Abstract
J Clin Hypertens (Greenwich). 2011;13:910–916. ©2011 Wiley Periodicals, Inc.
Anti‐inflammatory and pain therapies have been associated with blood pressure (BP) destabilization. Hence, the effects on BP of sumatriptan/naproxen sodium in fixed‐dose combination, sumatriptan 85 mg, and naproxen sodium 500 mg administered intermittently for the acute treatment of migraine attacks were assessed. Patients with migraine with or without aura and no history of hypertension were randomized to sumatriptan/naproxen sodium (n=135), sumatriptan (n=136), or naproxen sodium (n=136) to treat migraine attacks for 6 months in a double‐blind, parallel‐group trial. Following a treated migraine attack, patients performed 2 consecutive days of self‐measured BPs beginning ≥24 hours after the last dose of study medication and transmitted them by a transtelephonic modem. The primary end point was the change from baseline in self‐measured BP at 6 months. Changes in self‐measured BP from baseline to 6 months for sumatriptan/naproxen sodium were −2.1/−1.5 mm Hg (95% confidence intervals, −3.4 to −0.8 for systolic and −2.6 to −0.3 for diastolic). Mean changes from baseline in self‐measured BP did not differ among the 3 treatment groups. Additional categorical analyses did not show increases from baseline with sumatriptan/naproxen sodium relative to either of the monotherapy groups. Intermittent acute migraine treatment with sumatriptan/naproxen sodium for up to 6 months was associated with clinically insignificant decreases in self‐measured BP that were similar to those with sumatriptan or naproxen alone in normotensive patients with migraine. J Clin Hypertens (Greenwich). 2011;00:00–00. ©2011 Wiley Periodicals, Inc.
Sumatriptan/naproxen sodium is a single‐tablet combination therapy for the acute treatment of migraine that contains sumatriptan 85 mg formulated with rapid‐release technology (for fast disintegration and rapid release) and the nonsteroidal anti‐inflammatory drug (NSAID) naproxen sodium 500 mg. 1 , 2 , 3 Each of the individual components of sumatriptan/naproxen sodium has the potential to increase blood pressure (BP). Sumatriptan, a 5‐hydroxytryptamine receptor agonist, can cause elevations in BP of 4% to 6% in patients with or without a history of hypertension because of its vasoconstrictor effects. 4 , 5 Naproxen, an NSAID, can increase BP by 2 mm Hg to 4 mm Hg through renal sodium and water retention, an effect that can be accompanied by peripheral edema and weight gain. 6
In a single‐dose, crossover study in healthy volunteers, BP changes observed during 10 hours following sumatriptan/naproxen sodium did not differ from those observed after 100‐mg sumatriptan tablets. 7 , 8 The present study was conducted to extend these observations by determining whether sumatriptan/naproxen sodium affects BP with intermittent administration for the acute treatment of migraine during a prolonged period.
Methods
Design
The trial was a randomized, double‐blind, active‐controlled, parallel‐group study (Clinicaltrials.gov NCT00792636). Patients underwent a screening visit to ascertain eligibility and, after obtaining baseline BP measurements using a home monitoring system described below, a baseline visit to randomize to treatment. Following randomization, patients started a 6‐month treatment period during which they treated migraine episodes with study medication and performed self‐measured BPs starting at least 24 hours after dosing. The double‐blind treatment period was followed by a post‐treatment visit approximately 1 week after completion of the treatment period to evaluate safety to coincide with the elapse of approximately 5 half‐lives of naproxen.
Procedures
Patients were randomly assigned to sumatriptan/naproxen sodium 85 mg/500 mg, sumatriptan tablets 85 mg, or naproxen sodium tablets 500 mg and asked to use study medication to treat migraine episodes during the ensuing 6 months. For a given migraine episode, a second dose of study medication could be administered if relief of pain was inadequate and at least 2 hours had elapsed from the initial dose. If at least 2 hours had elapsed following a second dose of study medication and acceptable relief was not achieved, an alternative, noninvestigational, protocol‐permitted therapy designated by the investigator could be administered. Clinical assessments were performed in the clinic at months 1, 3, and 6 of the treatment period via a standard aneroid BP manometer. Self‐BP measurements were performed at baseline on nonmigraine days and during the treatment period according to the development of a migraine attack.
Patients
Patients eligible for the study were men or nonpregnant, nonlactating women aged 18 to 65 years with migraine headaches meeting International Headache Society (IHS) criteria for migraine with or without aura 9 and a history of ≥2 but ≤8 migraine attacks per month on average for the 6 months prior to the screening visit. Patients were excluded if they had ≥15 headache days per month; a history of hypertension or a screening BP of ≥130 mm Hg systolic or ≥85 mm Hg diastolic in 2 of 3 measurements; known or suspected cardiovascular or cerebrovascular disease; a history of cardiac arrhythmias requiring medication or of clinically significant electrocardiogram (ECG) abnormalities that, in the investigator’s opinion, contraindicated study participation; or a history of basilar, retinal, or hemiplegic migraine or secondary headaches. Other exclusion criteria included use within 3 months before trial initiation of ergots or ergot‐derived medications for migraine prophylaxis; use of a monoamine oxidase inhibitor, preparations containing St John’s Wort, or central nervous system stimulants from 2 weeks before the screening visit through 2 weeks after the end of study treatment; and current use of any anticoagulant or antiplatelet agent (except aspirin ≤325 mg/d). All patients provided written informed consent prior to study participation, and the trial was approved by institutional review boards for the 44 study sites in the United States.
Measures and Data Analysis
BP Monitoring Assessments At baseline, patients were trained to use a transtelephonic self‐measured BP device (A&D Medical UA‐767PBT, Abingdon, United Kingdom) to transfer BP measurement data from the patients’ homes to a central server via a wireless communications device port. Patients were instructed to use the same arm throughout the study and to refrain from consumption of substances that could induce a vasoactive effect in the 24‐hour period involving self‐BP measurements (eg, oral corticosteroids, nasal decongestants, alcohol, caffeine‐containing substances, and tobacco products).
Baseline self‐BP measurements were taken on 3 migraine‐free consecutive days at least 24 hours after use of any acute migraine treatment. On each of the 3 days of baseline measurements, patients performed 3 BP measurements in the morning and 3 in the evening for a total of 18 values. During the double‐blind treatment period, self‐BP measurements commenced a minimum of 24 hours after the last dose of study medication for each migraine attack. Patients then obtained 3 measures in the morning and 3 in the evening during a 2‐day period for a total of 12 values per migraine attack. Measurements were initiated in the morning or the evening as long as ≥24 hours had elapsed since the last dose of study medication. If a new migraine attack requiring treatment occurred during the 2‐day on‐treatment BP monitoring period, patients treated the migraine and delayed self‐BP measurements until 24 hours had elapsed since the last dose of study medication, at which point measurements were re‐initiated for 2 consecutive days. BP was also measured in duplicate in the clinic at months 1, 3, and 6 of the treatment period via a standardized auscultatory method.
Safety Assessments Safety measures included treatment‐emergent adverse events (any untoward medical occurrence with onset after the first dose of study medication) and results of ECGs, physical examinations, and clinical laboratory tests.
Statistical Analyses The primary end point was the mean change from baseline in self‐BP measurements at 6 months. The primary analysis examined treatment effects of sumatriptan/naproxen sodium using a mixed‐model repeated‐measures (MMRM) longitudinal analysis of the mean change from baseline in systolic BP at 6 months. Analyses were conducted on the intent‐to‐treat (ITT) population, defined as patients who took ≥1 dose of study medication and provided ≥1 valid post‐baseline self‐BP reading. The model included covariates of age, sex, baseline BP, and number of baseline migraine attacks per month. Because estimation of change in BP with clinically relevant precision (ie, the half‐width) was the study purpose, construction of 95% CIs was implemented as the method of statistical inference. Secondary key analyses were performed to examine changes in BP for each treatment group and to compare differences vs sumatriptan/naproxen sodium. Four subpopulation analyses of the ITT population were created and examined to demonstrate the robustness of primary analysis results. These were based on (1) baseline number of migraine attacks per month; (2) number of sumatriptan/naproxen sodium doses per migraine attack; (3) number of sumatriptan/naproxen sodium doses per month; and (4) total number of sumatriptan/naproxen sodium doses. For the primary BP end points, a separate MMRM analysis with corresponding 95% CIs was computed within each of the subgroups within the subpopulations defined by each of the 4 criteria above.
Other secondary end points included the percentage of patients withdrawn from the study for any reason related to BP and the proportion of patients with the following BP changes for any given 2‐day block during the study: (1) increase of ≥5 mm Hg from baseline systolic BP; (2) increase of ≥3 mm Hg from baseline diastolic BP; (3) systolic BP ≥140 mm Hg; or (4) diastolic BP ≥90 mm Hg. These values were derived as follows: (1) most antihypertensive agents are considered effective if they lower BP by ≥5/3 mm Hg, and (2) 140/90 mm Hg has been the conventional cut‐off value for hypertension. 10 Logistic regression analyses were performed to assess treatment differences using the 95% CI for the odds ratio (OR). Other secondary end points included the time to the first increase in daily average systolic BP ≥5 mm Hg or diastolic BP of ≥3 mm Hg from the baseline pressure. Evaluation of differences in treatments was performed using proportional hazards regression and the 95% CI for the hazard ratio.
Based on the assumption that the standard deviation for change in systolic BP from baseline would be 10 mm Hg both within and between treatment groups, it was estimated that 280 patients would be required to obtain a 95% CI half‐width of 4.0 mm Hg (a clinically relevant level of precision). Given the assumption that approximately 33% of randomized patients (20% of those randomized to sumatriptan/naproxen sodium, 30% of those randomized to sumatriptan 85 mg, and 50% of those randomized to naproxen sodium 500 mg) would discontinue the study before the end of the 6‐month treatment period, 2 it was estimated that 420 patients would need to be randomized for 280 patients to complete.
Results
Patient Enrollment and Disposition
Of 407 patients randomized, 135 received sumatriptan/naproxen sodium, 136 received sumatriptan, and 136 received naproxen (Figure 1). The proportion of patients who completed the study was higher in the sumatriptan/naproxen sodium group (50%) than the sumatriptan group (40%) or the naproxen group (40%). Reasons for premature withdrawal were similar among treatment groups with the exception of a higher frequency of withdrawal because of lack of efficacy with naproxen (10%) than with sumatriptan/naproxen sodium (2%) or sumatriptan (3%).
Figure 1.
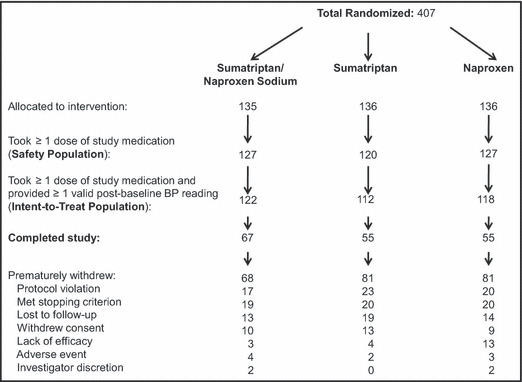
Disposition of patients during trial. BP indicates blood pressure.
Baseline characteristics including migraine type, age at migraine onset, frequency of migraine attacks, and previous treatments used for migraine were similar among treatment groups (Table I). Additionally, exposure to study medication and the mean number of doses of study medication used by patients in the 3 treatment groups were comparable.
Table I.
Demographics, Baseline Clinical Characteristics, and Exposure to Study Medication (Safety Population)
| Sumatriptan/Naproxen Sodium | Sumatriptan | Naproxen | |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Mean age, y (SD) | 38.9 (11.6) | 39.7 (11.2) | 39.2 (11.7) |
| Women, No. (%) | 98 (77) | 104 (87) | 105 (83) |
| Race, No. (%) | |||
| White | 112 (88) | 104 (87) | 113 (89) |
| Black | 13 (10) | 11 (9) | 11 (9) |
| Asian | 2 (2) | 3 (3) | 3 (2) |
| American Indian/Alaska native | 0 (0) | 1 (<1) | 0 (0) |
| Asian and white | 0 (0) | 1 (<1) | 0 (0) |
| Migraine type, No. (%) | |||
| With aura only | 32 (25) | 30 (25) | 37 (29) |
| Without aura only | 70 (55) | 67 (56) | 75 (59) |
| Both with and without aura | 25 (20) | 23 (19) | 15 (12) |
| Mean migraine attacks per mo (SD) | 4.5 (1.8) | 4.1 (1.7) | 4.6 (1.8) |
| Mean age at onset of migraine, y (SD) | 21.1 (10.8) | 20.0 (10.4) | 24.3 (11.7) |
| Mean clinic BP, mm Hg (SD) | |||
| Systolic | 108.3 (9.3) | 109.6 (9.6) | 108.6 (9.2) |
| Diastolic | 71.0 (6.9) | 71.6 (7.2) | 71.4 (7.3) |
| Mean self‐BP, mm Hg (SD) | |||
| Systolic | 111.7 (9.0) | 110.8 (9.2) | 110.1 (9.2) |
| Diastolic | 76.0 (6.8) | 75.7 (7.4) | 74.7 (7.0) |
| Exposure to study medication | |||
| Mean number of doses (SD) | 18.3 (15.5) | 19.0 (19.6) | 16.6 (13.6) |
| Mean number of days on treatment (SD) | 14.1 (11.4) | 14.8 (15.3) | 11.6 (9.3) |
Abbreviations: BP, blood pressure; SD, standard deviation.
Self‐BP Findings
Baseline mean systolic and diastolic BPs were similar among the treatment groups (Table I). The self‐BP monitoring data by month of treatment are shown in Figure 2. Mean (standard error) changes from baseline to 6 months in systolic and diastolic BPs are shown in Figure 3. Based on the least squares means from the MMRM analysis, small reductions from baseline to 6 months in BP were observed in all treatment groups. In the sumatriptan/naproxen sodium group, least squares mean changes from baseline to 6 months (95% CI) were −2.1 mm Hg (−3.4 to −0.8) for systolic and −1.5 mm Hg (−2.6 to −0.3) for diastolic. The respective values for the sumatriptan group were −1.7 mm Hg (−3.2 to −0.3) for systolic and −0.9 mm Hg (−2.2 to 0.3) for diastolic. The respective values for the naproxen group were −1.9 mm Hg (−3.4 to −0.4) for systolic and −0.7 mm Hg (−2.0 to 0.6) for diastolic. These 95% CIs for the least squares means are consistent with a small reduction in BP (ie, CI upper limit less than zero), with the exception of diastolic BP for sumatriptan and naproxen. Least squares mean changes from baseline to 6 months did not differ between sumatriptan/naproxen sodium and naproxen or between sumatriptan/naproxen sodium and sumatriptan for either systolic or diastolic BP, as shown by the 95% CIs for the difference in mean change between groups, which included zero for all comparisons (−1.7 to 2.2) mm Hg systolic and (−0.9 to 2.5) mm Hg diastolic and (−1.6 to 2.4) mm Hg systolic and (−1.2 to 2.2) mm Hg diastolic, respectively. Results in the subpopulations defined by numbers of migraine attacks per month at baseline, sumatriptan/naproxen sodium doses per migraine attack, sumatriptan/naproxen doses per month, and total sumatriptan/naproxen sodium doses are supportive of the primary results. Hence, migraine frequency, dose per attack (1 vs 2 tablets), cumulative dosing per month, and cumulative overall dosing did not show increased BP associated with increased dosing or increased attack frequency.
Figure 2.
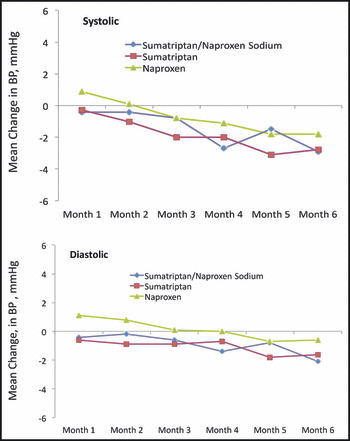
Mean changes from baseline in systolic and diastolic blood pressure (BP) over time by treatment group (intent‐to‐treat population).
Figure 3.
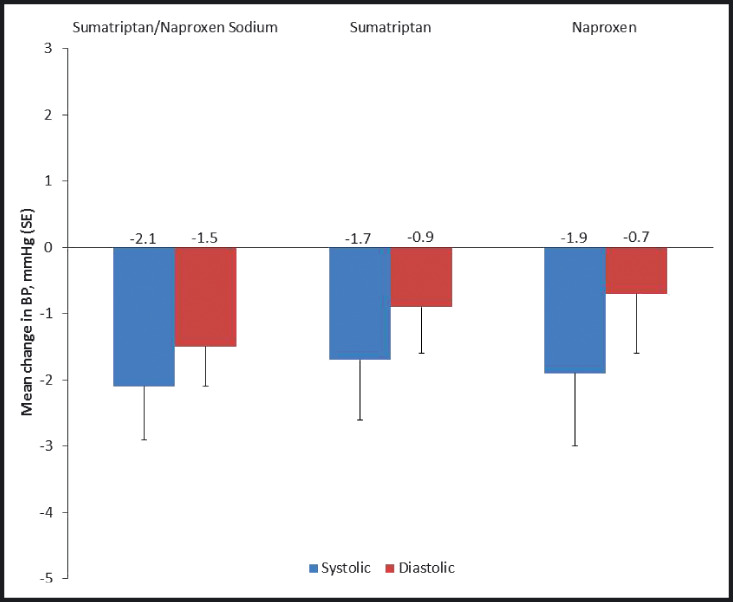
Mean (±standard deviation) changes from baseline in blood pressure (BP) at 6 months by treatment group (intent‐to‐treat population).
The time to the first day with an average systolic BP increase of ≥5 mm Hg from the baseline systolic BP was comparable between the sumatriptan/naproxen group and the sumatriptan group, with a hazard ratio of 1.14 and 95% CI of 0.84 to 1.54. The hazard ratio for naproxen vs sumatriptan/naproxen sodium was 1.36, with a 95% CI of 1.01 to 1.83. The time to the first day with an increase from baseline of ≥3 mm Hg in average diastolic BP was comparable between sumatriptan/naproxen sodium and each of the active comparators, with a hazard ratio of 0.88 and a 95% CI of 0.65 to 1.21 for sumatriptan and a hazard ratio of 1.18 with a 95% CI of 0.88 to 1.60 for naproxen.
Proportions of Patients With BP Elevations
The proportions of patients who had changes from baseline in BP ≥5/3 mm Hg or BP ≥140/90 mm Hg are shown in Table II. No differences in the proportions of patients with elevated BPs were observed between sumatriptan/naproxen sodium and either sumatriptan or naproxen sodium based on the 95% CIs for the OR, which included unity in both cases.
Table II.
Percentage of Patients With BP Values Exceeding Predefined Thresholds During the Double‐Blind Treatment Period (Intent‐to‐Treat Population)
| Average for 2‐Day Time Period | Treatment Group | Proportion n/N (%) | Difference vs Sumatriptan/Naproxen Sodium |
|---|---|---|---|
| ≥5 mm Hg increase in systolic BP | Sumatriptan/naproxen sodium | 53/120 (44) | – |
| Sumatriptan | 57/109 (52) | 8% | |
| Naproxen | 63/115 (55) | 11% | |
| ≥3 mm Hg increase in diastolic BP | Sumatriptan/naproxen sodium | 72/120 (60) | – |
| Sumatriptan | 65/109 (60) | 0% | |
| Naproxen | 77/115 (67) | 7% | |
| ≥140 mm Hg systolic BP | Sumatriptan/naproxen sodium | 2/122 (2) | – |
| Sumatriptan | 2/112 (2) | 0% | |
| Naproxen | 3/118 (3) | 1% | |
| ≥90 mm Hg diastolic BP | Sumatriptan/naproxen sodium | 10/122 (8) | – |
| Sumatriptan | 11/112 (10) | 2% | |
| Naproxen | 11/118 (9) | 1% |
Valid blood pressure (BP) measurements included those taken ≥24 hours following and ≤96 hours after each dose of study medication used to treat a migraine attack and taken prior to the onset of a subsequent individual migraine.
Adverse Events and Other Safety/Tolerability Findings
The percentages of patients with ≥1 treatment‐emergent adverse event were similar among the 3 treatment groups (Table III). Adverse events leading to discontinuation from the study occurred in 3% of the sumatriptan/naproxen sodium group, 2% in the sumatriptan group, and 2% in the naproxen group. No clinically meaningful changes in laboratory tests, vital signs, or ECGs were observed in any of the 3 treatment groups.
Table III.
Patients With Treatment‐Emergent Adverse Events Reported in ≥2% of Patients by Treatment Group (Safety Population)
| Sumatriptan/Naproxen Sodium (n=127) | Sumatriptan (n=120) | Naproxen (n=127) | |
|---|---|---|---|
| Any adverse event | 46 (36) | 46 (38) | 37 (29) |
| Adverse events in ≥2% of patients | 20 (16) | 29 (24) | 18 (14) |
| Nasopharyngitis | 4 (3) | 6 (5) | 5 (4) |
| Urinary tract infection | 1 (<1) | 6 (5) | 3 (2) |
| Sinusitis | 3 (2) | 2 (2) | 1 (<1) |
| Upper respiratory tract infection | 0 (0) | 5 (4) | 1 (<1) |
| Dizziness | 5 (4) | 5 (4) | 1 (<1) |
| Somnolence | 2 (2) | 3 (3) | 2 (2) |
| Back pain | 2 (2) | 3 (3) | 2 (2) |
| Neck pain | 2 (2) | 4 (3) | 0 (0) |
| Nausea | 4 (3) | 2 (2) | 1 (<1) |
| Seasonal allergy | 3 (2) | 1 (<1) | 3 (2) |
| Sinus congestion | 0 (0) | 3 (3) | 1 (<1) |
Values are expressed as number (percentage).
Discussion
Sumatriptan/naproxen sodium used for intermittent treatment of migraine during a 6‐month period was associated with small, clinically insignificant decreases in systolic and diastolic home BPs at 6 months in patients with baseline BP values ≤130/80 mm Hg. Reductions in BP of similar magnitudes were observed for sumatriptan and naproxen sodium monotherapy. Furthermore, no differences in the proportions of patients with elevated BPs (defined as changes from baseline ≥5/3 mm Hg or ≥140/90 mm Hg) were observed between sumatriptan/naproxen sodium and either sumatriptan or naproxen sodium. The tendency for home BP to decline over time across treatment groups may reflect regression to the mean, 11 , 12 a possibility that cannot be excluded in the absence of a placebo arm. Alternatively, lower home BP values may have been a beneficial effect of regularly aborting the migraine process and reducing migraine pain and associated symptoms. Effective treatment of migraine attacks over time could also reduce BP by alleviating anxiety associated with uncontrolled migraine—a possible dual CNS effect of serotonergic intervention.
Strengths and Limitations
The use of multiple readings through transtelephonic home BP recordings allowed for the collection of numerous BP readings (>55,000), which maximized the likelihood of detecting small changes in BP. This technology has been shown to be more reproducible and representative of 24‐hour average BP than clinic measurements. 13 , 14 There is consensus that self‐measurement of BP is useful for clinical trials because it allows multiple recordings of BP; allows for evaluation in association with acute, intermittent dosing of drugs or following symptoms of disease or illness; and is associated with satisfactory compliance by study patients. 15 , 16 Self‐monitoring of BP was an ideal tool for the present study since the individual patients could trigger a set of readings at any time during the trial based on the need to administer study drug acutely to treat a given migraine attack.
Criteria for participation in the present study were similar to those of previous sumatriptan/naproxen sodium studies, and the demographic and clinical characteristics of the study population were representative of the general population of migraine patients. 17 , 18 As most migraine patients are relatively young women who are otherwise healthy and have a low rate of use of antihypertensive therapies, 18 the exclusion of antihypertensive drugs should have little impact on the generalizability of the trial to a broader patient population.
While the study findings are limited by a dropout rate that was higher than expected, the sample size remained sufficient to accomplish the specific study objectives to support clinical interpretation. An unusually and unexpectedly large proportion of patients met the protocol‐defined stopping criteria of insufficient migraine attacks, defined as experiencing <2 migraines per month during the first 3 months of the trial (13% sumatriptan/naproxen sodium, 10% sumatriptan, and 13% naproxen sodium). Patients experiencing up to 8 migraines per month were eligible for this study. However, the exclusion of patients using antihypertensives may have reduced the number of those experiencing a higher monthly frequency of attacks within the study population as antihypertensives are common migraine prophylactic agents and prophylaxis is generally recommended for migraine patients experiencing ≥4 attacks per month. The incidence of withdrawal in this study was higher than that observed in a previous adult sumatriptan/naproxen sodium 12‐month safety trial. 19 The target sample size for completed patients was not obtained (target of 280 vs actual of 177). Nevertheless, the goal of estimating the mean change in home BP with adequate precision to allow small changes in BP to be detected was maintained for the individual treatment groups, as well as for the between‐group treatment differences, by using a model‐based analytical approach that imputes data missing due to dropouts. In addition, smaller‐than‐expected standard deviations (5.2 mm Hg actual vs 10.0 mm Hg predicted) contributed to maintaining adequately narrow CIs. These reductions in variance are due to the overall large volume of home BP measurements collected and used to compute monthly average BPs. Another clinical limitation of the study is the exclusion of patients with higher ranges of “prehypertension” (ie, 131–139/81–89 mm Hg). While it is likely that these patients would respond similarly to the study patients with lower baseline BPs, this cannot be proven based on our findings.
Conclusions
The use of sumatriptan/naproxen sodium for the acute, intermittent treatment of migraine for up to 6 months did not produce clinically important changes in BP among nonhypertensive migraine patients, and the effects on BP by the combination agent were not different from the individual components.
Acknowledgments
Acknowledgements and disclosures: The authors acknowledge Jane Saiers, PhD, for assistance in manuscript preparation. The editorial assistance was funded by GlaxoSmithKline. Dr William White, lead author of the paper, has received research funding from the National Institutes of Health; Novartis Pharmaceuticals, Inc; and Pfizer, Inc (both independent research grants) between 2008 and present. He has also received consulting fees for participation in steering committees, data safety monitoring boards, and cardiovascular adjudication and advisory committees from the following sponsors: Abbott Immunology, Astra‐Zeneca, Astellas, Biosante, Forest Research Institute, GlaxoSmithKline, Roche, Takeda Global Research. Dr Derosier, Ms Thompson, Dr Adams, and Mr Goodman are all full‐time employees of GlaxoSmithKline. All authors had full access to the data and participated in the writing and/or editing of this paper. No author received payment for manuscript writing/editing. This study was funded by GlaxoSmithKline, Research Triangle Park, NC.
References
- 1. Cleves C, Tepper SJ. Sumatriptan/naproxen sodium combination for the treatment of migraine. Expert Rev Neurother. 2008;8:1289–1297. [DOI] [PubMed] [Google Scholar]
- 2. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan‐naproxen for acute treatment of migraine. JAMA. 2007;297:1443–1454. [DOI] [PubMed] [Google Scholar]
- 3. Winner P, Cady RK, Ruoff GE, et al. Twelve‐month tolerability and safety of sumatriptan‐naproxen sodium for the treatment of acute migraine. Mayo Clin Proc. 2007;82:61–68. [DOI] [PubMed] [Google Scholar]
- 4. Fowler PA, Lacey LF, Thomas M, et al. The clinical pharmacology, pharmacokinetics and metabolism of sumatriptan. Eur Neurol. 1991;31:291–294. [DOI] [PubMed] [Google Scholar]
- 5. Imitrex (sumatriptan tablets) prescribing information. GlaxoSmithKline, Research Triangle Park, NC. February 2010.
- 6. Antman EM, Bennett JS, Daugherty A, et al. Use of nonsteroidal anti‐inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. [DOI] [PubMed] [Google Scholar]
- 7. Treximet (sumatriptan and naproxen sodium) prescribing information. GlaxoSmithKline, Research Triangle Park, NC. December 2009. Accessed at : http://www.gsk‐clinicalstudyregister.com/quick‐search‐list.jsp?tab=results&letterrange=All&type=GSK+Study+ID&item=106396&studyType=All&phase=All&status=All&population=All&marketing=All&country=All&studyId=106396 18Sept2011.
- 8. Haberer LJ, Walls CM, Lener SE, et al. Distinct pharmacokinetic profile and safety of a fixed‐dose tablet of sumatriptan and naproxen sodium for the acute treatment of migraine. Headache. 2010;50:357–373. [DOI] [PubMed] [Google Scholar]
- 9. Headache Classification Committee of the International Headache Society . The international classification of headache disorders, 2nd ed. Cephalalgia. 2004;1:9–160. [DOI] [PubMed] [Google Scholar]
- 10. Chobanian AV, Bakris GL, Black HR, et al . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 11. Morton V, Torgerson DJ. Regression to the mean: treatment effect without the intervention. J Eval Clin Pract. 2005;11:59–65. [DOI] [PubMed] [Google Scholar]
- 12. White WB, Mehrotra DV, Black HR, et al. Effects of controlled‐onset extended‐release verapamil on nocturnal blood pressure (dippers vs nondippers). COER‐Verapamil Study Group. Am J Cardiol. 1997;80:469–474. [DOI] [PubMed] [Google Scholar]
- 13. Denolle T, Waeber B, Kieldsen S, et al. Self‐measurement of blood pressure in clinical trials and therapeutic applications. Blood Press Monit. 2000;5:145–149. [PubMed] [Google Scholar]
- 14. Pickering TG, White WB. ASH position paper. When and how to use self (home) and ambulatory blood pressure monitoring. J Clin Hypertens. 2008;10:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baquet JP, Mallion JM. Self‐monitoring of blood pressure should be used in clinical trials. Blood Press Monit. 2002;7:55–59. [DOI] [PubMed] [Google Scholar]
- 16. White WB, Salzman P, Schwid SR, et al. Transtelephonic home blood pressure to assess the monoamine oxidase‐B inhibitor rasagiline in Parkinson disease. Hypertension. 2008;52:587–593. [DOI] [PubMed] [Google Scholar]
- 17. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention Study. Headache. 2007;47:355–363. [DOI] [PubMed] [Google Scholar]
- 18. Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol. 2008;7:354–361. [DOI] [PubMed] [Google Scholar]
- 19. Winner P, Cady RK, Ruoff GE, et al. Twelve‐month tolerability and safety of sumatriptan‐naproxen sodium for the treatment of acute migraine. Mayo Clin Proc. 2007;82:61–68. [DOI] [PubMed] [Google Scholar]


