Abstract
J Clin Hypertens (Greenwich). 2011;13:873–880. ©2011 Wiley Periodicals, Inc.
This 12‐week, multicenter, randomized, double‐blinded, 4‐arm study in 440 patients with moderate to severe hypertension compared ambulatory blood pressure (ABP) responses with a triple‐combination regimen (olmesartan medoxomil [OM] 40 mg, amlodipine besylate [AML] 10 mg, and hydrochlorothiazide [HCTZ] 25 mg) and its component dual‐combination regimens at similar doses. At week 12, the triple combination resulted in a greater reduction in mean 24‐hour systolic and diastolic blood pressure (−30.3/−18.0 mm Hg) compared with the 3 dual‐combination regimens (OM 40 mg/AML 10 mg: −23.5/−13.9, OM 40 mg/HCTZ 25 mg: −23.9/−14.5, and AML 10 mg/HCTZ 25 mg: −18.5 mm Hg/−10.7 mm Hg; P<.0001 each). Greater efficacy was also found during daytime and nighttime hours and during the last 6, 4, or 2 hours of the dosing interval. The authors conclude that the triple combination of OM 40 mg/AML 10 mg/HCTZ 25 mg demonstrated superior efficacy and sustained reductions in ABP compared with its dual‐combination components.
Approximately half of the people with hypertension in the United States do not have adequate blood pressure (BP) control (systolic BP [SBP] <140 mm Hg and diastolic BP [DBP] <90 mm Hg). 1 Achieving this level of control typically requires the use of ≥2 classes of antihypertensive drugs 2 , 3 , 4 that have complementary mechanisms of action. Ideal combinations should also incorporate component drugs with sufficient durations of action in order to reduce the morning surge in BP and the early morning peak in myocardial infarction and stroke. 5 , 6 , 7 In order to study such effects, it is necessary to use ambulatory BP monitoring (ABPM), which not only distinguishes sustained hypertension from white‐coat hypertension, but also allows the study of diurnal variation and true 24‐hour drug efficacy. 2 , 7 , 8 , 9 , 10 , 11
The Triple Therapy With Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients Study (TRINITY) demonstrated that a 3‐drug regimen of olmesartan medoxomil (OM) 40 mg, amlodipine besylate (AML) 10 mg, and hydrochlorothiazide (HCTZ) 25 mg reduced office seated DBP (SeDBP) and SBP (SeSBP) to a greater degree and achieved a higher rate of BP goal attainment than any of the 3 component dual‐combinations. 12 The objective of this prespecified TRINITY substudy was to compare 24‐hour BP patterns and control rates at baseline and week 12 in a nested subgroup. Specific measured variables included mean daytime (8 am–4 pm) and nighttime (10 pm–6 am) BP values, with additional comparisons among treatments during the final 6, 4, and 2 hours of the dosing interval.
Methods
Study Design
Methods for TRINITY have previously been described in detail. 12 This multicenter (N=317), randomized, double‐blind, 12‐week, parallel‐group study compared the triple‐combination regimen (OM 40 mg/AML 10 mg/HCTZ 25 mg) with its component dual‐combination regimens in patients with moderate to severe hypertension. To be eligible for randomization, all patients had to be 18 years or older and had to have a mean SeBP ≥140/100 mm Hg (SeSBP ≥140 mm Hg and SeDBP ≥100 mm Hg) or SeBP ≥160/90 mm Hg (SeSBP ≥160 mm Hg and SeDBP ≥90 mm Hg) at two consecutive visits during the washout period. Excluded were patients with secondary hypertension, recent (≤6 months) cerebrovascular or coronary artery disease, New York Heart Association class III or IV heart failure, uncontrolled diabetes (defined as glycated hemoglobin >9%), or severe renal insufficiency (defined as creatinine clearance <30 mL/min).
After providing written informed consent, eligible patients (stratified by age, race, and diabetes status) were randomized to 1 of 4 treatment groups that included active drug therapy between weeks 4 and 12. Preparations used were OM 40 mg/AML 10 mg administered as a fixed‐dose combination, OM 40 mg/HCTZ 25 mg administered as a fixed‐dose combination, AML 10 mg plus HCTZ 25 mg, or OM 40 mg/AML 10 mg/HCTZ 25 mg administered as OM 40 mg/HCTZ 25 mg fixed‐dose combination plus AML 10 mg. 12 During the first 2 weeks of the double‐blind treatment period, patients received either a dual‐combination regimen or placebo. All patients receiving placebo were switched at week 2 to one of the dual‐combination regimens until week 4. Patients initially assigned to a dual‐combination regimen continued this treatment until week 4, at which time patients either continued the dual‐combination regimen or were switched to the triple‐combination regimen (OM 40 mg/AML 10 mg/HCTZ 25 mg) until the final observation at week 12. Patients were instructed to take study medications at the same time each day (±2 hours). Both investigators and patients remained blinded as to which medication was being administered at any given time.
Safety assessments included vital signs, physical examinations, 12‐lead electrocardiograms, clinical laboratory tests, and adverse event monitoring, and were reported previously for the TRINITY study population. In general, the safety profile demonstrated for the ABPM substudy cohort was similar to the TRINITY population. 12
ABP Substudy
Patients who volunteered to be a part of the ABPM substudy remained within the main TRINITY analysis, the results of which have been reported previously. The ABPM substudy was performed only at selected sites with sufficient training and experience. Enrollment was voluntary, with 440 patients assigned randomly to 1 of the 4 treatment groups.
BP was measured using a validated automated BP monitor (Omron HEM‐705CP; Omron Healthcare, Inc, Bannockburn, IL). At each visit, there were 3 cuff BP values; the mean of these 3 evaluations was used as the SeBP for that visit. Spacelabs HealthCare, Inc (Issaquah, WA) provided ABPM equipment and support. All patients were fitted for an appropriately sized ABPM device. All ABP assessments were made at baseline (prior to randomization) and at the end of the 12‐week active treatment period. Baseline ABP was obtained in patients off all antihypertensive medication for ≥3 weeks. The concluding assessment was obtained 1 day prior to the last scheduled visit in patients completing the trial and at the time of study discontinuation in patients who did not complete the trial but did complete ≥6 weeks of randomized therapy. During week 12, patients were instructed to withhold their morning study medication until after initiation of ABPM.
Statistical Analysis
Prespecified efficacy end points included the mean change from baseline to week 12 in mean 24‐hour, daytime (8 am–4 pm), and nighttime (10 pm–6 am) ABP; mean change from baseline to week 12 in mean ABP during the last 6, 4, and 2 hours of the dosing interval; and the percentage of patients who achieved various ABP targets at the end of the 12‐week treatment period (24‐hour target: <130/80 mm Hg; daytime targets: <135/85 mm Hg and <130/80 mm Hg; nighttime targets: <130/80 mm Hg and <120/80 mm Hg). In addition, mean change from baseline to week 12 in seated cuff BP in the subset of patients with valid ABP measurements at both baseline and trial conclusion was assessed in a post hoc analysis.
Data from all patients who participated in the ABPM substudy and had valid ABP measurements prior to and after randomization were analyzed. Treatment comparisons for the change from baseline in ABP were performed using an analysis of covariance (ANCOVA) model with a baseline ABP value as a covariate and treatment as a fixed effect. All treatment comparisons were calculated as OM 40 mg/AML 10 mg/HCTZ 25 mg minus the respective dual‐combination regimens. Efficacy in achieving ABP targets for the triple‐combination regimen compared with each dual‐combination regimen was assessed using the Cochran–Mantel–Haentszel test. Early termination measurements during the double‐blind treatment period were included in the analyses.
Results
Demographic and Baseline Characteristics
Of the 6724 patients screened in TRINITY, 2492 were randomized and 2116 completed the evaluation. Of these, 440 patients who consented to participate in the ABPM substudy, in whom valid ABP measurements could be obtained prior to and after randomization, were included in the ABPM cohort. The mean age of this cohort was 57.1 years and the mean duration of hypertension was 11.0 years. Overall, 54.5% were men, 71.1% were white, and 25.7% were black. Baseline characteristics of the ABPM cohort were similar to those of the total TRINITY cohort except for a slightly lower proportion of black patients. 12 , Table I summarizes demographic and baseline characteristics for the ABPM cohort by treatment regimen. Values are nearly identical to the main study cohort. 12
Table I.
Demographic and Baseline Characteristics by Treatment Group: ABPM Cohort
| OM 40/AML 10 mg (n=112) | OM 40/HCTZ 25 mg (n=116) | AML 10/HCTZ 25 mg (n=95) | OM 40/AML 10/HCTZ 25 mg (n=117) | |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 57.9 (11.4) | 55.8 (9.2) | 57.0 (10.3) | 57.6 (11.1) |
| ≥65, No. (%) | 30 (26.8) | 18 (15.5) | 20 (21.1) | 31 (26.5) |
| Male sex, No. (%) | 67 (59.8) | 64 (55.2) | 55 (57.9) | 54 (46.2) |
| Race/ethnicity, No. (%) | ||||
| Hispanic or Latino | 19 (17.0) | 17 (14.7) | 16 (16.8) | 24 (20.5) |
| White | 83 (74.1) | 78 (67.2) | 69 (72.6) | 83 (70.9) |
| Black | 27 (24.1) | 33 (28.4) | 24 (25.3) | 29 (24.8) |
| BMI, kg/m2 | ||||
| Mean (SD) | 32.2 (6.1) | 33.2 (6.4) | 32.4 (6.5) | 31.7 (5.9) |
| BMI ≥30, No. (%) | 70 (62.5) | 76 (65.5) | 61 (64.2) | 66 (56.4) |
| Diabetes, No. (%) | 24 (21.4) | 15 (12.9) | 13 (13.7) | 19 (16.2) |
| Duration of hypertension, mean (SD), y | 11.5 (10.2) | 10.3 (9.1) | 12.0 (10.4) | 10.4 (10.0) |
| Baseline cuff BP, mean (SD), mm Hga | ||||
| Systolic | 169.5 (14.7) | 166.3 (13.0) | 167.8 (13.3) | 168.3 (12.5) |
| Diastolic | 100.4 (7.7) | 100.9 (8.0) | 100.1 (7.0) | 100.1 (6.4) |
Abbreviations: ABPM, ambulatory blood pressure monitoring; AML, amlodipine; BMI, body mass index; BP, blood pressure; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation. aBaseline for cuff BP was defined as the mean of the randomization visit and the visit immediately preceding randomization.
ABPM Measures
During the entire 24‐hour period, there was a greater reduction in ambulatory systolic and diastolic BP with the triple‐combination regimen compared with each of the respective dual‐combination regimens (Figure 1). Treatment differences in mean 24‐hour, daytime, and nighttime systolic and diastolic ABP between the triple‐combination regimen group and the dual‐combination regimen groups at week 12 were all statistically significant (P≤.018 for all comparisons, Figure 2). At week 12, the triple‐combination resulted in a greater reduction in mean 24‐hour systolic and diastolic BP (−30.3 mm Hg/−18.0 mm Hg) compared with the dual‐combination regimens (OM 40 mg/AML 10 mg, −23.5/−13.9; OM 40 mg/HCTZ 25 mg, −23.9/−14.5; and AML 10 mg/HCTZ 25 mg, −18.5/−10.7 mm Hg; P<.0001 for each comparison). Mean 24‐hour BP at week 12 was 117.1 mm Hg/69.2 mm Hg with the triple combination, 126.2 mm Hg/74.7 mm Hg for OM 40 mg/AML 10 mg, 123.4 mm Hg/74.2 mm Hg for OM 40 mg/HCTZ 25 mg, and 128.5 mm Hg/77.9 mm Hg for AML 10 mg/HCTZ 25 mg (Table II). Similarly, reductions in BP during the last 6, 4, and 2 hours of the dosing interval were significantly greater with the triple combination than with any dual‐combination therapy (P≤.0157 for all comparisons) (Figure 3). The triple combination lowered BP to a greater degree during the last 2 hours of the dosing interval than any of the dual combinations (−27.7 mm Hg/16.8 mm Hg vs a range of −23.2 mm Hg/−14.1 mm Hg to −20.2 mm Hg/−11.6 mm Hg, respectively; P≤.0090 vs each dual combination). Overall, mean BP during the last 2 hours of the dosing interval was 123.4 mm Hg/74.4 mm Hg for the triple‐combination regimen group, 132.2 mm Hg/80.2 mm Hg for the OM 40‐mg/AML 10‐mg group, 127.9 mm Hg/78.6 mm Hg for the OM 40‐mg/HCTZ 25‐mg group, and 129.7 mm Hg/81.0 mm Hg for the AML 10‐mg/HCTZ 25‐mg group (Table II).
Figure 1.
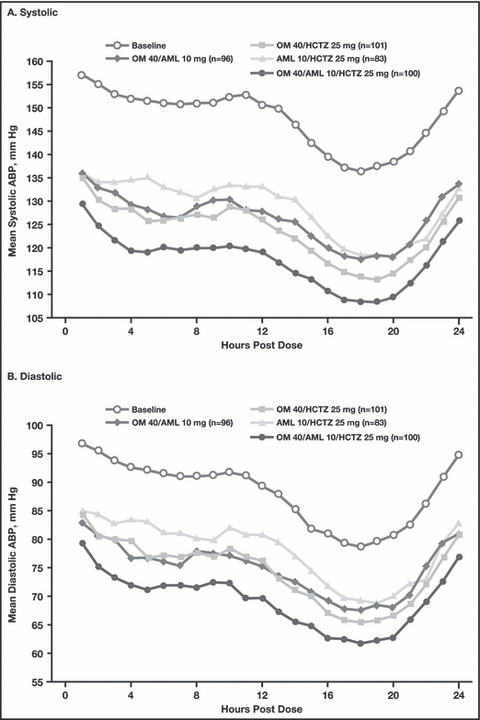
Hourly mean systolic (A) and diastolic (B) 24‐hour ambulatory blood pressure (ABP) by regimen. AML indicates amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil.
Figure 2.
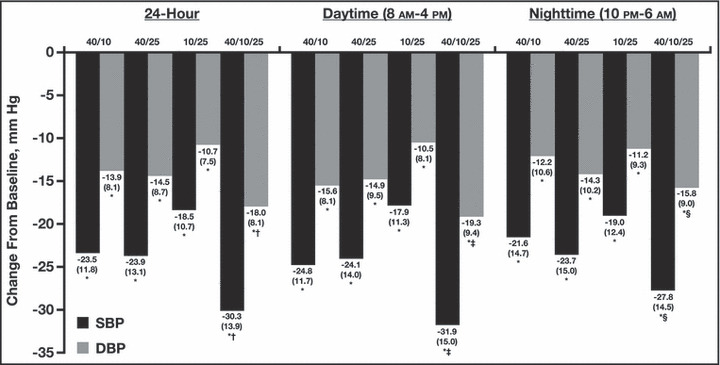
Change from baseline to week 12 in mean (standard deviation [SD]) 24‐hour, daytime, and nighttime systolic and diastolic ambulatory blood pressure (ABP) by regimen. *P<.0001 vs baseline; † P<.0001 vs each dual‐combination regimen; ‡ P≤.0001 vs each dual‐combination regimen; § P≤.018 vs each dual‐combination regimen. Doses (mg) are OM/AML (n=96), OM/HCTZ (n=101), AML/HCTZ (n=83), and OM/AML/HCTZ (n=100), respectively. AML indicates amlodipine besylate; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SBP, systolic blood pressure.
Table II.
Mean (SD) 24‐Hour, Daytime, and Nighttime Baselinea and Week 12 ABP
| OM 40/AML 10 mg (n=96) | OM 40/HCTZ 25 mg (n=101) | AML 10/HCTZ 25 mg (n=83) | OM 40/AML 10/HCTZ 25 mg (n=100) | |
|---|---|---|---|---|
| 24‐hour ABP | ||||
| Baseline | 149.7 (13.9)/88.6 (10.4) | 147.3 (13.6)/88.7 (10.2) | 147.0 (12.1)/88.6 (10.1) | 147.4 (13.6)/87.2 (9.4) |
| Week 12 | 126.2 (12.4)/74.7 (8.7) | 123.4 (15.3)/74.2 (10.4) | 128.5 (12.1)/77.9 (8.3) | 117.1 (10.3)/69.2 (7.2) |
| Daytime ABP (8 am–4 pm) | ||||
| Baseline | 155.2 (14.0)/94.0 (10.8) | 152.5 (14.4)/94.0 (10.4) | 151.6 (12.8)/93.4 (10.6) | 153.7 (13.9)/92.9 (9.3) |
| Week 12 | 130.4 (12.8)/78.5 (8.9) | 128.4 (16.0)/79.1 (11.0) | 133.7 (13.3)/82.9 (8.9) | 121.8 (11.9)/73.6 (8.6) |
| Nighttime ABP (10 pm–6 am) | ||||
| Baseline | 141.3 (17.2)/81.4 (12.1) | 139.5 (15.2)/81.6 (11.1) | 139.6 (12.8)/82.2 (11.1) | 138.2 (15.9)/79.4 (11.5) |
| Week 12 | 119.7 (14.2)/69.2 (10.2) | 115.8 (16.4)/67.3 (10.9) | 120.6 (12.2)/70.9 (9.3) | 110.5 (11.2)/63.6 (8.0) |
| Last 6 hour ABP | ||||
| Baseline | 147.4 (16.9)/87.1 (11.6) | 142.8 (14.3)/85.5 (10.3) | 142.1 (12.7)/85.6 (10.5) | 143.7 (15.1)/84.8 (10.8) |
| Week 12 | 124.2 (15.0)/73.5 (10.4) | 119.8 (16.8)/71.5 (11.7) | 122.9 (12.5)/74.1 (9.2) | 115.2 (11.9)/68.1 (8.8) |
| Last 4 hour ABP | ||||
| Baseline | 151.3 (17.3)/90.4 (12.1) | 145.5 (14.3)/88.0 (10.4) | 144.6 (13.2)/88.0 (10.9) | 146.8 (15.8)/87.7 (11.1) |
| Week 12 | 127.5 (15.9)/76.3 (11.1) | 123.0 (17.4)/74.2 (12.3) | 125.3 (12.8)/76.7 (9.1) | 118.6 (12.7)/71.0 (9.5) |
| Last 2 hour ABP | ||||
| Baseline | 155.4 (17.2)/94.3 (12.1) | 150.1 (14.1)/92.3 (10.4) | 149.9 (14.4)/92.6 (11.3) | 151.1 (15.8)/91.5 (11.4) |
| Week 12 | 132.2 (15.5)/80.2 (10.8) | 127.9 (18.4)/78.6 (12.8) | 129.7 (14.6)/81.0 (9.7) | 123.4 (13.1)/74.7 (9.7) |
Abbreviations: ABP, ambulatory blood pressure; AML, amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SD, standard deviation. Data are mean (SD), mm Hg. Week 12 values include early termination. aBaseline for mean ABP was measured prior to randomization.
Figure 3.
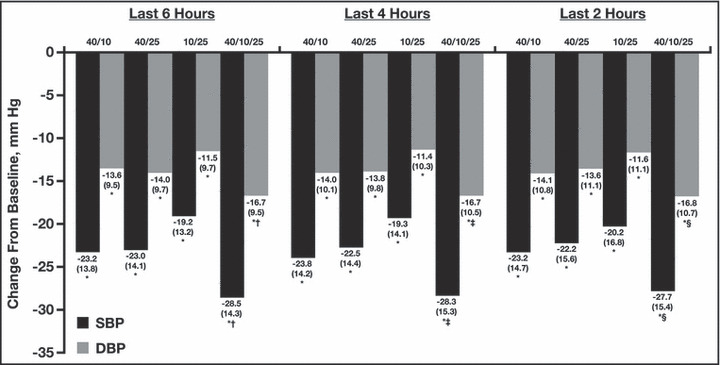
Change from baseline to week 12 in mean (standard deviation [SD]) systolic and diastolic ambulatory blood pressure (ABP) during the last 6, 4, and 2 hours of the dosing interval by regimen. *P<.0001 vs baseline; † P≤.0111 vs each dual‐combination regimen; ‡ P≤.0157 vs each dual‐combination regimen; § P≤.0090 vs each dual‐combination regimen. Doses (mg) are OM/AML (n=96), OM/HCTZ (n=101), AML/HCTZ (n=83), and OM/AML/HCTZ (n=100), respectively. AML indicates amlodipine besylate; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SBP, systolic blood pressure.
Consistent with these findings, a significantly greater percentage of patients in the triple‐combination regimen group achieved BP targets for 24‐hour mean, daytime, and nighttime ABP compared with each dual‐combination regimen group (P≤.0032; P≤.0402; and P≤.0273, respectively for mean 24‐hour, daytime, and nighttime BP targets) (Table III). The mean 24‐hour BP target of <130/80 mm Hg was achieved in 86.5% of patients receiving the triple‐combination regimen compared with 58.4%, 68.9%, and 51.2% of patients receiving OM 40 mg/AML 10 mg, OM 40 mg/HCTZ 25 mg, and AML 10 mg/HCTZ 25 mg, respectively (P≤.0032 vs each dual‐combination regimen).
Table III.
Percentage of Patients Achieving ABP Targets at Week 12 by Treatment Group
| ABP Target | Patients Achieving ABP Target (%) | |||
|---|---|---|---|---|
| OM 40/AML 10 mg (n=101) | OM 40/HCTZ 25 mg (n=106) | AML 10/HCTZ 25 mg (n=86) | OM 40/AML 10/HCTZ 25 mg (n=104) | |
| Mean 24 hour | ||||
| <130/80 mm Hg | 58.4 | 68.9 | 51.2 | 86.5a |
| Daytime (8 am–4 pm) | ||||
| <135/85 mm Hg | 61.4 | 65.1 | 41.9 | 79.8b |
| <130/80 mm Hg | 40.6 | 51.9 | 25.6 | 67.3c |
| Nighttime (10 pm–6 am) | ||||
| <130/80 mm Hg | 72.3 | 81.1 | 75.6 | 93.3d |
| <120/80 mm Hg | 52.5 | 64.2 | 52.3 | 79.8e |
Abbreviations: ABP, ambulatory blood pressure; AML, amlodipine besylate; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil. n, number of patients with valid ABP measurements at endpoint. a P≤.0032; b P≤.0204; c P≤.0402; d P≤.0273; e P≤.0121 vs each dual‐combination regimen.
Clinic BP Measures
Seated cuff BP measurements at week 12 were consistently higher than mean 24‐hour ABP measurements at week 12. At week 12 (last observation carried forward), mean seated cuff BP measurements in patients with valid ABPM recordings were 127.4/77.5 mm Hg, 137.0/81.6 mm Hg, 134.4/83.8 mm Hg, and 137.2/84.9 mm Hg in the triple‐combination regimen, OM 40‐mg/AML 10‐mg, OM 40‐mg/HCTZ 25‐mg, and AML 10‐mg/HCTZ 25‐mg groups, respectively. Least‐squares mean reduction from baseline to week 12 in seated cuff BP was significantly greater in the triple‐combination regimen group (−39.9 mm Hg/22.9 mm Hg) compared with the OM 40‐mg/AML 10‐mg (−30.7 mm Hg/19.0 mm Hg), OM 40‐mg/HCTZ 25‐mg (−31.5/17.6 mm Hg), and AML 10‐mg/HCTZ 25‐mg groups (−28.9/15.4 mm Hg) (P≤.001 for all treatment comparisons).
Discussion
Like the parent protocol, the TRINITY ABPM substudy in patients with moderate to severe hypertension demonstrates the antihypertensive superiority of the triple‐combination regimen (OM 40 mg/AML 10 mg/HCTZ 25 mg daily) compared with any of its component dual‐combination regimens. The mean reduction in 24‐hour BP with the triple regimen was −30.3 mm Hg/18.0 mm Hg, which is about 6 to 12 mm Hg/4 to 7 mm Hg greater than any of the 3 dual‐combination regimens. Accordingly, the number of individuals reaching target BP values was greater with the triple‐drug combination: the ABP target of <130/80 mm Hg was achieved by 57.7% of patients receiving OM 40 mg/AML 10 mg/HCTZ 25 mg, compared with 34.7% of patients receiving OM 40 mg/AML 10 mg, 50.9% of patients receiving OM 40 mg/HCTZ 25 mg, and 40.7% of patients receiving AML 10 mg/HCTZ 25 mg during the last 2 hours of the dosing interval. Furthermore, the greater efficacy of the triple‐combination regimen was maintained throughout the day, including the last 6, 4, and 2 hours of the dosing period.
ABPM is an essential component of pharmaceutical development studies and large clinical trials 7 , 13 , 14 , 15 , 16 due in large part to its reproducibility. 17 Intrinsically, 24‐hour ABP assessment includes a large number of determinations and therefore provides a more stable approximation of the true mean BP. Traditional office‐based cuff BP measurements reflect only a brief period in time during which the BP is affected by both patient‐related factors (eg, prior caffeine ingestion, presence of white‐coat hypertension pattern) and non‐patient factors (eg, number of BP recordings obtained, variations in instruments and technique between staff members). 16 Consequently, single BP measurements in the clinic have relatively low reproducibility (±10 mm Hg); improving the accuracy (to ±5 mm Hg) would require many more (10–13) replications. 16 , 17 ABPM is currently recommended by the American Society of Hypertension as the best method for assessing cardiovascular risk in individuals with hypertension. 7 End‐organ damage (left ventricular mass and urinary albumin excretion rate) and adverse outcomes (ischemic heart disease, stroke, and cardiovascular mortality) correlate more strongly with ABP values than those obtained by traditional means. 10 , 18 , 19 , 20 , 21 , 22 As observed in the current substudy, ABP values are usually lower than office‐based BP values. 23 As a result, ABP goals recommended by experts are also lower (mean 24‐hour BP should generally be <130/80 mm Hg; mean daytime BP <135/85 mm Hg and mean nighttime BP <125/75 mm Hg). 2 , 7
In order to achieve adequate BP control, most individuals with hypertension require ≥2 antihypertensive agents. 2 , 3 , 4 Based on their complementary mechanisms of action, combining an angiotensin receptor blocker, a dihydropyridine calcium channel blocker, and a thiazide‐type diuretic is a rational approach to improving efficacy and BP control rates, in part through enhanced medication adherence and shorter time to achieve BP control. 24 , 25 Clinical trials have demonstrated that thiazide diuretics and amlodipine significantly decrease the risk of cardiovascular events and there is evidence that angiotensin receptor blockers may have similar benefits, 26 , 27 , 28 although improved disease outcomes was not a feature of TRINITY. Another potential virtue of rational combination therapy is better tolerability. No new safety concerns were identified during the double‐blind treatment period for either the triple‐combination treatment or any of the dual‐combination regimens. The safety profile in this ABPM substudy population was similar to that observed in the entire TRINITY population. 12
Limitations
Limitations to the current study must be mentioned. Sites were selected based on their prior experience in conducting ABPM and the voluntary participation of the participants. Selection bias is therefore possible, but the ABPM subgroup faithfully represented the general demographic characteristics of the main cohort, and the magnitude of the antihypertensive effects in the ABPM substudy participants at week 12 were similar to those seen in the main study. 12 A greater weakness is the use of only the highest doses of each of the 3 agents in the dual‐ and triple‐combination regimens. More complete dosing information might be particularly useful in managing specific adverse outcomes such as amlodipine‐induced edema or thiazide‐induced hyponatremia. Finally, the study excluded individuals with symptomatic heart failure and therefore it cannot be determined whether the product is appropriate for this population.
Conclusions
In summary, the TRINITY ABPM substudy verifies that, during a 12‐week period in individuals with moderate to severe hypertension, a once‐daily triple combination (OM 40 mg, AML 10 mg, and HCTZ 25 mg) reduces systolic and diastolic BP to a greater degree than any of the 3 combinations of any 2 components at equivalent doses over the entire 24‐hour dosing interval. The triple‐combination regimen also resulted in a greater percentage of patients reaching ABP targets, including mean 24‐hour, daytime, and nighttime BP values.
Acknowledgments and disclosures:
Research funds for this study and preparation of the manuscript were provided by Daiichi Sankyo, Inc, Parsippany, New Jersey. Editorial support for this article was provided by Vrinda Mahajan, PharmD, of Peloton Advantage, LLC, Parsippany, New Jersey. Editorial oversight was provided by the authors and the final draft of the paper was written by the lead author (JLI). The opinions expressed in the current article are those of the authors, who received no compensation or other form of financial support for their participation in the preparation of this article. Dr Izzo has served as a consultant or investigator for Daiichi Sankyo, Inc, Boehringer‐Ingelheim, Forest Laboratories, Novartis Pharmaceuticals, GlaxoSmithkline, and Takeda Pharmaceuticals. Dr Chrysant has served as a consultant and speakers’ bureau member for and received grant/research support and honoraria from Daiichi Sankyo, Inc. Dr Kereiakes reports no disclosure information. Dr Oparil has received grant/research support from Amgen Inc and Merck & Co, Inc; is a member of the speakers’ bureau for Daiichi Sankyo, Inc and Novartis; has served as a consultant for Boehringer‐Ingelheim, Daiichi Sankyo, Inc, Eli Lilly, Forest Laboratories, NicOx, Novartis, and Omron Healthcare; and has received honorarium from Daiichi Sankyo, Inc, Novartis, and Pfizer Inc. Drs Melino, Lee, and Heyrman and Mr Fernandez are employees of Daiichi Sankyo, Inc.
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 3. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 4. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. [DOI] [PubMed] [Google Scholar]
- 6. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 7. Pickering TG, White WB. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4:56–61. [DOI] [PubMed] [Google Scholar]
- 8. Gardner SF, Schneider EF. 24‐Hour ambulatory blood pressure monitoring in primary care. J Am Board Fam Pract. 2001;14:166–171. [PubMed] [Google Scholar]
- 9. Marchiando RJ, Elston MP. Automated ambulatory blood pressure monitoring: clinical utility in the family practice setting. Am Fam Physician. 2003;67:2343–2350. [PubMed] [Google Scholar]
- 10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 11. Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension. 2000;35:844–851. [DOI] [PubMed] [Google Scholar]
- 12. Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double‐blind, 12‐week, parallel‐group study. Clin Ther. 2010;32:1252–1269. [DOI] [PubMed] [Google Scholar]
- 13. Cohen DL, Townsend RR. Is ambulatory blood pressure monitoring finally catching on? J Clin Hypertens (Greenwich). 2010;12:193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickering TG, Shimbo D, Haas D. Ambulatory blood‐pressure monitoring. N Engl J Med. 2006;354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 15. Staessen JA, Asmar R, De Buyzere M, et al. Task Force II: blood pressure measurement and cardiovascular outcome. Blood Press Monit. 2001;6:355–370. [DOI] [PubMed] [Google Scholar]
- 16. Wexler R. Ambulatory blood pressure monitoring in primary care. South Med J. 2010;103:447–452. [DOI] [PubMed] [Google Scholar]
- 17. Warren RE, Marshall T, Padfield PL, et al. Variability of office, 24‐hour ambulatory, and self‐monitored blood pressure measurements. Br J Gen Pract. 2010;60:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansen TW, Jeppesen J, Rasmussen S, et al. Ambulatory blood pressure and mortality: a population‐based study. Hypertension. 2005;45:499–504. [DOI] [PubMed] [Google Scholar]
- 19. Hansen TW, Jeppesen J, Rasmussen S, et al. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–250. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Zanchetti A, Agebiti‐Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment‐induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation. 1997;95:1464–1470. [DOI] [PubMed] [Google Scholar]
- 21. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 22. Verdecchia P, Angeli F, Gattobigio R. Clinical usefulness of ambulatory blood pressure monitoring. J Am Soc Nephrol. 2004;15(suppl 1):S30–S33. [DOI] [PubMed] [Google Scholar]
- 23. Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens. 1995;13:1377–1390. [PubMed] [Google Scholar]
- 24. Rosenthal T, Gavras I. Fixed‐drug combinations as first‐line treatment for hypertension. Prog Cardiovasc Dis. 2006;48:416–425. [DOI] [PubMed] [Google Scholar]
- 25. Chrysant SG. Using fixed‐dose combination therapies to achieve blood pressure goals. Clin Drug Investig. 2008;28:713–734. [DOI] [PubMed] [Google Scholar]
- 26. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 27. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 28. Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]


