Abstract
J Clin Hypertens (Greenwich). 2012; 14:623–629. © 2012 Wiley Periodicals, Inc.
Indirect evidence suggests that chlorthalidone may be more effective than hydrochlorothiazide (HCTZ), but direct comparisons are lacking. Using national Veterans Administrative pharmacy data from 2003 to 2008, the authors performed a retrospective cohort study examining the effectiveness of chlorthalidone and HCTZ among new thiazide users. For 1 year following the thiazide start, rates of persistence of thiazide use, adequacy of response (defined as the absence of additional new antihypertensive medications following thiazide initiation), and an overall composite of treatment effectiveness incorporating both outcomes were examined. In this cohort of >125,000 individuals, persistence of thiazide use was higher for HCTZ than chlorthalidone (72.0% vs 62.0%; P<.001), but among thiazide‐persistent users, more HCTZ users had additional antihypertensives added compared with chlorthalidone (76.4% vs 70.1%; P<.001). The overall composite treatment response (thiazide persistence and no antihypertensive additions) revealed a slight advantage for HCTZ over chlorthalidone (50.7% vs 47.4%; P=.002). These findings remained consistent after adjustment using multivariable logistic regression. This study provides real‐world clinical data supporting a potential efficacy advantage of chlorthalidone among patients who tolerate the drug and remain persistent with treatment; however, strategies to optimize the way chlorthalidone is prescribed in clinical practice are necessary to increase its overall effectiveness relative to HCTZ.
Thiazide diuretics are often considered a homogeneous therapeutic class, where all agents reduce cardiovascular event risk equally as a direct consequence of antihypertensive effects. While hydrochlorothiazide (HCTZ) is the prototype thiazide, chlorthalidone is a phthalimidine possessing distinct pharmacokinetics. 1 Whether its unique effects result in meaningful pharmacodynamic differences in hypertensive patients is actively debated. Retrospective analyses of the Multiple Risk Factor Intervention Trial (MRFIT) and one small randomized trial comparing ambulatory blood pressures (BPs) provide evidence suggesting that chlorthalidone is a more effective antihypertensive than HCTZ at contemporary doses and may provide superior cardiovascular risk reduction. 2 , 3 , 4 , 5 Moreover, the preponderance of clinical trials demonstrating reductions in stroke and heart failure deaths with a diuretic regimen have intentionally used chlorthalidone. 1
Despite abundant outcome evidence with chlorthalidone, prescriptions for HCTZ in the United States outnumber chlorthalidone by nearly 20‐fold. 6 A traditional randomized, outcome‐based trial between HCTZ and chlorthalidone could determine efficacy under idealized conditions, but even if chlorthalidone was more effective than HCTZ in such a study, dissemination of this finding into practice may not be assured. 7 In the case of chlorthalidone, potential efficacy advantages over HCTZ could be offset by practical use limitations. HCTZ is easier to prescribe in clinical practice. It is more widely available in fixed‐dose combinations with other popular antihypertensive classes, as well as potassium‐sparing agents, which may reduce laboratory monitoring burden. This dilemma highlights the need for studies of comparative effectiveness performed under real‐world practice conditions to compliment traditional efficacy trials. 8 Therefore, we conducted an observational cohort study to examine the comparative effectiveness of HCTZ and chlorthalidone using national administrative data from the Veterans Health Administration (VA). We constructed pragmatic outcome measures designed to reflect the end result of provider and patient‐related decision‐making and provide a proxy of treatment effectiveness in real‐world clinical practice.
Methods
This retrospective cohort study examined the comparative effectiveness of chlorthalidone and HCTZ among veterans initiating therapy with either agent. For purposes of this paper, both HCTZ and chlorthalidone are referred to as thiazides. Administrative VA pharmacy data were obtained from the Austin Information Technology Center for fiscal years (FYs) 2003 through 2008. This research was approved by the University of Iowa’s institutional review board and the Iowa City VA Research and Development Committee.
Study Cohort
Patients initiating thiazide diuretic treatment during FY 2004 with chlorthalidone or HCTZ were selected. New users were identified by an initial thiazide fill during FY 2004 that was preceded by a 1‐year period with evidence of regular VA medication use but where no thiazide fills occurred. 6 These criteria excluded patients transferring care to the VA who may have been receiving a thiazide outside the VA system prior to the first VA fill. To ensure adequate follow‐up data, patients were further required to have regular VA medication use during the 2 years following thiazide initiation.
Clinical Outcomes
Two primary treatment outcomes were considered over the 1‐year period following thiazide initiation: nonpersistence and insufficient response. These outcomes were selected to represent the real‐world effectiveness of thiazide use in the chronic management of hypertension, where effectiveness is a balance of tolerability and benefit. The first outcome was persistence of thiazide use, defined as a pattern of refills that extended beyond the 1‐year follow‐up period with no significant interruptions. An interruption occurred if an interval between two consecutive thiazide fills exceeded more than two times the days supply value of the first fill; this was deemed nonpersistence. The second outcome was adequate response, defined as the absence of any new antihypertensive medications started within the 1‐year period following thiazide initiation. Initiation of new antihypertensive medication during this period was termed insufficient response. Finally, we determined composite treatment effectiveness, which incorporated both treatment outcomes, and was defined as persistent thiazide use without the need for additional antihypertensive therapy during the 1‐year follow‐up period.
Covariates
Use of nonthiazide antihypertensive medications was assessed during several time periods in relation to thiazide initiation. Medications received in the year prior to thiazide initiation, but not refilled after initiation, were considered prior medication failures. Medications filled both prior to and following thiazide initiation without significant interruption in use were considered as ongoing antihypertensive therapy. Medications started on the same day or within 14 days of thiazide initiation were considered as started concurrently with the thiazide diuretic. The use of potassium‐sparing diuretics (ie, amiloride, spironolactone, or triamterene) at the time of thiazide initiation was determined separately from other antihypertensives and included either ongoing use or concurrent initiation. Antihypertensives started more than 14 days following thiazide initiation were considered new medications and, as previously noted, comprised the primary outcome measure definition of insufficient response.
The daily dose of thiazide at initiation was estimated by dividing the product of the unit drug strength and quantity dispensed fields by the days supply field. Daily doses were categorized as micro‐dose (≤12.5 mg), low‐dose (12.5 mg<dose ≤25 mg), and high‐dose (>25 mg).
Thiazide medication adherence was determined among patients with persistent thiazide use during the follow‐up period and estimated by MED‐OUT, a validated index based on medication refill patterns. 9 In brief, this index uses the days supply dispensed and the time interval between individual refills to determine the proportion of days in which the patient was without medication. For all statistical procedures, the MED‐OUT index was transformed by the expression 2*arcsine (√X). This transformation is used with variables expressed as percentages to eliminate the tendency of the mean and the variance to be correlated, which violates regression assumptions. For ease of interpretation, adherence was expressed as (1 − MED‐OUT), which reflects the proportion of days with available medication.
Statistical Analysis
Clinical characteristics were compared between chlorthalidone and HCTZ treatment groups using chi‐square tests for categorical variables and t tests for continuous variables. Differences between treatment groups in the unadjusted frequency of categorical outcomes and in the time course of nonpersistence were compared using chi‐square tests. Multivariable logistic regression was used to adjust for observed confounding factors in the relationship between thiazide treatment group and effectiveness outcomes. Separate multivariable analyses were conducted for the two primary outcome measures, where ineffectiveness was defined as nonpersistence to thiazide treatment in the first model and insufficient treatment response (ie, requiring the addition of new antihypertensive medication) in the second model. A replication analysis was conducted using identical methods applied to patients initiating thiazide treatment during FY 2006. All statistical procedures were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC), with P<.05 for the statistical significance threshold.
Results
Study Cohort
The stepwise selection of study patients is shown in Figure 1. Nearly 1 million veterans received thiazide diuretics during FY 2004 and 145,354 (16.0%) patients were new thiazide starters. Of these, 126,808 (87.2%) had sufficient 2‐year follow‐up data and comprised the final study population. The clinical characteristics of new users of chlorthalidone and HCTZ are contrasted in Table I. Several important differences were observed and consistent with selective use of chlorthalidone among patients with more treatment‐refractory hypertension. At the time of thiazide initiation, chlorthalidone starters were taking more antihypertensive medications, more likely to have another antihypertensive agent started concurrently, and more likely to have previous antihypertensive treatment failure. Chlorthalidone starters were also less likely to receive micro‐dose therapy (≤12.5 mg/d) or a potassium‐sparing diuretic concurrently at thiazide initiation.
Figure 1.
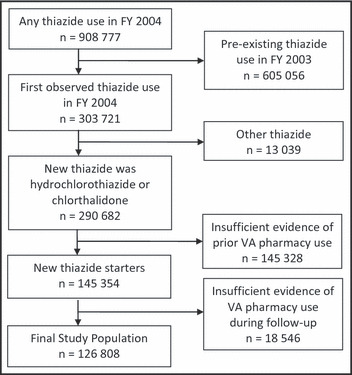
Cohort selection. FY indicates fiscal year; VA, Veterans Health Administration.
Table I.
Clinical Characteristics of New Thiazide Users in FY 2004
| Clinical Characteristic | Chlorthalidone (n=2257) | Hydrochlorothiazide (n=124,551) | Statistics |
|---|---|---|---|
| Men, No. (%) | 2180 (96.6%) | 119,392 (95.9%) | χ2=2.99; DF=1; P=.084 |
| Age, mean (SD), y | 66.7 (11.4) | 66.7 (11.2) | t=0.08; DF=126,806; P=.96 |
| AH failure in prior year, No. (%) | 618 (27.4%) | 29,382 (23.6%) | χ2=17.6; DF=1; P<.001 |
| Thiazide monotherapy, No. (%)a | 221 (9.8%) | 20,943 (16.8%) | χ2=78.6; DF=1; P<.001 |
| K‐sparing at index, No. (%) | 34 (1.5) | 14,343 (11.5) | χ2=221; DF=1; P<.001 |
| Concurrent AH started, No. (%) | 576 (25.5) | 19,290 (15.5) | χ2=169; DF=1; P<.001 |
| Ongoing AH drug therapy, No. (%) | χ2=33.9; DF=3; P<.001 | ||
| None | 466 (20.6) | 29,310 (23.5) | |
| 1 | 886 (39.3) | 51,178 (41.1) | |
| 2 | 635 (28.1) | 32,862 (26.4) | |
| ≥3 | 270 (12.0) | 11,201 (9.0) | |
| Initial thiazide dose, No. (%) | χ2=302; DF=2; P<.001 | ||
| Micro (≤12.5 mg) | 638 (28.3) | 58,118 (46.7) | |
| Low (12.5 mg<dose ≤25 mg) | 1509 (66.9) | 61,722 (49.6) | |
| High (>25 mg) | 110 (4.9) | 4711 (3.8) |
Abbreviations: AH, antihypertensive drug; DF, degrees of freedom; FY, fiscal year; K‐sparing, potassium‐sparing diuretic (eg, spironolactone, amiloride, triamterene); SD, standard deviation. aThiazide monotherapy indicates no active AH drugs at baseline and no new AH drugs started concurrently with the thiazide.
Unadjusted Clinical Outcomes
The unadjusted frequencies of clinical outcomes are presented in Figure 2. The persistence of thiazide use for 1 year following initiation was significantly higher for HCTZ (72.4%) than for chlorthalidone (62.0%) (χ2=118; degrees of freedom [DF]=1; P<.001). However, patients treated with HCTZ were significantly less likely to achieve an adequate response (70.1%) compared with chlorthalidone (76.4%) (χ2=26.1; DF=1; P<.001) among patients who remained persistent. When the two outcomes were considered simultaneously, the composite treatment response revealed a small but statistically significant advantage for HCTZ (50.7%) over chlorthalidone (47.4%) (χ2=9.9; DF=1; P=.002).
Figure 2.
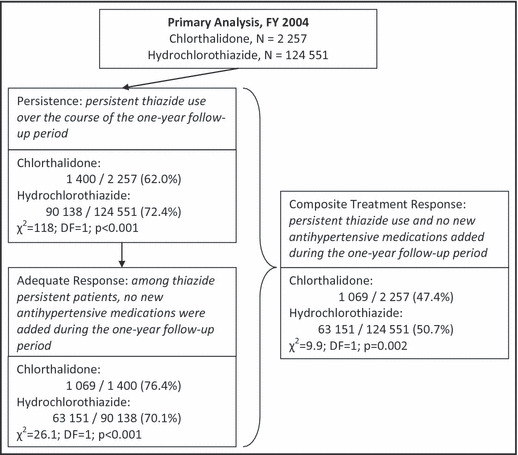
Unadjusted frequency of primary and composite treatment outcomes.
The time courses for nonpersistence or insufficient response are compared in Table II. Overall, 35,270 patients were nonpersistent with thiazide treatment over the 1‐year follow‐up period. Of these, 37.9% had no refills following initiation, 31.2% had at least 2 medication fills but none beyond 180 days after initiation, and the remaining 30.9% discontinued after 180 days. The time course of nonpersistence was similar between HCTZ and chlorthalidone users. Among thiazide‐persistent patients, 27,318 had an insufficient response, as defined by the initiation of a new antihypertensive medication. The largest proportion occurred more than 180 days following thiazide initiation (46.3%). The time course did not differ between HCTZ and chlorthalidone groups.
Table II.
Time Course of Nonpersistence and Insufficient Response
| Time course group | Overall | Chlorthalidone | Hydrochlorothiazide |
|---|---|---|---|
| Nonpersistence, No.a | 35,270 | 857 | 34,413 |
| After single fill | 13,370 (37.9%) | 316 (36.9%) | 13,054 (37.9%) |
| Two fills, but within 180 days | 11,002 (31.2%) | 298 (34.8%) | 10,704 (31.1%) |
| Within 1 y | 10,898 (30.9%) | 243 (28.4%) | 10,655 (31.0%) |
| Inadequate response, No.b | 27,318 | 331 | 26,987 |
| 15–30 d | 1900 (7.0%) | 27 (8.2%) | 1927 (7.1%) |
| 31–90 d | 5529 (20.2%) | 71 (21.5%) | 5458 (20.2%) |
| 91–180 d | 7216 (26.4%) | 81 (24.4%) | 7135 (26.4%) |
| 181–365 d | 12,646 (46.3%) | 152 (45.9%) | 12,494 (46.3%) |
aTest for difference in time course distribution between hydrochlorothiazide and chlorthalidone: χ2=5.7; degrees of freedom (DF)=2; P=.0580. bTest for difference in time course distribution between hydrochlorothiazide and chlorthalidone: χ2=1.31; DF=3; P=.7268.
Multivariable Clinical Outcome Predictors
In order to adjust for important sources of confounding, multivariable logistic regression models were created for the two primary effectiveness outcomes. In univariate analysis, chlorthalidone use was associated with a significantly greater likelihood for nonpersistence, compared with HCTZ (odds ratio [OR], 1.60; 95% confidence interval [CI], 1.47–1.75), and this relationship remained consistent after statistical adjustment (OR, 1.62; 95% CI, 1.48–1.76) (Table III). Other factors that were independently associated with thiazide nonpersistence included female sex, age, prior antihypertensive failure, concurrent antihypertensive initiation, use of a potassium‐sparing diuretic, and initial thiazide dose. The number of antihypertensives used in ongoing pharmacotherapy was U‐shaped, where the lowest risk for nonpersistence was observed for patients receiving 1 or 2 agents, with elevated risk among patients with no ongoing hypertension treatment and those taking multiple antihypertensives (≥3). The FY 2006 replication analysis yielded highly consistent findings, where the adjusted likelihood of nonpersistence remained significantly elevated for chlorthalidone compared with HCTZ (OR, 1.49; 95% CI, 1.37–1.62).
Table III.
Predictors of Thiazide Nonpersistence
| Primary Analysis, FY 2004 (N=126,808) | Replication Analysis, FY 2006 (N=113,994) | ||
|---|---|---|---|
| Clinical Characteristic | Univariate Models OR (95% CI)a | Adjusted Model OR (95% CI)b | Adjusted Model OR (95% CI)b |
| Chlorthalidone | 1.60 (1.47–1.75) | 1.62 (1.48–1.76) | 1.49 (1.37–1.62) |
| Women | 1.05 (0.99–1.12) | 1.11 (1.04–1.18) | 1.15 (1.074–1.22) |
| Age | 1.009 (1.008–1.010) | 1.010 (1.009–1.011) | 1.014 (1.013–1.015) |
| AH failure in prior year | |||
| None | Reference | Reference | Reference |
| ≥1 | 1.04 (1.01–1.07) | 1.02 (0.98–1.05) | 1.04 (1.01–1.08) |
| ≥2 | 1.22 (1.14–1.29) | 1.19 (1.12–1.27) | 1.15 (1.08–1.22) |
| K‐sparing at index | 1.23 (1.19–1.28) | 1.19 (1.15–1.24) | 1.20 (1.14–1.25) |
| Concurrent AH started | 1.11 (1.07–1.15) | 1.05 (1.01–1.09) | 1.01 (0.97–1.04) |
| Ongoing AH drug therapy | |||
| None | 1.15 (1.12–1.19) | 1.17 (1.14–1.21) | 1.20 (1.16–1.24) |
| 1 or 2 | Reference | Reference | Reference |
| ≥3 | 1.13 (1.08–1.18) | 1.10 (1.06–1.15) | 1.07 (1.02–1.11) |
| Initial thiazide dose | |||
| Micro (≤12.5 mg) | 0.95 (0.93–0.97) | 0.95 (0.93–0.97) | 0.96 (0.94–0.99) |
| Low (12.5 mg<dose ≤25 mg) | Reference | Reference | Reference |
| High (>25 mg) | 1.19 (1.120–1.27) | 1.19 (1.11–1.26) | 1.07 (1.00–1.16) |
Abbreviations: AH, antihypertensive drug; FY, fiscal year; K‐sparing, potassium‐sparing diuretic (eg, spironolactone, amiloride, triamterene). aOdds ratios (ORs) and 95% confidence intervals (CIs) for univariate models separately examining the association between nonpersistence and each individual clinical characteristic. bORs and 95% CIs for a single multivariable logistic regression model controlling for all clinical characteristics specified in the Table.
Independent predictors of insufficient treatment response among thiazide‐persistent patients are presented in Table IV. For this outcome, chlorthalidone use was less likely to be associated with an insufficient treatment response, compared with HCTZ (OR, 0.71; 95% CI, 0.63–0.80). Of note, greater thiazide adherence was independently protective against insufficient treatment response (OR, 0.85; 95% CI, 0.83–0.87). The FY 2006 replication analysis yielded highly consistent findings, where the adjusted likelihood of insufficient treatment response remained significantly lower for chlorthalidone compared with HCTZ (OR, 0.72; 95% CI, 0.63–0.81).
Table IV.
Predictors of Insufficient Response Among Thiazide‐Persistent Patients
| Primary Analysis, FY 2004 (N=91,538) | Replication Analysis, FY 2006 (N=82,230) | ||
|---|---|---|---|
| Clinical Characteristic | Univariate Models OR (95% CI)a | Adjusted Model OR (95% CI)b | Adjusted Model OR (95% CI)b |
| Chlorthalidone | 0.73 (0.64–0.82) | 0.71 (0.63–0.80) | 0.72 (0.63–0.81) |
| Women | 0.88 (0.81–0.94) | 0.79 (0.73–0.85) | 0.76 (0.71–0.83) |
| Age | 0.995 (0.994–0.996) | 0.996 (0.995–0.997) | 0.995 (0.993–0.996) |
| AH failure in prior year | |||
| None | Reference | Reference | Reference |
| ≥1 | 1.35 (1.31–1.40) | 1.40 (1.35–1.45) | 1.32 (1.27–1.37) |
| ≥2 | 1.26 (1.18–1.36) | 1.28 (1.19–1.37) | 1.20 (1.12–1.29) |
| K‐sparing at index | 0.90 (0.86–0.94) | 0.77 (0.73–0.81) | 0.78 (0.74–0.83) |
| Concurrent AH started | 0.85 (0.82–0.89) | 0.72 (0.69–0.75) | 0.71 (0.68–0.74) |
| Ongoing AH drug therapy | |||
| None | 1.29 (1.25–1.33) | 1.34 (1.29–1.39) | 1.40 (1.35–1.45) |
| 1 or 2 | Reference | Reference | Reference |
| ≥3 | 0.90 (0.85–0.95) | 0.88 (0.84–0.93) | 0.90 (0.85–0.95) |
| Initial thiazide dose | |||
| Micro (≤12.5 mg) | 0.83 (0.81–0.86) | 0.83 (0.81–0.86) | 0.80 (0.780–0.83) |
| Low (12.5 mg< dose ≤25 mg) | Reference | Reference | Reference |
| High (>25 mg) | 1.00 (0.93–1.08) | 1.05 (0.97–1.13) | 1.11 (1.02–1.21) |
| Adherencec | 0.85 (0.83–0.87) | 0.85 (0.83–0.87) | 0.84 (0.82–0.87) |
Abbreviations: AH, antihypertensive drug; FY, fiscal year; K‐sparing, potassium‐sparing diuretic (eg, spironolactone, amiloride, triamterene). aOdds ratios (ORs) and 95% confidence intervals (CIs) for univariate models separately examining the association between insufficient response and each individual clinical characteristic. bORs and 95% CIs for a single multivariable logistic regression model controlling for all clinical characteristics specified in the Table. cAdherence measured by 1 MED‐OUT. 9
Sensitivity Analyses
The primary analyses were conducted using similar dosing categories for chlorthalidone and HCTZ. However, because there is evidence that chlorthalidone is approximately twice as potent as HCTZ, we conducted sensitivity analyses using an alternative potency assumption of chlorthalidone being twice as potent as HCTZ. Two different approaches were used: (1) a continuous dose variable, where dose was adjusted for the chlorthalidone group to account for the potency difference; and (2) separate dosing thresholds for chlorthalidone and HCTZ for the micro‐, low‐, and high‐dose categories. Both approaches produced numerically different parameter estimates for the main effect (ie, chlorthalidone vs HCTZ), but these differences were not outside the bounds of the 95% CI of the primary analysis (ie, the sensitivity analyses did not yield statistically different results). Similarly, the sensitivity analyses yielded slightly different parameter estimates across the covariates in the multivariable models, but the overall results were unaltered (data not shown).
Discussion
The comparative effectiveness of HCTZ and chlorthalidone is a key question in hypertension management, and our study is the first to investigate the question using a real‐world cohort of thiazide users. We found that patients are more likely to remain persistent with HCTZ than chlorthalidone following initiation of either agent. However, for those who remain persistent on chlorthalidone, there is an apparent efficacy advantage in that they are less likely to require further additional antihypertensives. Our study was conducted in a national sample comprising more than 125,000 patients, and findings remained consistent when the analysis was replicated in a separate cohort using identical methods.
There are several potential explanations for lower persistence rates among patients initiating chlorthalidone. The most direct interpretation is that HCTZ is better tolerated than chlorthalidone. However, this observation is complicated by several factors, including differences in dosing and potency and in the selection bias of which patients are prescribed chlorthalidone. Historically, HCTZ was often used at doses in excess of 50 mg/d, but contemporary once‐daily administration of 12.5 to 25 mg/d produces lower rates of electrolyte disturbances and other adverse events. 1 Chlorthalidone is about twice as potent as HCTZ at these contemporary doses. 10 Despite this potency difference, chlorthalidone starters in our cohort received significantly higher initial doses than those initiating HCTZ. Moreover, HCTZ users were more likely to be prescribed a concurrent potassium‐sparing diuretic, which may have protected against electrolyte disturbances. While we attempted to control for differences in dosing and potassium‐sparing diuretic use, residual confounding could partially explain our findings. Differences in persistence rates could also reflect the tendency of prescribers to reserve chlorthalidone for more difficult‐to‐treat patients, who may have treatment‐refractory hypertension and be at higher risk for adverse effects. This assertion was supported in our cohort, as chlorthalidone starters were taking more antihypertensive medications, were more likely to have another antihypertensive agent started concurrently, and were more likely to have previous antihypertensive treatment failure. We adjusted for known sources of selection bias, but randomization is the only methodological tool that can fully annul these biases.
Among thiazide‐persistent patients, chlorthalidone demonstrated a clinical advantage over HCTZ, as fewer patients required additional antihypertensive medications during the first year of thiazide therapy. This finding contributes to a growing body of literature suggesting that chlorthalidone may be more effective because of a longer half‐life and more prolonged duration of action, greater effect in lowering nighttime BP, or possible pleiotropic effects unrelated to BP. 2 , 3 , 4 , 5 , 11 , 12 Answering the question of whether to use HCTZ or chlorthalidone may seem relatively insignificant; however, hypertension affects one quarter to one third of adults, and approximately 32% of patients with hypertension are prescribed thiazide diuretics. 13 , 14 Small clinical differences could therefore translate into meaningful effects on millions of individuals.
Limitations
We acknowledge several important limitations of our analysis. The lack of a randomized design increases the potential for bias and unmeasured confounding, although our findings are strengthened by the multivariable and replication analyses. Furthermore, the direction of the selection bias we observed from known confounding factors, such as initial dosing and concurrent use of potassium‐sparing diuretics, was toward finding a better composite outcome in patients starting HCTZ. If confounding due to unknown factors followed the same directionality, it is likely that the higher persistence observed among HCTZ users may be at least partly attributable to selection bias. However, applying this same directionality suggests that the greater efficacy of chlorthalidone among medication‐persistent patients is not due to selection bias and may actually be underestimated in this observational cohort. A second potential limitation is that we did not assess actual BP control rates or cardiovascular‐related end points. Determination of BP control utilizing electronic records has been shown to differ by almost 25% from actual visits under research protocol, 15 while comparing long‐term cardiovascular outcomes in observational studies of hypertension is particularly difficult because of frequent and unrestricted medication exposures. As an example, more than 50% of patients initially treated with a thiazide in our cohort discontinued the drug or were started on a new antihypertensive within just 1 year.
While a randomized controlled design would clearly be superior in determining the comparative efficacy or effectiveness of HCTZ vs chlorthalidone, sample size and cost considerations make this unlikely to occur, and the external validity of such a study may suffer if the treatment arms are not constructed in a way that reflects how care is actually delivered in practice. In the absence of these data, pragmatic designs such as ours focusing on the real‐world utilization of these agents are a viable alternative for exploring the comparative effectiveness of these agents.
Conclusions
Our study provides real‐world clinical data supporting the premise that chlorthalidone is more effective among patients who remain persistent on the drug. However, this advantage may be offset from an effectiveness standpoint given underlying barriers to prescribing chlorthalidone, as well as in the selection biases in how it is used clinically. We suggest that future studies focus on strategies to optimize how chlorthalidone is prescribed in clinical practice in order to increase its real‐world effectiveness.
Disclosures: The research reported here was supported by the Department of Veterans Affairs Health Services Research and Development (VA HSR&D) Service through the Center for Comprehensive Access & Delivery Research and Evaluation (REA 09‐220) at the Iowa City VA Medical Center. Dr Lund is supported by a VA HSR&D Career Development Award (CDA 10‐017). All authors report no conflicts of interest in the publication of this manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1. Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. [DOI] [PubMed] [Google Scholar]
- 2. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. [DOI] [PubMed] [Google Scholar]
- 3. Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta‐analysis of dose‐response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23:440–446. [DOI] [PubMed] [Google Scholar]
- 4. Dorsch MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694. [DOI] [PubMed] [Google Scholar]
- 5. Ernst ME, Neaton JD, Grimm RH Jr, et al. Long‐term effects of chlorthalidone versus hydrochlorothiazide on electrocardiographic left ventricular hypertrophy in the Multiple Risk Factor Intervention Trial. Hypertension. 2011;58:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avorn J. Transforming trial results into practice change: the final translational hurdle: comment on “Impact of the ALLHAT/JNC7 Dissemination Project on thiazide‐type diuretic use.” Arch Intern Med. 2010;170:858–860. [DOI] [PubMed] [Google Scholar]
- 8. IOM (Institute of Medicine) . Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 9. Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Med Care. 1998;26:814–823. [DOI] [PubMed] [Google Scholar]
- 10. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. [DOI] [PubMed] [Google Scholar]
- 11. Temperini C, Cecchi A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: sulfonamide diuretics revisited – old leads for new applications? Org Biomol Chem. 2008;6:2499–2506. [DOI] [PubMed] [Google Scholar]
- 12. Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension. 2010;56:463–470. [DOI] [PubMed] [Google Scholar]
- 13. Egan BM, Zhao Y, Axon RN. US Trends in prevalence, awareness, treatment, and control of hypertension, 1998–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 14. Stafford RS, Monti V, Furberg CD, Ma J. Long‐term and short‐term changes in antihypertensive prescribing by office‐based physicians in the United States. Hypertension. 2006;48:213–218. [DOI] [PubMed] [Google Scholar]
- 15. Fishman PA, Anderson ML, Cook AJ, et al. Accuracy of blood pressure measurements reported in an electronic medical record during routine primary care visits. J Clin Hypertens (Greenwich). 2011;13:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]


