Abstract
J Clin Hypertens (Greenwich). 2011;13:658–661. ©2011 Wiley Periodicals, Inc.
Key Points
-
•
Central sympatholytic drugs reduce blood pressure mainly by stimulating central α2‐adrenergic receptors in the brainstem centers, thereby reducing sympathetic nerve activity and neuronal release of norepinephrine to the heart and peripheral circulation.
-
•
This class of drugs, however, is currently used mainly as fourth‐line (or beyond) drug therapy for hypertension because of side effects of drowsiness, fatigue, and dry mouth.
-
•
Rebound hypertension is also another major concern in certain drugs with a short half‐life, particularly in patients who are nonadherent to the regimen. Therefore, their use on a “PRN” basis for treatment of blood pressure surge in the absence of symptoms or acute target complications should also be avoided.
Central sympatholytic agents were first introduced into clinical use in the 1960s. α‐Methyldopa, the first drug to be widely used, is the only prodrug in this class. It is converted to α‐methylnorepinephrine, which was at first thought to act peripherally as a false neurotransmitter. Clonidine, the prototype of the second‐generation drugs in this class, all of which are imidazoline derivatives, was initially developed as a nasal decongestant because of its potential vasoconstrictor effect via postsynaptic α2‐adrenergic receptor (AR) stimulation but was surprisingly found to have antihypertensive effects via activation of presynaptic α2‐AR in the brainstem. 1 A similar effect of centrally formed α‐methylnorepinephrine is now understood to account for the blood pressure (BP)–lowering effect of methyldopa. Subsequently, other direct‐acting central sympatholytic drugs such as guanfacine and guanabenz were approved for treatment of hypertension in the United States. Moxonidine and rilmenidine are also centrally acting drugs used in England and other European countries but are not available in the United States. In contrast to clonidine and other α2 agonists, moxonidine and rilmenidine predominantly reduce sympathetic nerve activity (SNA) and BP by stimulating imidazoline‐1 (I1) receptor, rather than α2‐AR in the brainstem. 2
Pharmacology
The pharmacokinetic profile of central sympatholytic agents is shown in the Table. Central sympatholytic drugs reduce BP mainly by activation of α2‐AR in the rostral ventrolateral medulla (Figure), resulting in decreased SNA 3 , 4 and, to a lesser extent, by inhibition of presynaptic release of norepinephrine from peripheral sympathetic nerve terminals. 3 Activation of I1 receptors in the brainstem centers also contributes to sympatholytic action and BP‐lowering effects of all the second‐generation drugs (ie, except for methyldopa), independent of central α2‐ARs. 5 Clonidine activates both I1 receptors and α2‐ARs. Guanfacine and guanabenz are more selective for α2‐AR than clonidine.
Table TABLE.
Dose Range and Pharmacokinetics of Central Sympatholytic Drugs
| Drug | Total Dose Range, mg/d | Doses Per Day | Tmax, h | Half‐Life, h | Renal Elimination, % |
|---|---|---|---|---|---|
| Clonidine | 0.2–1.2 | 2–3 | 1–4 | 6–16 | 40–60 |
| Clonidine patch | 0.1–0.6 | Weekly | 72 | 14–26 | 40–60 |
| Guanabenz | 8–32 | 2 | 2–5 | 6–14 | <5 |
| Guanfacine | 1–3 | 1 | 1–4 | 10–30 | 50 |
| Methyldopa | 250–2000 | 2 | 2–4 | 1–2 | 70 |
| Moxonidinea | 0.2–0.6 | 1–2 | 1.0–1.5 | 2–3 | 50–75 |
| Rilmenidinea | 1–2 | 1 | 1.7 | 8.5 | 52–93 |
Abbreviation: Tmax, time to peak plasma concentration. aNot available in the United States.
Figure FIGURE.
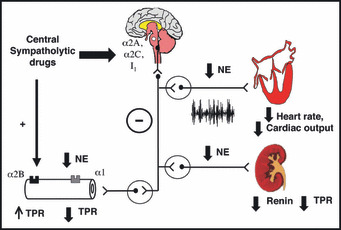
Illustration of the mechanisms underlying cardiovascular effects of central sympatholytic drugs. Central sympatholytic drugs inhibit central sympathetic discharge via activation of α2A, α2C, or imidazoline 1 (I1) receptors in the brainstem, resulting in decreased heart rate, cardiac output, and total peripheral resistance (TPR). Activation of prejunctional α2A and α2C receptors causing inhibition of norepinephrine (NE) release from peripheral sympathetic nerve terminals also contributes to reduction in blood pressure (not shown in the Figure). Activation of α2B receptors, which are located postjunctionally in the vascular smooth muscle may cause transient vasoconstriction and blood pressure elevation during rapid drug administration.
Clonidine
Clonidine has been approved for treatment of hypertension in the United States since 1974. Following oral administration, plasma levels of clonidine peak in approximately 1 to 4 hours and the plasma half‐life ranges from 6 to 16 hours. 6 , 7 The half‐life increases up to 41 hours in patients with severe impairment of renal function. About 40% to 60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. About 50% of the absorbed dose is metabolized in the liver. An antihypertensive effect of clonidine is usually observed within 30 to 60 minutes after an oral dose. The peak effect occurs within 2 to 4 hours, with a duration of action lasting for approximately 6 to 8 hours. 6 Therefore, the drug should be given 2 to 3 times a day.
Treatment with oral clonidine has been shown to decrease resting cardiac output by 15% to 20% without affecting total peripheral resistance (TPR) in hypertensive patients. 8 , 9 Reduction in heart rate, rather than stroke volume, is mainly responsible for decreased resting cardiac output. Reduction in plasma renin activity and urinary aldosterone excretion is also observed after clonidine, likely related to reduction in renal SNA. 9 , 10 , 11
Clonidine can also be administered via a 7‐day transdermal system. Plasma clonidine levels increase more gradually with the clonidine patch than with oral clonidine, reaching steady‐state concentration within 3 days of application. 12 When the patch is removed, plasma clonidine concentration remains unchanged for 8 to 12 hours, suggesting that skin acts as a temporary drug reservoir. 11 , 12 BP‐lowering effects of the transdermal system are comparable to that with the oral route. However, skin reactions are a troublesome side effect of the clonidine patch, occurring in 20% to 25% of patients. 13 Pretreatment with hydrocortisone cream may reduce the incidence of dermatitis without affecting clonidine delivery to the circulation. 14
Abrupt cessation of clonidine is known to precipitate symptoms of sympathetic overactivity, such as anxiety, tremor, headache, palpitation, and rebound hypertension within 36 to 72 hours of discontinuation. 15 , 16 A rise in BP above pretreatment values is less common. Nevertheless, oral clonidine should be gradually weaned when therapy is discontinued and it should not be given as a once‐daily regimen or on an as‐needed basis. Rebound hypertension occurs less frequently with the transdermal system because of more gradual reduction in clonidine levels. The rebound phenomenon may be exaggerated in patients concurrently treated with β‐AR blockers due to unopposed α‐adrenergic vasoconstriction, and β‐AR blockade should never be initiated by itself in withdrawing patients. Therapy for rebound includes treatment with α‐AR blockers (or with combined α‐ and β‐AR blockers if tachycardia is severe) or reinstitution of clonidine.
Guanfacine
Guanfacine is an α2 agonist with 12 to 25 times higher selectivity than clonidine for the α2‐AR vs the α1‐AR. 17 , 18 In individuals with normal renal function, the average elimination half‐life is approximately 17 hours. Peak plasma concentrations occur from 1 to 4 hours after single oral doses. Steady‐state blood levels are achieved within 4 days in most patients. In contrast to clonidine, studies in hypertensive patients have suggested that guanfacine reduces BP mainly by reducing total vascular resistance rather than cardiac output. 19 , 20 Guanfacine reduces resting heart rate with minimal effect on heart rate during exercise. 19 In contrast, guanfacine reduces TPR similarly both at rest and during exercise with minimal effects on cardiac output. 19 Guanfacine is typically administered once daily at bedtime. Most of the side effects of guanfacine, such as dry mouth, sedation, and fatigue, are similar to those seen with clonidine. Rebound hypertension, however, occurs less frequently than clonidine because of its longer half‐life. 21 Although guanfacine is US Food and Drug Administration–approved only for treatment of hypertension, it has also been used for treatment of attention deficit/hyperactivity disorder in children and young adults. 22
α‐Methyldopa
In contrast to clonidine and guanfacine, α‐methyldopa does not directly reduce BP but, as noted earlier, first requires conversion to α‐methylnorepinephrine in the central nervous system, which, in turn, leads to activation of central α2‐ARs and inhibition of sympathetic outflow. Oral bioavailability of methyldopa is about 25%, with an average time to reach maximum plasma concentration of 2 hours. Half‐life on average is between 1 and 2 hours in healthy patients and hypertensive patients 23 , 24 but is increased to 4 to 6 hours in patients with renal failure, resulting in a prolonged hypotensive action in these patients. 25 Following oral administration, an effect of α‐methyldopa on BP is detectable within 1 hour, reaching a peak effect within 6 to 8 hours. 23 Plasma renin activity is also reduced during α‐methyldopa treatment. 26 The usual daily dosage of α‐methyldopa is 500 mg to 2 g, divided into 2 to 4 doses. In hypertensive patients, methyldopa reduces BP mainly by reducing TPR with minimal effects on heart rate or cardiac output. 23 Although once a mainstay of antihypertensive therapy, α‐methyldopa is currently used mainly in pregnant women with hypertension because of lack of teratogenicity or fetal side effects. Maternal cardiac output, uterine blood flow, and renal blood flow are also unaffected by α‐methyldopa. 27 Side effects of α‐methyldopa requiring drug discontinuation are relatively infrequent. Sedation and fatigue may occur but are usually transient. Other side effects include positive Coombs test, drug‐induced fever, and pancreatitis, hemolytic anemia, hepatic dysfunction, nasal congestion, exacerbation of Parkinsonism, hyperprolactinemia, and gynecomastia.
Guanabenz
Guanabenz is another direct central α2‐agonist, which is predominantly eliminated via hepatic biotransformation. 28 , 29 Thus, unlike clonidine, dose adjustment is not required in patients with renal failure but required in those with chronic liver diseases. Guanabenz has been shown to be effective in reducing left ventricular hypertrophy in hypertensive patients 4 and in attenuating morning hypertension when administered at nighttime. 30 The half‐life of guanabenz is between 12 and 14 hours and the drug is typically given twice daily at a dosage between 8 mg/d and 32 mg/d. 31 Side effects of guanabenz are similar to those of clonidine. 31
Summary
Central sympatholytic drugs are effective in lowering BP by decreasing sympathetic outflow to the heart and peripheral circulation. Long‐term use of central sympatholytic drugs has been shown to reduce target organ complications but it is unclear whether these drugs reduce cardiovascular risk beyond their BP‐lowering effect. Perioperative use of central α2‐agonists in patients with high cardiovascular risk undergoing cardiac or noncardiac surgery appears to reduce cardiac complications and should be considered in patients who cannot tolerate β‐blockers. Because of lack of clear‐cut cardiovascular benefit and several major side effects, including fatigue, sedation, and dry mouth, these drugs should be used as fourth‐line drug therapy for hypertension. Central sympatholytic drugs should also be used cautiously in patients with autonomic failure with supine hypertension due to a potential direct vasoconstrictor effect mediated by vascular postsynaptic α2 receptors. Central sympatholytic drug use should be avoided in patients who are nonadherent to treatment because of precipitation of withdrawal symptoms on abrupt drug discontinuation. Their use on a “PRN” basis for treatment of episodes of BP elevation in the absence of symptoms or acute target complications should also be discouraged because of potential rebound hypertension. In patients who are adherent to treatment, central sympatholytic drugs remain an important add‐on therapy for hypertension, particularly if associated with overactivation of the sympathetic nervous system (usually characterized by elevated heart rate or cardiac output) 32 or for hypertension associated with impairment in carotid or aortic baroreceptor–mediated inhibition of central sympathetic outflow. 33
Other Sympatholytic Drugs
Reserpine has been extensively used in the past as an effective antihypertensive agent especially at lower doses and combined with thiazide‐type diuretics. 34 , 35 Unlike sympatholytic drugs mentioned above, reserpine reduces BP by depleting norepinephrine stores in the peripheral postganglionic sympathetic nerve terminals without reducing central sympathetic discharge. 36 Although higher doses (0.75 mg/d to 10 mg/d) have been associated with significant side effects, including nasal stuffiness, peptic ulcer disease, and depression, its long action and effectiveness make it an available useful agent for antihypertensive therapy, particularly at lower doses (0.1 mg/d to 0.5 mg/d). 34 , 35
Disclosure: The authors received no honoraria for their contribution to this issue.
References
- 1. DeQuattro V, Li D. Sympatholytic therapy in primary hypertension: a user friendly role for the future. J Hum Hypertens. 2002;16(Suppl 1):S118–S123. [DOI] [PubMed] [Google Scholar]
- 2. Fenton C, Keating GM, Lyseng‐Williamson KA. Moxonidine: a review of its use in essential hypertension. Drugs. 2006;66:477–496. [DOI] [PubMed] [Google Scholar]
- 3. Wallin BG, Frisk‐Holmberg M. The antihypertensive mechanism of clonidine in man. Evidence against a generalized reduction of sympathetic activity. Hypertension. 1981;3:340–346. [DOI] [PubMed] [Google Scholar]
- 4. Miyajima E, Shigemasa T, Endo T, et al. Guanabenz combination therapy inhibits sympathetic nerve activity and regresses left ventricular hypertrophy. Cardiovasc Drugs Ther. 2000;14:61–66. [DOI] [PubMed] [Google Scholar]
- 5. Szabo B. Imidazoline antihypertensive drugs: a critical review on their mechanism of action. Pharmacol Ther. 2002;93:1–35. [DOI] [PubMed] [Google Scholar]
- 6. Anavekar SN, Howes LG, Jarrott B, et al. Pharmacokinetics and antihypertensive effects of low dose clonidine during chronic therapy. J Clin Pharmacol. 1989;29:321–326. [DOI] [PubMed] [Google Scholar]
- 7. Louis WJ, McNeil JJ, Anavekar SN, et al. Comparison of pharmacokinetics and pharmacodynamics of adrenoceptor agonists and antagonists as antihypertensive agents. J Cardiovasc Pharmacol. 1987;10(Suppl 12):S100–S103. [PubMed] [Google Scholar]
- 8. Lund‐Johansen P. Hemodynamic changes at rest and during exercise in long‐term clonidine therapy of essential hypertension. Acta Med Scand. 1974;195:111–115. [DOI] [PubMed] [Google Scholar]
- 9. Farsang C, Varga K, Vajda L, et al. Effects of clonidine and guanfacine in essential hypertension. Clin Pharmacol Ther. 1984;36:588–594. [DOI] [PubMed] [Google Scholar]
- 10. Weber MA, Drayer JI, Laragh JH. The effects of clonidine and propranolol, separately and in combination, on blood pressure and plasma renin activity in essential hypertension. J Clin Pharmacol. 1978;18:233–240. [DOI] [PubMed] [Google Scholar]
- 11. Lowenthal DT, Matzek KM, MacGregor TR. Clinical pharmacokinetics of clonidine. Clin Pharmacokinet. 1988;14:287–310. [DOI] [PubMed] [Google Scholar]
- 12. MacGregor TR, Matzek KM, Keirns JJ, et al. Pharmacokinetics of transdermally delivered clonidine. Clin Pharmacol Ther. 1985;38:278–284. [DOI] [PubMed] [Google Scholar]
- 13. One year efficacy and tolerability of clonidine administered by the transdermal route in patients with mild to moderate essential hypertension – a multicentre open label study. The Antihypertensive Patch Italian Study (APIS) Investigators. Clin Auton Res. 1993;3:379–383. [PubMed] [Google Scholar]
- 14. Ito MK, O’Connor DT. Skin pretreatment and the use of transdermal clonidine. Am J Med. 1991;91:42S–49S. [DOI] [PubMed] [Google Scholar]
- 15. Weber MA. Discontinuation syndrome following cessation of treatment with clonidine and other antihypertensive agents. J Cardiovasc Pharmacol. 1980;2(Suppl 1):S73–S89. [DOI] [PubMed] [Google Scholar]
- 16. Karachalios GN, Charalabopoulos A, Papalimneou V, et al. Withdrawal syndrome following cessation of antihypertensive drug therapy. Int J Clin Pract. 2005;59:562–570. [DOI] [PubMed] [Google Scholar]
- 17. Sorkin EM, Heel RC. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31:301–336. [DOI] [PubMed] [Google Scholar]
- 18. Seedat YK. Clonidine and guanfacine‐‐comparison of their effects on haemodynamics in hypertension. S Afr Med J. 1985;67:557–559. [PubMed] [Google Scholar]
- 19. Magometschnigg D, Hitzenberger G, Bonelli J. Haemodynamic effects of guanfacine. Br J Clin Pharmacol. 1980;10(Suppl 1):125S–131S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schafer N, Rauh J, Rosenthal J. Haemodynamics in hypertensive patients before and during guanfacine treatment. Br J Clin Pharmacol. 1980;10(Suppl 1):133S–135S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson MF, Haring O, Lewin A, et al. Comparison of guanfacine versus clonidine for efficacy, safety and occurrence of withdrawal syndrome in step‐2 treatment of mild to moderate essential hypertension. Am J Cardiol. 1986;57:43E–49E. [DOI] [PubMed] [Google Scholar]
- 22. Kisicki JC, Fiske K, Lyne A. Phase I, double‐blind, randomized, placebo‐controlled, dose‐escalation study of the effects on blood pressure of abrupt cessation versus taper down of guanfacine extended‐release tablets in adults aged 19 to 24 years. Clin Ther. 2007;29:1967–1979. [DOI] [PubMed] [Google Scholar]
- 23. Bobik A, Jennings G, Jackman G, et al. Evidence for a predominantly central hypotensive effect of alpha‐methyldopa in humans. Hypertension. 1986;8:16–23. [DOI] [PubMed] [Google Scholar]
- 24. Myhre E, Rugstad HE, Hansen T. Clinical pharmacokinetics of methyldopa. Clin Pharmacokinet. 1982;7:221–233. [DOI] [PubMed] [Google Scholar]
- 25. Myhre E, Stenbaek O, Rugstad HE, et al. Pharmacokinetics of methyldopa in renal failure and bilaterally nephrectomized patients. Scand J Urol Nephrol. 1982;16:257–263. [DOI] [PubMed] [Google Scholar]
- 26. Mohammed S, Fasola AF, Privitera PJ, et al. Effect of methyldopa on plasma renin activity in man. Circ Res. 1969;25:543–548. [DOI] [PubMed] [Google Scholar]
- 27. Montan S, Anandakumar C, Arulkumaran S, et al. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy‐induced hypertension. Am J Obstet Gynecol. 1993;168:152–156. [DOI] [PubMed] [Google Scholar]
- 28. Sica DA. Centrally acting antihypertensive agents: an update. J Clin Hypertens (Greenwich). 2007;9:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lasseter KC, Shapse D, Pascucci VL, Chiang ST. Pharmacokinetics of guanabenz in patients with impaired liver function. J Cardiovasc Pharmacol. 1984;6(Suppl 5):S766–S770. [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto J, Chonan K, Aoki Y, et al. Therapeutic effects of evening administration of guanabenz and clonidine on morning hypertension: evaluation using home‐based blood pressure measurements. J Hypertens. 2003;21:805–811. [DOI] [PubMed] [Google Scholar]
- 31. Holmes B, Brogden RN, Heel RC, et al. Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs. 1983;26:212–229. [DOI] [PubMed] [Google Scholar]
- 32. Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–988. [DOI] [PubMed] [Google Scholar]
- 33. Robertson D, Hollister AS, Biaggioni I, et al. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329:1449–1455. [DOI] [PubMed] [Google Scholar]
- 34. Shamon SD, Perez MI. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst Rev. 2009:CD007655. [DOI] [PubMed] [Google Scholar]
- 35. Kostis JB, Berge KG, Davis BR, et al. Effect of atenolol and reserpine on selected events in the systolic hypertension in the elderly program (SHEP). Am J Hypertens. 1995;8:1147–1153. [DOI] [PubMed] [Google Scholar]
- 36. Pernow J, Thoren P, Millberg BI, et al. Renal sympathetic nerve activation in relation to reserpine‐induced depletion of neuropeptide Y in the kidney of the rat. Acta Physiol Scand. 1988;134:53–59. [DOI] [PubMed] [Google Scholar]


