Abstract
J Clin Hypertens (Greenwich). 2012;14:760–766. ©2012 Wiley Periodicals, Inc.
This study analyzed the cost‐effectiveness of a patient hypertension education intervention that provided patient education through interactive voice response technology and distribution of automated blood pressure monitors to high‐risk plan members with uncontrolled hypertension. A total of 17,318 members were identified with hypertension in an administrative database. The study sample consisted of all 534 high‐risk hypertensive plan members who received blood pressure monitors. Using data on activity‐based program costs and changes in hypertension control, this study modeled the intervention’s cost‐effectiveness relative to no intervention. The intervention was estimated to have brought hypertension under control in 151 patients during the study year. Across all 534 participants in 1 year, 0.3 events (acute myocardial infarction, stroke, congestive heart failure, and renal failure) were avoided and 2.77 life‐years were gained (LYG). The incremental cost‐effectiveness ratio (ICER) for the intervention compared with no intervention was $767 per person brought under control or $41,927 per LYG. If the gains in hypertension control from 1 year’s investment were assumed to last 10 years, the 10‐year ICER relative to no intervention was $1857 per LYG. The intervention is a cost‐effective strategy to address hypertension and can serve as a model for future innovations.
Hypertension is the leading risk factor for cardiovascular disease (CVD) including heart attacks, stroke, and congestive heart failure. 1 Recent data indicate that only 50% of all hypertensive individuals and 51% of treated hypertensive patients have their blood pressure (BP) under control. 2 Thus, there is considerable opportunity for improvements in cardiovascular health through effective hypertension prevention and control.
This study analyzed the cost‐effectiveness of a collaborative hypertension intervention conducted by the Utah Department of Health, Heart Disease and Stroke Prevention Program (HDSPP) and SelectHealth, a commercial health maintenance organization that is part of the Intermountain Healthcare integrated delivery system. The intervention’s key features were the use of interactive voice response (IVR) software to contact and educate hypertensive members and the provision of automated BP monitors to high‐risk plan members with uncontrolled hypertension for self‐monitoring. Using data on program costs and changes in hypertension control, this study modeled the cost‐effectiveness of the intervention.
Overview of the HDSPP
Since 2004, the Utah HDSPP has partnered with SelectHealth with the goal of increasing the control of high BP among health plan members with hypertension. This study focuses solely on patient education to promote self‐management of high BP. In consultation with both physicians and patients, Utah HDSPP and SelectHealth developed patient self‐management kits that included basic information about BP and the importance of managing it, a tool for keeping track of BP readings, a 10‐minute DVD aimed at motivating and promoting awareness of hypertension, tools to help patients take their medication as prescribed, a nutritional guide based on the Dietary Approaches to Stop Hypertension diet, and a pedometer and brochure that teaches about a walking program sponsored by the Utah HDSPP.
Every 6 months, plan members with a new diagnosis of hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) in the claims files were contacted by phone. The plan determined which members had uncontrolled hypertension (systolic BP >140 mm Hg or diastolic BP >90 mm Hg) via questions using IVR. Members with hypertension were assessed for current hypertension self‐management activities such as monitoring their BP, medication compliance, diet, and exercise and were asked whether they would like to receive the patient self‐management kit. SelectHealth also provided automated BP cuffs funded by HDSPP for self‐monitoring to participants who (1) reported that their BP was not under control or did not know whether their BP was under control, and (2) indicated that they were willing to use the cuffs to monitor their BP twice a week. SelectHealth reviewed patient charts to obtain the most recent BP measurements for the subset of participants who received the BP cuffs. Chart reviews were conducted 6 months after the information kit and BP cuffs were sent.
Methods
Data on Intervention Participants and Costs
We collected data for the 2007 round of the intervention. The HDSPP and SelectHealth provided summary details of the program structure, participants, and BP control at follow‐up drawn from an administrative database. The study sample consisted of all 534 plan members with hypertension who received BP monitors through the process described above. This was the only group reached by the intervention for which information about their BP control was available after intervention; 422 of the 534 had follow‐up information about hypertension control, prescription drug claims, and physician visits. The analysis below implicitly assumed that these 534 members were the only members to benefit from the entire patient education program; any improvement in hypertension among other members would lead to more favorable cost‐effectiveness ratios.
We conducted a site visit to collect program cost information at the primary activity level. The activity‐based approach assesses labor, materials, and contracted costs required to provide each primary activity. The four primary activities associated with the patient education program were development of materials (eg, creation of self‐management kits), recruitment of participants (eg, IVR), patient communication (eg, purchase and mailing of BP cuffs), and administration.
Cost‐Effectiveness Model
Figure 1 illustrates the major components of the cost‐effectiveness model. We had data for the implementation costs of the intervention, the number of insurance claims for prescription drugs for the participants, physician visits among the participants, and hypertension control rates for participants before and after the intervention.
Figure 1.
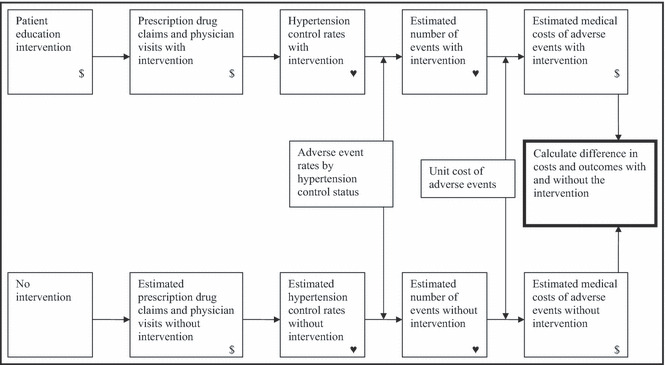
Model structure. $, cost;  , measure of effectiveness.
, measure of effectiveness.
The first step of the model was to estimate what prescription drug claims, physician visits, and hypertension control rates would have been among participants in the absence of the intervention. The second step was to estimate the number of adverse events (acute myocardial infarction [AMI], stroke, congestive heart failure [HF], and renal failure) expected among participants (Table SI). These events represent the main risks that hypertension poses. 1 Adverse event rates by hypertension control status from the literature were applied to the group of participants using both observed control rates with the intervention and estimated control rates without the intervention. The third step was to multiply the number of adverse events by the average cost of each event to estimate the total medical costs of events, again with and without the intervention (Table SII). Finally, the difference in costs (implementation plus medical) with and without the intervention provided an estimate of incremental costs. Incremental effectiveness was measured by the difference in (1) hypertension control, (2) number of adverse events, and (3) life‐years gained (LYG) with and without the intervention (Tables SIII and SIV). Because we do not have long‐term follow‐up data and therefore do not know how long the benefits of the intervention last, we report results assuming that the control rates from the intervention are maintained for 1 year and for 10 years.
Prescription Drug Claims, Physician Visits, and Control Rates Without the Intervention. Two key modeling assumptions must be made to construct counterfactual estimates of what would have happened to (1) prescription drug claims and physician visits and (2) control rates in the absence of the intervention. Among intervention participants, the value of decreased provider visits outweighed the increase in prescription expenditures, leading to a net decrease in medical management costs before until after intervention (see Table SII for medical costs for prescription drug claims and physician visits). We considered two extreme assumptions for medical management costs: (A1) none of the changes in prescriptions and provider visits was due to the intervention (ie, medical management costs would have changed as observed without the intervention), and (A2) all changes in prescriptions and provider visits in the participant group were due to the intervention (ie, medical management costs would have remained constant without the intervention).
Second, we considered two assumptions regarding control rates without the intervention: (B1) hypertension control rates improved by the same amount as in the overall plan HEDIS measures 2004–2005 (by 2.2 percentage points) and (B2) hypertension control rates remained constant at initial rates. The former assumption acknowledged that other influences could have also affected control rates. The latter assumed all of the improvement was due to the intervention.
Control Rates for Missing Data. Two types of missing data also required modeling assumptions to calculate control rates. First, in 2006, 55.6% of participants initially reported that they were unsure of their hypertension control status. We considered two assumptions regarding the actual control status at the beginning of the intervention: (C1) participants initially reporting they were unsure of their hypertension control status had control rates equal to that of the national average control among people with hypertension 1999–2002 (17.6% for patients aged 18–44 years and 31.4% for those aged 45–85 years), 2 and (C2) participants initially reporting they were unsure of their hypertension control status were not under control.
Second, 112 participants did not have follow‐up chart review. We considered two assumptions about the control status of these participants: (D1) participants without follow‐up chart review were not under control, and (D2) participants without follow‐up chart review had the same control rates as those with follow‐up (ie, missing at random).
In order to show the sensitivity of the results to these modeling assumptions, we present estimates for three sets of modeling assumptions: base, pessimistic, and optimistic models. The base model assigned average behavior to outcomes that were not observed and did not attribute the unanticipated reduction in provider visits to the intervention. Specifically, the base model assumed that (A1) the intervention had no impact on prescription drug claims and provider visits; (B1) without the intervention, hypertension control rates among the participants improved by the same amount as in the overall plan HEDIS measures; (C1) participants initially reporting they were unsure of their hypertension control status had control rates equal to that of the national average control among people with hypertension; and (D2) participants without follow‐up chart review had the same control rates as those with follow‐up.
The pessimistic model included assumptions that minimized the benefits attributed to the intervention. It used the same set of assumptions as the base model except for D1, which assumes participants without follow‐up chart review were not under control.
The optimistic model included assumptions that maximized the benefits attributed to the intervention. It assumed that (A2) all changes in prescription drug claims and provider visits were due to the intervention; (B2) without the intervention, hypertension control rates among the participants remained zero; (C2) participants initially reporting they were unsure of their hypertension control status were not under control; and (D2) participants without follow‐up chart review had the same control rates as those with follow‐up. Reporting both the pessimistic and optimistic models shows the sensitivity of the results to the key modeling and data assumptions regarding hypertension control and medical management costs in the absence of the intervention. Details of the rest of the cost‐effectiveness model and sensitivity analysis are provided in the Appendix.
Results
Recipients of BP monitors initially either did not have their BP under control or did not know whether it was under control. At 6‐month follow‐up, 45% had controlled BP (Table I). Hypertension control was higher for women than for men and, within sexes, higher for younger patients (18–44 years) than for older patients (45–85 years). Compared with the 6 months prior to receiving the BP cuff, participants’ claims for antihypertension prescriptions increased and participants’ number of primary care and specialist visits decreased in the 6 months after receiving the cuff.
Table I.
2007 Program Participants
| Group | Number |
|---|---|
| Diagnosis of hypertension identified in claims | 17,318 |
| Successfully contacted via interactive voice response | 7904 |
| Requested resource material | 3811 |
| Mailed blood pressure monitor | 534 |
| Data in follow‐up chart review | 422 |
| Among those with chart review | |
| Controlled blood pressure (<140/90 mm Hg) at follow‐up | Percent |
| Age 18–44 y, women (n=69) | 59.4 |
| Age 18–44 y, men (n=81) | 43.2 |
| Age 45–85 y, women (n=138) | 47.8 |
| Age 45–85 y, men (n=134) | 36.6 |
| Change in the 6‐month period prior to intervention to 6 months after the intervention | Change |
| Mean number of antihypertension prescription claims | +0.41 claims |
| Mean number of primary care provider and specialist visits | −1.10 visits |
The total annual cost of the entire patient education program, including outreach to members who did not ultimately receive a BP monitor, was $122,403 (Table II). These costs represent an average year for the ongoing patient education component. The two largest parts of program costs were contract costs for the IVR ($22,403 per year including development of the script and survey, telecommunication costs, transcription, and enrollment outreach) and materials costs to purchase the BP monitors ($43,686 per year; roughly $50 per monitor).
Table II.
2007 Program Costsa
| Development | Recruitment | Patient Communication | Program Oversight/Administration | Total | |
|---|---|---|---|---|---|
| Labor | $5304 | $171 | $7952 | $13,406 | $26,833 |
| SelectHealth | |||||
| Data analyst | $4286 | $171 | $0 | $686 | $5143 |
| Registered nurse | $0 | $0 | $6214 | $0 | $6214 |
| Clerical | $103 | $0 | $823 | $0 | $926 |
| Quality consultant | $915 | $0 | $915 | $0 | $1831 |
| HDSPP | |||||
| Health program specialist II | $0 | $0 | $0 | $432 | $432 |
| Health program specialist III | $0 | $0 | $0 | $6624 | $6624 |
| Program manager | $0 | $0 | $0 | $5664 | $5664 |
| Material/supplies | $6275 | $0 | $66,892 | $0 | $73,166 |
| SelectHealth | |||||
| BP basics | $0 | $0 | $385 | $0 | $385 |
| BP tracker | $0 | $0 | $385 | $0 | $385 |
| BP cuffs | $0 | $0 | $43,686 | $0 | $43,686 |
| Mailer boxes | $0 | $0 | $3002 | $0 | $3002 |
| DVD development | $6275 | $0 | $0 | $0 | $6275 |
| DVDs | $0 | $0 | $2227 | $0 | $2227 |
| Postage | $0 | $0 | $8995 | $0 | $8995 |
| HDSPP | |||||
| BP cuffs | $0 | $0 | $1093 | $0 | $1093 |
| BP basics | $0 | $0 | $7118 | $0 | $7118 |
| Contract servicesb | $2509 | $15,283 | $4611 | $0 | $22,403 |
| SelectHealth | |||||
| Phone script and survey | $2509 | $0 | $0 | $0 | $2509 |
| Telecommunication | $0 | $0 | $3217 | $0 | $3217 |
| Transcription | $0 | $0 | $1394 | $0 | $1394 |
| Enrollment outreach | $0 | $15,283 | $0 | $0 | $15,283 |
| Total | $14,087 | $15,455 | $79,455 | $13,406 | $122,403 |
Abbreviation: BP, blood pressure. aProgram costs include Utah Department of Health, Heart Disease and Stroke Prevention Program (HDSPP, fiscal year 2007) and SelectHealth expenditures (average annual costs January 1, 2004–June 30, 2007). bHDSPP expenditures that went to SelectHealth in the form of contracts have been listed for their ultimate purpose under SelectHealth.
Assuming the intervention’s gains in hypertension control were maintained for 1 year, under the base model assumptions, the incremental cost of the intervention was approximately $116,000 (Table III). This was a few thousand dollars less than the program costs (Table II), indicating some savings from the reduction in adverse events and their associated costs within the first year. The intervention was estimated to have brought 151 people’s hypertension under control. Across all 534 participants in 1 year, 0.3 events were avoided and 2.77 LYG. The ICER for the intervention compared with no intervention was $767 per person brought under control, $404,705 per event avoided, or $41,927 per LYG.
Table III.
ICERs Relative to Usual Care (95% Sensitivity Range): Base,a Optimistic,b and Pessimisticc Modeling Assumptions
| 1‐Year | 10‐Year | |
|---|---|---|
| Incremental cost | $116,154 ($113,272–$118,357) | $38,098 (−$3874 to $69,627) |
| Optimistic | $100,514 ($86,698–$114,246) | −$118,996 (−$253,192 to $5966) |
| Pessimistic | $118,546 ($116,859–$119,861) | $69,976 ($44,198–$89,280) |
| IE Measure | IE | ICER | IE | ICER |
|---|---|---|---|---|
| Number brought under control | 151 | $767d ($748–$782) | 151 | $252 (−$26 to $460) |
| Optimistic | 242 | $416 ($359–$473) | 242 | −$492 (−$1048 to $25) |
| Pessimistic | 101 | $1177 ($1160–$1190) | 101 | $695 ($439–$887) |
| Adverse events avoided | 0.29 (0.20–0.38) | $404,705 ($298,254–$592,959) | 3.92 (2.75–5.19) | $9720 (−$810 to $24,434) |
| Optimistic | 0.51 (0.35–0.70) | $196,350 ($132,853–$306,431) | 6.94 (4.79–9.31) | −$17,146 (−$34,533 to $1020) |
| Pessimistic | 0.18 (0.13–0.24) | $659,905 ($495,628–$947,981) | 2.46 (1.74–3.24) | $28,402 ($14,206–$50,212) |
| Life‐years gained | 2.77 (1.51–3.56) | $41,927 ($32,574–$76,569) | 20.51 (11.31–27.70) | $1857 (−$209 to $4488) |
| Optimistic | 5.10 (2.76–6.61) | $19,709 ($14,604–$36,790) | 37.34 (20.36–50.93) | −$3187 (−$8491 to $171) |
| Pessimistic | 1.70 (0.93–2.18) | $69,701 ($54,462–$127,891) | 12.68 (6.98–17.09) | $5518 ($3311–$10,705) |
aAssumes that (A1) the intervention has no impact on prescription drug claims and provider visits; (B1) without the intervention, hypertension control rates among the participants improve by the same amount as in the overall plan HEDIS measures 2004 to 2005 (ie, by 2.2 percentage points); (C1) participants initially reporting they were unsure of their hypertension control status (55.6%) have control rates equal to that of the national average control among people with hypertension 1999–2002 (17.6% for ages 18–44 y and 31.4% for ages 45–85 y); 3 and (D2) participants without follow‐up chart review (n=112) have the same control rates as those with follow‐up. bAssumes that (A2) all changes in prescription drug claims and provider visits are due to the intervention; (B2) without the intervention, hypertension control rates among the participants remain zero; (C2) participants initially reporting they were unsure of their hypertension control status (55.6%) are not under control; and (D2) participants without follow‐up chart review (n=112) have the same control rates as those with follow‐up. cUses same set of assumptions as the base model except for (D1), which assumes participants without follow‐up chart review are not under control. dIncremental cost‐effectiveness ratios (ICERs) do not exactly equal incremental cost/incremental effectiveness (IE) due to rounding of incremental cost and IE.
If hypertension control were maintained for 10 years, and the program costs were fixed at 1 year, the ICERs improve dramatically. Relative to no intervention, the 10‐year ICER was $9720 per event avoided and $1857 per LYG. The 95% sensitivity range included negative ICER, indicating that under some parameter combinations, the intervention was cost‐saving relative to no intervention.
Comparisons of the optimistic and pessimistic models in Table III demonstrate that the key modeling assumptions concerning medical management costs and control rates were important. In a 1‐year horizon, the ICERs from the optimistic model were roughly one third of those from the pessimistic model across effectiveness measures. For example, the optimistic ICER was $19,709 per LYG while the pessimistic ICER was $69,701 per LYG. In a 10‐year horizon, the optimistic model generated negative ICERs for all effectiveness measures, indicating that the intervention would save money on net from reduced expenditures on provider visits and adverse events. The pessimistic model did not show cost savings, but the 10‐year ICERs were generally low.
One‐way sensitivity analysis revealed that the results of the base model in terms of LYG with a 1‐year time horizon were most sensitive to the parameters governing the probability of CVD death for uncontrolled BP (Figure 2). For example, over the range of probability of CVD death within 1 year for women aged 45 to 85 years with uncontrolled BP, the ICER ranged from $33,000/LYG to $81,000/LYG. Parameters for other outcomes and costs, when varied one at a time, generated much smaller ranges in the ICER. All parameters not listed in Figure 2 generated ranges of <$1000 around the point estimate.
Figure 2.
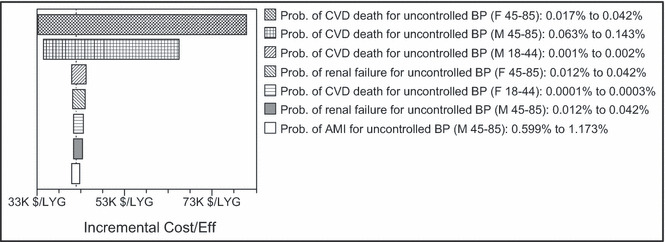
One‐way sensitivity analysis of base model with 1‐year time horizon: most influential parameters. Prob. indicates probability; CVD, cardiovascular disease; BP, blood pressure; F, female; M, male, AMI, acute myocardial infarction; LYG, life‐years gained.
Discussion
The collaborative intervention between the HDSPP and SelectHealth is a cost‐effective approach for reducing CVD risk among plan members with hypertension, especially if improvements in hypertension control are sustainable when program participation concludes. As discussed below, the cost‐effectiveness of the collaborative intervention is comparable to other hypertension therapies and lifestyle intervention programs aimed at reducing CVD risk.
Randomized control trials have shown that self‐monitoring of BP can generate small improvements in BP. 4 , 5 , 6 Two of these studies reported that self‐monitoring was cost neutral or slightly cost saving relative to standard care. 4 , 6 Our results did not show cost savings except under favorable model assumptions. However, our results indicated a larger level of effectiveness (improvement in hypertension control) than implied in the trials, suggesting that greater health benefits were gained for the additional costs of the program.
The cost‐effectiveness ratios are similar to those reported for pharmacologic hypertension therapies. In one review, the cost‐effectiveness ratios, all relative to no intervention and updated to 2007 dollars, ranged from $11,000 per LYG to more than $600,000 per quality‐adjusted life‐years, with a mean ratio across studies of $68,000 per LYG. 7 Even our most pessimistic model with a 1‐year time horizon had an ICER of about $67,000 per LYG. With a 10‐year horizon, our pessimistic ICER of roughly $5500 per LYG is lower than any of the reviewed pharmacologic interventions.
Our results also compare favorably to other cost‐effective lifestyle interventions to reduce CVD risk. The Center for Disease Control and Prevention’s WISEWOMAN program has reported cost‐effectiveness ratios, updated to 2007 dollars, of $5000 per LYG for a 10‐year horizon and $50,000 per LYG for a 1‐year horizon. 8 Our base model results were approximately $1900 per LYG and $42,000 per LYG, respectively. Physician‐counseled smoking cessation strategies for women had cost‐effectiveness ratios of $2900 to $5000 per LYG. 9 , 10
The model was sensitive to key modeling assumptions and parameter estimates. The 95% sensitivity ranges were often wide, especially for effectiveness measures events avoided and LYG, which required additional modeling assumptions. The model was also sensitive to the assumed length of time the gains in hypertension control were maintained. The 1‐year horizon assumed that the gains were lost soon after the intervention follow‐up was complete, a conservative assumption but one with support in the literature on self‐monitoring. 6 The 10‐year results, even if unlikely, illustrated how important maintenance of hypertension control can be. Even at 1 year, an ICER of $42,000 per LYG compares favorably with other prevention and medical interventions.
Several features of the intervention were crucial to its success. The partners reported that the ability to pool resources was integral to launching and maintaining the program. Gains in hypertension control were likely improved through the use of IVR technology to contact and educate plan members and by focusing on high‐risk and motivated plan members. Since the evaluation, SelectHealth has modified the IVR to combine hypertension with cholesterol and diabetes management into one program called “Healthy Heart.” Members are flagged using tailored claims data with hypertension, cholesterol, and/or diabetes and receive a tailored call addressing BP, cholesterol, and/or diabetes testing.
Limitations
Several limitations can be noted with this analysis. First, the ICERs are relative to no intervention and do not provide a comparison to other possible interventions. Second, the improvement in BP control in the intervention group could be at least partly due to selection bias (ie, motivated participants would have had above‐average improvement in control without the intervention). However, the target population had uncontrolled hypertension, indicating that these members might have been hard to manage patients with below‐average expected improvement in control rates without the intervention. Finally, it is not clear how accurately risk calculators track changes in risk resulting from changes in input risk factors. 11 Despite this limitation, the use of risk calculators to measure effectiveness of CVD interventions is necessary given the short period available for data collection in most risk reduction programs.
Additionally, several limitations, if possible to address, would likely lead to lower cost‐effectiveness ratios than those reported. First, the program costs included efforts to reach more members with educational materials than those that received the BP cuffs. Any improvement in hypertension among this group would lead to lower cost‐effectiveness ratios. Second, effectiveness was measured by hypertension control rates. The literature suggests that hypertension has a continuous effect on the events modeled here, 12 implying that lowering of BP that does not result in a change in control status produces health benefits not measured in the model. Finally, the costs of events did not include long‐term care costs. The savings in medical costs from events avoided due to the intervention would have been even larger had long‐term care costs been included.
Conclusions
The intervention conducted by the HDSPP and SelectHealth is a cost‐effective strategy to control hypertension and potentially reduce CVD in a real‐world setting. Identifying cost‐effective interventions is vital for dissemination of promising practices for improving hypertension control. This intervention partnership can serve as a model for future innovations in hypertension prevention.
Funding Sources: This research was supported by contract number 200‐2002‐00776 TO 39 from the Centers for Disease Control and Prevention. The views expressed in this presentation are solely those of the authors.
Disclosures: None.
Supporting information
Table SI. Adverse Events: Probability of Event per 1,000 Persons (Ranges in Sensitivity Analysis*).
Table SII. Medical Costs of Events (2007 $)*.
Table SIII. Cardiovascular Disease (CVD) Death: Percent Reduction in the Probability of CVD Death* From Change From Uncontrolled Blood Pressure to Controlled Blood Pressure (Ranges in Sensitivity Analysis†).
Table SIV. Life Expectancy Calculations: 10‐Year CVD Risk Reduction for Women Aged 45 to 85.
Supporting info item
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 2. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 3. Hajjar I, Kotchen JM, Kotchen TA. Hypertension: trends in prevalence, incidence and control. Annu Rev Public Health. 2006;27:465–490. [DOI] [PubMed] [Google Scholar]
- 4. Soghikian K, Casper SM, Fireman BH, et al. Home blood pressure monitoring. Effect on use of medical services and medical care costs. Med Care. 1992;30:855–865. [PubMed] [Google Scholar]
- 5. Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta‐analysis of randomised trials. BMJ. 2004;329:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McManus RJ, Mant J, Roalfe A, et al. Targets and self monitoring in hypertension: randomised controlled trial and cost effectiveness analysis. BMJ. 2005;331:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown AD, Garber AM. Cost effectiveness of coronary heart disease prevention strategies in adults. Pharmacoeconomics. 1998;14:27–48. [DOI] [PubMed] [Google Scholar]
- 8. Finkelstein EA, Khavjou OA, Will J. Cost‐effectiveness of WISEWOMAN, a program aimed at reducing heart disease risk among low‐income women. J Women’s Health. 2006;15:379–389. [DOI] [PubMed] [Google Scholar]
- 9. Kupersmith J, Holmes‐Rovner M, Hogan A, et al. Cost‐effectiveness analysis in heart‐disease, Part II: preventive therapies. Prog Cardiovasc Dis. 1995;37:243–271. [DOI] [PubMed] [Google Scholar]
- 10. Cummings SR, Rubin SM, Oster G. The cost‐effectiveness of counseling smokers to quit. JAMA. 1989;261:75–79. [PubMed] [Google Scholar]
- 11. Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Full Report (NIH Publication No. 02‐5215). Washington, DC: National Institutes of Health, National Heart, Lung and Blood Institute. http://www.nhlbi.nih.gov/guidelines/cholesterol/. Retrieved June 4, 2004. [Google Scholar]
- 12. Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334:13–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Adverse Events: Probability of Event per 1,000 Persons (Ranges in Sensitivity Analysis*).
Table SII. Medical Costs of Events (2007 $)*.
Table SIII. Cardiovascular Disease (CVD) Death: Percent Reduction in the Probability of CVD Death* From Change From Uncontrolled Blood Pressure to Controlled Blood Pressure (Ranges in Sensitivity Analysis†).
Table SIV. Life Expectancy Calculations: 10‐Year CVD Risk Reduction for Women Aged 45 to 85.
Supporting info item


