (n=3207) | ACE Inhibitor (n=19,337) | SD | VR | ARB (n=3207) | ACE Inhibitor (n=3207) | SD | VR | |
| Female | 38.2% | 50.2% | .24 | 38.2% | 38.2% | .00 | ||
| Race, white | 98.3% | 97.9% | .03 | 98.3% | 98.4% | .00 | ||
| Race, black | 0.9% | 1.0% | .01 | 0.9% | 1.0% | .01 | ||
| Race, other | 0.7% | 1.0% | .03 | 0.7% | 0.6% | .02 | ||
| Age | 67.8 (13.3) | 65.4 (13.7) | .18 | 0.94 | 67.8 (13.3) | 67.7 (13.2) | .01 | 0.99 |
| BMI | 32.1 (7.0) | 31.9 (7.0) | 0.04 | 1.02 | 32.1 (7.0) | 32.3 (7.4) | .02 | 1.10 |
| Encounters in the past year, No. | 5.7 (5.5) | 5.7 (5.5) | 0.00 | 0.98 | 5.7 (5.5) | 5.7 (5.5) | .00 | 1.00 |
| Years between ACE‐ARB date and data pull | 5.3 (1.8) | 4.8 (1.9) | 0.24 | 0.91 | 5.3 (1.8) | 5.2 (1.8) | .01 | 1.02 |
| Systolic BP | 143.7 (20.6) | 144.2 (20.2) | 0.03 | 1.05 | 143.7 (20.6) | 143.8 (21.0) | .00 | 1.04 |
| Diastolic BP | 80.9 (12.0) | 81.9 (12.5) | 0.09 | 0.93 | 80.9 (12.0) | 80.7 (12.4) | .01 | 1.05 |
| Pulse | 74.2 (11.7) | 74.2 (12.2) | 0.00 | 0.92 | 74.2 (11.7) | 74.0 (11.9) | .02 | 1.04 |
| BUN observed | 86.0% | 88.5% | 0.08 | 86.0% | 85.3% | .02 | ||
| BUN value | 18.7 (9.0) | 17.5 (7.2) | 0.16 | 1.55 | 18.7 (9.0) | 18.4 (8.3) | .04 | 0.85 |
| LDL observed | 72.2% | 78.5% | 0.15 | 72.2% | 70.4% | .04 | ||
| LDL value | 110.4 (34.7) | 110.7 (34.1) | 0.01 | 1.03 | 110.4 (34.7) | 110.2 (34.0) | .01 | 0.96 |
| HDL observed | 72.2% | 78.4% | 0.14 | 72.2% | 71.0% | .03 | ||
| HDL value | 51.3 (14.8) | 50.4 (14.7) | 0.06 | 1.01 | 51.3 (14.8) | 51.2 (14.9) | .01 | 1.02 |
| Cholesterol observed | 73.7% | 80.1% | 0.15 | 73.7% | 72.4% | .03 | ||
| Cholesterol value | 196.5 (41.7) | 195.1 (41.1) | 0.03 | 1.02 | 196.5 (41.7) | 197.1 (42.0) | .02 | 1.02 |
| GFR observed | 38.3% | 47.3% | 0.18 | 38.3% | 38.4% | .00 | ||
| GFR <15 | 0.7% | 0.5% | 0.03 | 0.7% | 0.7% | .00 | ||
| GFR 15–30 | 2.0% | 0.7% | 0.12 | 2.0% | 1.2% | .06 | ||
| GFR 30–60 | 20.5% | 13.3% | 0.19 | 20.5% | 20.8% | .01 | ||
| GFR >60 | 76.7% | 85.5% | 0.23 | 76.7% | 77.2% | .01 | ||
| HbA1c observed | 0.32 | 0.35 | 0.08 | 31.6% | 32.1% | .01 | ||
| HbA1c value | 7.0 (1.4) | 7.1 (1.5) | 0.07 | 0.84 | 7.0 (1.4) | 7.0 (1.4) | .02 | 1.04 |
| Triglycerides observed | 71.4% | 77.6% | 0.14 | 71.4% | 70.2% | .03 | ||
| Triglycerides value | 184.2 (112.0) | 180.7 (130.2) | 0.03 | 0.74 | 184.2 (112.0) | 187.2 (111.8) | .03 | 1.00 |
| Potassium observed | 86.2% | 89.0% | 0.08 | 86.2% | 85.7% | .01 | ||
| Potassium value | 4.3 (0.45) | 4.3 (0.43) | 0.04 | 1.09 | 4.3 (0.45) | 4.3 (0.43) | .01 | 0.92 |
| Prior use of β‐blockers | 26.8% | 26.7% | 0.00 | 26.8% | 26.6% | .00 | ||
| Prior use of calcium channel blockers | 17.4% | 12.4% | 0.14 | 17.4% | 17.7% | .01 | ||
| Prior use of thiazides | 16.5% | 21.2% | 0.12 | 16.5% | 15.9% | .02 | ||
| Comorbidity history | ||||||||
| Hyperlipidemia | 53.6% | 54.3% | 0.01 | 53.6% | 52.4% | .02 | ||
| Ischemic HD | 19.0% | 18.5% | 0.01 | 19.0% | 18.9% | .00 | ||
| Asthma | 9.3% | 8.0% | 0.04 | 9.3% | 8.4% | .03 | ||
| COPD | 6.7% | 6.1% | 0.03 | 6.7% | 6.3% | .02 | ||
| CKD | 3.4% | 1.9% | 0.09 | 3.4% | 3.4% | .00 | ||
| MI | 1.2% | 1.7% | 0.04 | 1.2% | 1.2% | .00 | ||
| CHF | 5.9% | 5.2% | 0.03 | 5.9% | 5.8% | .01 | ||
| PVD | 3.9% | 4.0% | 0.00 | 3.9% | 3.6% | .01 | ||
| CVD | 8.6% | 7.7% | 0.03 | 8.6% | 9.1% | .02 | ||
| Chronic pulmonary disease | 15.6% | 14.5% | 0.03 | 15.6% | 14.4% | .03 | ||
| Connective tissue disease‐rheumatic disease | 3.3% | 2.6% | 0.05 | 3.3% | 3.2% | .01 | ||
| Diabetes | 24.9% | 28.1% | 0.07 | 24.9% | 26.2% | .03 | ||
| Cancer | 9.6% | 10.9% | 0.04 | 9.6% | 9.5% | .00 | ||
| CAD | 17.6% | 17.0% | 0.01 | 17.6% | 17.6% | .00 | ||
| Cardiomyopathy | 0.7% | 0.8% | 0.02 | 0.7% | 0.7% | .01 | ||
| Atrial fibrillation | 5.0% | 4.6% | 0.02 | 5.0% | 5.1% | .01 | ||
| Hypertensive heart disease with heart failure | 2.4% | 1.6% | 0.06 | 2.4% | 2.1% | .02 | ||
| Chronic pulmonary heart disease | 0.4% | 0.3% | 0.02 | 0.4% | 0.5% | .00 | ||
| Dysrhythmias | 7.3% | 6.6% | 0.03 | 7.3% | 7.1% | .01 | ||
| Respiratory symptoms | 22.3% | 22.9% | 0.01 | 22.3% | 22.9% | .01 | ||
| Stroke (hemorrhagic or ischemic) | 4.3% | 3.8% | 0.03 | 4.3% | 4.9% | .03 | ||
| Angina | 2.0% | 2.1% | 0.01 | 2.0% | 2.5% | .04 | ||
| Charlson score: 0 | 48.1% | 46.9% | 0.02 | 48.1% | 48.2% | .00 | ||
| Charlson score: 1–3 | 48.8% | 49.8% | 0.02 | 48.8% | 48.6% | .00 | ||
| Charlson score: 4+ | 3.2% | 3.4% | 0.01 | 3.2% | 3.2% | .00 | ||
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; BUN, serum urea nitrogen; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; HD, heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standardized difference; VR, variance ratio.
Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease formula. 8 We also created a variable that was a count of the number of encounters in the year prior to the index date, which is intended to capture recent utilization. To account for variations in the index date, we created a variable that was the time (in years) between the index date and the final date of observation (December 31, 2008). In addition to variables identified a priori, we also rank‐ordered lists of all diagnoses and all drugs in the ACE inhibitor and ARB groups to identify additional potential confounding factors.
To estimate the propensity score (ie, the predicted probability of new ARB vs ACE inhibitor given baseline variables), 9 we fitted a logistic regression model using the baseline variables listed in Table I. Unobserved laboratory data were handled using the approach described above. The propensity score was estimated using PROC GAM in SAS (SAS Institute Inc, Cary, NC), which allows for nonlinear relationships in the regression model. We attempted to match each patient taking an ARB to a patient taking an ACE inhibitor using 1:1 matching based on logit of the propensity score, using a greedy match algorithm (gmatch macro for SAS, Mayo Clinic College of Medicine http://ndc.mayo.edu/mayo/research/biostat/upload/gmatch.sas; last assessed July 14, 2010). A maximum caliper of 0.2 times the standard deviation of logit of the propensity score was used, to ensure similarity of matched patients. 10
To assess balance before and after matching, we calculated a standardized difference between ACE inhibitor and ARB groups for each covariate. The standardized difference is the absolute difference in sample means divided by the square root of the average standard deviation in the two groups. Unlike P values, the standardized difference does not depend on the sample size and has been advocated as a preferred measure of balance. 10 Standardized differences of <0.1 have been suggested as suggesting possible nontrivial imbalance. 11 For continuous covariates, we also calculated a variance ratio, 12 with values close to 1, suggesting similar variability in the variable between groups.
Similar to an intention‐to‐treat analysis, we chose to focus on the initial decision and ramifications of choosing an ACE inhibitor or ARB. Thus, patients remained in their index class group regardless of whether they later switched treatments.
We studied the following events of interest: all‐cause mortality, stroke, CAD, diabetes, and CKD. Deaths were identified using the Social Security Administration Death Master File. Stroke, CAD, diabetes, and CKD were each examined only in those free of that condition at the index date and were identified using the following diagnoses appearing on an outpatient encounter: stroke: 433.*1, 434.*1, 435, 436, 437.1*, or 437.9*; CAD: 414.*; diabetes: 250.*; CKD: 585.*. To be classified as an outcome, we required either two outpatient encounter diagnoses (and used earliest date as diagnosis date) or one medication order with the ICD‐9 code(s) of interest or where the disease appeared on the problem list. Birman‐Deych 13 has reported positive predictive values ≥0.95 based on inpatient ICD‐9 codes for hypertension, stroke, CAD, and diabetes. We relied on outpatient diagnoses because all of the primary care patients used Geisinger outpatient care but not all used Geisinger inpatient care.
Cox proportional hazards models14 were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), using ACE inhibitor users as the reference group. Patients were censored at the date of the data pull or at 4 years (whichever came first). As a sensitivity analysis, we also artificially censored patients at 1 year of follow‐up. For nonfatal outcomes, patients were censored at their mortality date. To account for matching, the model was stratified on the matching identification variable. An overall model was fitted for each outcome. We then fitted models stratified by age categories and sex to investigate whether there was treatment effect heterogeneity by demographic subgroups.
Results
Data were acquired from 115,064 adult patients who met criteria for hypertension during an outpatient encounter or as an associated diagnosis on a medication order during a 7‐year period (January 1, 2001, through December 31, 2008; Figure 1). After excluding patients who did not meet ≥1 of the previously described inclusion criteria, we were left with 113,281 (98.5%) patients. The medication orders for these patients were queried, and 67,326 had an order for an ACE inhibitor and/or an ARB. Sixty patients were excluded because the first medication order occurred at least 1 week prior to any signal for hypertension, leaving 67,266 for further evaluation.
Figure 1.
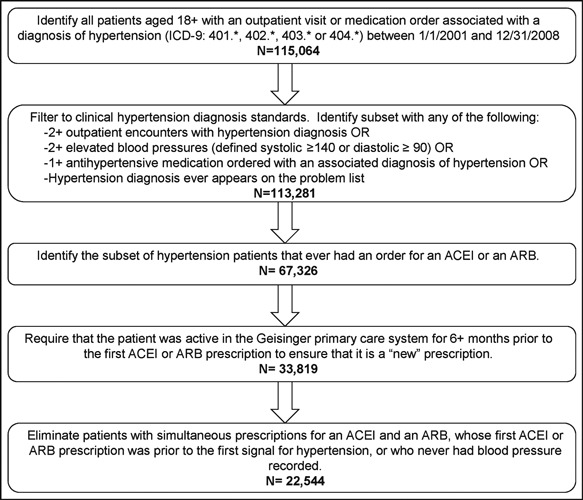
Patient selection diagram. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; OR, odds ratio.
To create a new user cohort, the study sample was limited to those who had been active Geisinger patients for at least 6 months prior to the first ACE inhibitor or ARB prescription. This resulted in 33,819 patients being considered in further analysis.
We excluded 11,275 patients because the first day of an ACE inhibitor/ARB order occurred before the first observed BP, the first diagnosis of hypertension, or included orders for both an ACE inhibitor and an ARB. Of the remaining 22,544 patients, 3207 were ARB users.
The first 4 columns of Table I display summary statistics of the key covariates, stratified by ACE inhibitor or ARB group, prior to matching. ARB users were more likely to be men, older, have higher estimated GFR, and to have prior calcium channel blocker orders and less likely to have prior orders for thiazides. In addition, there were differences in the prevalence of orders for particular laboratory tests. ACE inhibitor users were more likely to have cholesterol measured. The ARB group was 38% female, compared with 50% female in the ACE inhibitor group. The probability of ARB use (with ACE inhibitor as reference) was modeled using logistic regression (Table I). The odds of an ARB order was 1.57 times as high for men compared with women (95% CI, 1.44–1.71) and was the strongest predictor of ARB use. The only other clinically significant effect size observed was for prior use of thiazides, which had an OR of 0.70 (95% CI, 0.63–0.78). The C statistic from the propensity score model was 0.64, suggesting that the predictors capture some of the variation in treatment assignment, but much of the variation was determined by other factors.
Logit of the propensity score, stratified by treatment group, is displayed in Figure S1. There is a great deal of overlap in the propensity score between the two groups. As a result, we were able to propensity score–match all of the ARB patients to an ACE inhibitor patient, resulting in 3207 matched pairs. Summary statistics for important covariates for the two groups after matching are displayed in the last 4 columns of Table I, which shows that matching resulted in large increases in group similarities. In particular, the standardized differences are close to 0 and the variance ratios are close to 1.
The person‐time at risk and the number of events for each outcome, stratified by treatment group, age, and sex, are presented in Table II. Kaplan‐Meier survival curves were plotted for each outcome of interest, stratified by ACE inhibitor–ARB group for each outcome (Figure 2). The curves look very similar between the two groups, with the exception that the ARB group appeared to have shorter time‐to‐event values for new‐onset diabetes.
Table II.
Number of Events and Person‐Time at Risk for Each Outcome, Stratified by Treatment Group and Age and Sex Subgroups
| Outcome | ARB | ACE Inhibitor | ||||
|---|---|---|---|---|---|---|
| Events, No. | Person‐Years at Risk | Incidence Ratea | Events, No. | Person‐Years at Risk | Incidence Rate | |
| Mortality | 220 | 11,530 | 1.9 | 219 | 11,439 | 1.9 |
| Age 50–65 | 29 | 3283 | 0.9 | 26 | 3402 | 0.8 |
| Age 65+ | 182 | 7065 | 2.6 | 189 | 6884 | 2.8 |
| Male | 116 | 7140 | 1.6 | 116 | 7340 | 1.6 |
| Female | 104 | 4390 | 2.4 | 103 | 4099 | 2.5 |
| CAD | 141 | 9289 | 1.5 | 150 | 9309 | 1.6 |
| Age 50–65 | 35 | 2939 | 1.2 | 26 | 3033 | 0.9 |
| Age 65+ | 106 | 5330 | 2.0 | 119 | 5164 | 2.3 |
| Male | 81 | 5986 | 1.4 | 84 | 6348 | 1.3 |
| Female | 60 | 3303 | 1.8 | 66 | 2961 | 2.2 |
| CKD | 150 | 10,485 | 1.4 | 132 | 10,534 | 1.3 |
| Age 50–65 | 20 | 3094 | 0.6 | 8 | 3279 | 0.2 |
| Age 65+ | 127 | 6270 | 2.0 | 120 | 6152 | 2.0 |
| Male | 89 | 6420 | 1.4 | 81 | 6728 | 1.2 |
| Female | 61 | 4065 | 1.5 | 51 | 3806 | 1.3 |
| Diabetes | 220 | 8223 | 2.7 | 182 | 8044 | 2.3 |
| Age 50–65 | 57 | 2445 | 2.3 | 61 | 2384 | 2.6 |
| Age 65+ | 136 | 4826 | 2.8 | 102 | 4735 | 2.2 |
| Male | 136 | 5184 | 2.6 | 115 | 5265 | 2.2 |
| Female | 84 | 3039 | 2.8 | 67 | 2779 | 2.4 |
| Stroke | 94 | 11,066 | 0.8 | 87 | 11,047 | 0.8 |
| Age 50–65 | 10 | 3226 | 0.3 | 9 | 3368 | 0.3 |
| Age 65+ | 83 | 6652 | 1.2 | 77 | 6527 | 1.2 |
| Male | 61 | 6839 | 0.9 | 52 | 7090 | 0.7 |
| Female | 33 | 4227 | 0.8 | 35 | 3957 | 0.9 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CKD, chronic kidney disease. aIncidence rate is number of events per 100 person‐years.
Figure 2.
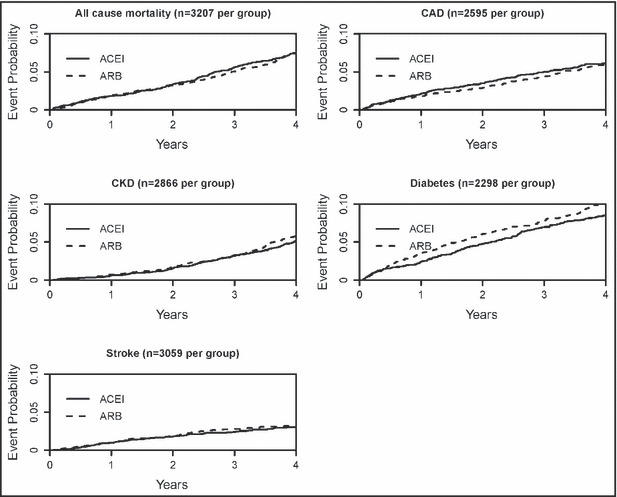
Cumulative distribution functions (1‐Kaplan‐Meier) for all outcomes, stratified by angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) group. OR indicates odds ratio; CAD, coronary artery disease; CKD, chronic kidney disease; ICD‐9, International Statistical Classification of Diseases and Related Health Problems–Ninth Revision.
Table III displays HRs and 95% CIs. HRs >1 favor ACE inhibitors and HRs <1 favor ARBs. For mortality, stroke, CAD, and CKD, the HR was close to 1 and the 95% CIs included 1, suggesting that ACE inhibitor and ARB were associated with similar outcome rates. For diabetes, the overall estimated HR was 1.28 (95% CI, 1.08–1.52), suggesting that the ARB group was at greater risk for diabetes. The estimated HR for diabetes was 1.39 (95% CI, 1.09–1.77) in men and 1.93 (95% CI, 1.22–3.07) in women, although the evidence for a sex difference was not strong (P=.32).
Table III.
Hazard Ratios (95% Confidence Interval) for ARB vs ACE Inhibitor for Study Outcomes, Stratified by Subgroup
| Mortality | Stroke | CAD | Diabetes | CKD | |
|---|---|---|---|---|---|
| All | 0.95 (0.82–1.09) | 1.09 (0.87–1.35) | 1.01 (0.84–1.22) | 1.28 (1.08–1.52) | 1.10 (0.92–1.32) |
| Age 50–65 | 1.60 (0.71–3.60) | 2.00 (0.71–5.62) | 1.14 (0.56–2.35) | 0.73 (0.38–1.40) | 3.00 (0.85–10.63) |
| Age 65+ | 0.85 (0.70–1.02) | 1.00 (0.75–1.34) | 1.15 (0.87–1.52) | 1.35 (1.03–1.77) | 1.03 (0.81–1.32) |
| Male | 1.02 (0.83–1.27) | 1.13 (0.83–1.54) | 1.19 (0.90–1.56) | 1.39 (1.09–1.77) | 1.19 (0.92–1.55) |
| Female | 0.88 (0.63–1.21) | 1.09 (0.61–1.95) | 0.73 (0.46–1.15) | 1.93 (1.22–3.07) | 0.77 (0.53–1.13) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CKD, chronic kidney disease.
We repeated the analyses but with artificial censoring at 12 months (thus, ensuring short‐term follow‐up after the initial treatment decision). The results of this sensitivity analysis are displayed in Table SII. As in the primary analysis, the HRs are not significantly different from 0, except for diabetes (HR, 1.56; 95% CI, 1.18–2.06).
Discussion
In a population of adults with hypertension from a large health system in Pennsylvania, we compared important clinical outcomes for patients taking ACE inhibitors or ARBs. We found no evidence of differences in rates of death, stroke, CAD, or CKD among users of these medications. A higher likelihood of new‐onset diabetes was observed for users of ARBs relative to ACE inhibitors (HR, 1.28; 95% CI, 1.08–1.52), especially among women (HR, 1.93; 95% CI, 1.22–3.07).
The finding of no differences between ARBs and ACE inhibitors for mortality, CAD, CKD, and stroke is consistent with ONTARGET 6 and other studies. 15 , 16
Our finding of a differential effect of ACE inhibitor vs ARB on diabetes incidence should be interpreted with caution. One reason is that we investigated 5 different outcomes, both overall and for 4 different subgroups (all planned). Thus, even if ACE inhibitors and ARBs were equally effective for all outcomes, we would expect to find about 1 statistically significant result (due to multiple comparisons). In addition, this finding has not been consistently observed in other studies. In a meta‐analysis of placebo‐controlled trials, Tocci and colleagues 5 found that both ACE inhibitors and ARBs reduce the risk of new‐onset diabetes, although the size of the effect (OR, 0.8) was about the same in the two groups. The ONTARGET trial, 6 which followed 25,620 patients for a median of 56 months, found that the ARB alone group had slightly higher incidence of new‐onset diabetes than the ACE inhibitor alone group, but the difference was not statistically significant (relative risk, 1.12; 95% CI, 0.97–1.29). A potential explanation for fewer diabetes occurrences in the ACE inhibitor users may be that ACE inhibitors (but not ARBs) potentiate bradykinin through blockade of kininase II (ie, ACE), 17 , 18 which may improve insulin sensitivity. 19
As stated previously, the majority of clinical trials of ACE inhibitors and ARBs had follow‐up times of ≤4 months. For rare outcomes such as those studied here, we would expect longer follow‐up times to be necessary in order to see a difference between groups. For example, clinical experience suggests that the onset of type 2 diabetes occurs slowly over many months, and, in fact, our Kaplan‐Meier curves diverged only after 6 months.
Study Strengths
This study had a number of strengths, including use of a new‐user cohort design, 20 , 21 propensity score matching, and a stable study population. Finally, we employed statistical methods (eg, first treatment carried forward) similar to those of randomized trials.
Study Limitations
Despite the strengths of the study design described above, there are potential limitations as well. First, we examined only associations between what was prescribed (medication orders) and relevant clinical outcomes, as nonadherence and nonpersistence are not accurately reflected by EHR data. We cannot, for example, determine whether ACE inhibitors and ARBs are equally effective among persistent users or if the apparent similarity in outcomes is due to some combination of differential exposure and differential effectiveness. Our intention‐to‐treat effect focuses instead on the downstream effect of the initial decision to treat with an ACE inhibitor or ARB, but not on mechanisms. Another limitation is that there could be variables that affect the treatment decision and the outcomes that are not captured in the medical record (ie, unmeasured confounding). While we attempted to minimize the risk of bias by including dozens of carefully selected potential confounders in the propensity score model, there is still the possibility that important variables were excluded. Similarly, while we controlled for recent past medication use in the propensity score model, we cannot capture the full history strictly from medical records. Finally, the ascertainment of outcomes was from outpatient medical records, which may lead to some misclassification. However, we did use standard and/or validated definitions for each measure as published in peer‐reviewed literature. 13 Naturally, this disadvantage does not apply to the analysis of all‐cause death.
Conclusions
Results from this study can help guide clinician decision‐making, as we compared ACE inhibitors and ARBs on several key outcomes, with long follow‐up times, for patient subgroups defined by sex and age. Our finding of a greater risk of new‐onset diabetes among ARB users relative to ACE inhibitor users, especially among women, warrants further investigation.
Acknowledgments and disclosures: We thank Olugbenga Ogedegbe, MD, MPH, for valuable advice, and Dr Mack Lipkin for careful review of the manuscript. Dr Shah has received research funding from Pfizer, Roche, AstraZeneca, GlaxoSmithKline, Novartis, and Merck. Dr Hennessy has received research funding from Takeda Pharmaceuticals North America, Inc, AstraZeneca, and Bristol‐Myers Squibb, and performed consulting for Abbott, Amgen, AstraZeneca, Endo Pharmaceuticals, Novartis, Teva, and Wyeth. Dr Roy has received research funding from Roche and Amgen. None of the other investigators have any affiliations or financial involvement that may create conflicts with the material presented in this report.
Funding: This project was funded under contract number HHSA29020050041l from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services.
Disclaimer: Identifiable information on which this report, presentation, or other form of disclosure is based is protected by Federal Law, Section 924(c) of the Public Halth Service Act, 42 USC 299c‐3(c). Any confidential information in this report or presentation that is knowingly disclosed is disclosed solely for the purpose for which it was provided. No identifiable information about any individuals or entities supplying the information or described in it will be knowingly used except in accordance with their prior consent.
Supporting information
Figure S1. Plot of logit of estimated propensity scores, stratified by treatment group.
Table SI. Logistic regression model for the probability of ARB (relative to ACEI).
Table SII. Sensitivity analysis based on censoring time. Hazard ratios (95% confidence interval) for ARB vs. ACEI for study outcomes.
Supporting info item
Supporting info item
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. National Center for Health Statistics . Health, United States, 2009. Hyattsville, MD: National Center for Health Statistics; 2010. Available at: http://www.cdc.gov/nchs/fastats/hyprtens.htm. Accessed December 10, 2010. [Google Scholar]
- 3. Matchar DB, McCrory DC, Orlando LA, et al. Comparative Effectiveness of Angiotensin‐Converting Enzyme Inhibitors (ACE Inhibitor) and Angiotensin II Receptor Antagonists (ARB) for Treating Essential Hypertension. (CER 10). Prepared by Duke EPC under Contract No. 290‐02‐0025. Rockville, MD: AHRQ; 2007. [PubMed] [Google Scholar]
- 4. Reboldi G, Angeli F, Cavallini C, et al. Comparison between angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: a meta‐analysis. J Hypertens. 2008;26:1282–1289. [DOI] [PubMed] [Google Scholar]
- 5. Tocci G, Paneni F, Palano F, et al. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers and diabetes: a meta‐analysis of placebo‐controlled clinical trials. Am J Hypertens. 2011;24:582–590. [DOI] [PubMed] [Google Scholar]
- 6. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 7. TRANSCEND Investigators , Yusuf S, Teo K, et al. Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin‐converting enzyme inhibitors: a randomised controlled trial. The Lancet. 2008;372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 9. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 10. Austin PC. Propensity‐score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134:1128–1135. [DOI] [PubMed] [Google Scholar]
- 11. Normand SLT, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 12. Imai K, King G, Stuart EA. Misunderstandings between experimentalists and observationalists about causal inference. J R Stat Soc Ser A. 2008;171:481–502. [Google Scholar]
- 13. Birman‐Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 14. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model, 2nd ed. New York, NY: Springer; 2000. [Google Scholar]
- 15. Kunz R, Friedrich C, Wolbers M, Mann JFE. Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. [DOI] [PubMed] [Google Scholar]
- 16. Setoguchi S, Shrank WH, Liu J, et al. Angiotensin receptor blockers and angiotensin‐converting enzyme inhibitors: challenges in comparative effectiveness using Medicare data. Clin Pharmacol Ther. 2011;89:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gainer JV, Morrow JD, Loveland A, et al. Effect of bradykinin‐receptor blockade on the response to angiotensin‐converting‐enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285–1292. [DOI] [PubMed] [Google Scholar]
- 18. Gavras I, Gavras H. Role of bradykinin in hypertension and the antihypertensive effect of angiotensin‐converting enzyme inhibitors. Am J Med Sci. 1988;295:305–307. [DOI] [PubMed] [Google Scholar]
- 19. Carvalho CR, Thirone AC, Gontijo JA, et al. Effect of captopril, losartan, and bradykinin on early steps of insulin action. Diabetes. 1997;46:1950–1957. [DOI] [PubMed] [Google Scholar]
- 20. Ray W. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 21. Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE principles: recognizing high‐quality observational studies of comparative effectiveness. Am J Manag Care. 2010;16:467–471. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plot of logit of estimated propensity scores, stratified by treatment group.
Table SI. Logistic regression model for the probability of ARB (relative to ACEI).
Table SII. Sensitivity analysis based on censoring time. Hazard ratios (95% confidence interval) for ARB vs. ACEI for study outcomes.
Supporting info item
Supporting info item


