Abstract
J Clin Hypertens (Greenwich). 2012; 14:593–600. © 2012 Wiley Periodicals, Inc.
The data on the risk of hypertension in human immunodeficiency virus (HIV)–infected patients, particularly in those with lipodystrophy, are controversial. The authors assessed the impact of lipodystrophy on hypertension in a cohort of HIV‐infected adults receiving combination antiretroviral therapy. This was a cross‐sectional study in which lipodystrophy (clinically and fat mass ratio [FMR]–defined), blood pressure, and body composition (dual‐energy x‐ray absorptiometry and computed tomography) were evaluated in 368 HIV adults. The prevalence of hypertension in HIV patients with or without clinically or FMR‐defined lipodystrophy was similar (with clinical lipodystrophy 35.3% vs without 32.9%, not significant; with FMR lipodystrophy 41.7% vs without 32.2%, not significant). When HIV‐infected patients were classified into 4 categories of fat distribution (based on the presence or absence of lipoatrophy and abdominal prominence), isolated lipoatrophy was not significantly associated with hypertension, but patients with isolated central fat accumulation and mixed forms of lipodystrophy had a significantly higher prevalence of hypertension. Hypertensive HIV patients had significantly higher total fat, central, and central/peripheral fat mass ratio than normotensive ones. After adjustment for age, sex, smoking, and body mass index, hypertension remains significantly associated with central/peripheral fat mass ratio (odds ratio, 1.258; 95% confidence interval, 1.008–1.569). Hypertension was not more prevalent in lipodystrophic HIV‐infected patients, but was significantly associated with central/peripheral fat mass ratio.
The widespread use of combination antiretroviral therapy (cART) has changed human immunodeficiency virus (HIV‐1) infection into a chronic manageable illness. However, together with chronic HIV infection and aging, lifelong cART is associated with adverse effects including cardiovascular disease and lipodystrophy. 1 , 2 , 3 , 4
In contrast with metabolic abnormalities, few and sometimes controversial studies are available on the effect of cART and lipodystrophy on blood pressure (BP) in HIV‐infected patients. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
Regular BP monitoring to rule out hypertension in HIV‐infected patients has been recently recommended, 14 and some authors have suggested a high prevalence of hypertension among these patients, 11 , 15 , 16 , 17 , 18 which was not confirmed by others. 12 , 16 , 19 , 20 In some studies, the higher risk for hypertension in HIV‐infected patients is associated with lipodystrophy, 21 , 22 , 23 , 24 , 25 which is in opposition to data from uninfected HIV patients with genetic lipoatrophies, for whom diabetes is a common feature but not hypertension. 21 , 22 , 23 , 24 , 25 Given this controversy, the aim of this study was to estimate the association between lipodystrophy (clinically and fat mass ratio [FMR]–defined) and body composition with the prevalence of hypertension in HIV‐1 patients on antiretroviral therapy.
Methods
Patients
As part of a cross‐sectional study, we evaluated all 364 noninstitutionalized, HIV‐1–infected Caucasian adult patients, consecutively referred from the Infectious Diseases Clinic to the Endocrinology Outpatient Clinic. Patients were included on the first visit, and all were taking cART. The study protocol was approved by the hospital ethics committee and all patients provided informed consent.
For each patient, the following information was collected using a standardized protocol: demographic data (age, sex), duration of HIV infection, probable transmission route of HIV infection, duration of cART, antihypertensive drug therapy, and characterization of the infection using the Centers for Disease Control and Prevention (CDC) staging criteria. 26
A blood sample was collected and CD4 cells, HIV‐1 RNA, and fasting glucose and insulin were determined. Insulin resistance was defined by the homeostatic model assessment. 27 Body weight was measured using TANITA (model TBF 300; Tanita, Tokyo, Japan) scale, with patients wearing light clothes without shoes, and height was measured to the nearest centimeter in the standing position using a wall stadiometer (Holtain Limited Crymych, Dyfed, UK). Neck, hip, waist, thigh, and arm circumferences were measured by the same observer with standard techniques. 28
BP was measured after the patient had remained seated for 5 minutes, with the elbow flexed at the heart, using a standard aneroid sphygmomanometer with the cuff on the upper right arm. Two BP readings were taken and the mean of the two readings was calculated. Hypertension was defined as systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg or self‐reported medication for high BP. 29
Clinical lipodystrophy (CL) was defined as peripheral lipoatrophy with or without central fat accumulation assessed by both practitioner and patient as we previously described. 30 , 31 , 32 , 33 Central fat accumulation or abdominal prominence was defined by the last International Diabetes Federation criteria for the metabolic syndrome 34 as having waist circumference ≥94 cm in men and 80 cm in women. 35 The clinical assessment was performed by the same practitioner (PF). Patients were classified into 4 different groups according to the presence or absence of either lipoatrophy or abdominal prominence: group 1, no lipodystrophy (patients without clinical lipoatrophy and without abdominal prominence); group 2, isolated central fat accumulation (patients without clinical lipoatrophy and with abdominal prominence); group 3, isolated lipoatrophy (patients with clinical lipoatrophy and without abdominal prominence); group 4, mixed forms of lipodystrophy (patients with clinical lipoatrophy and with abdominal prominence).
Body Composition and Laboratory Definitions
Body composition was assessed with whole‐body dual‐energy x‐ray absorptiometry (DXA). Measurements were performed while patients were in a supine position, with standard positioning of the arms and feet. Cut lines separating regions of interest were those indicated by the manufacturer. Regional fat mass values were grouped and analyzed for the following anatomical regions: arms, legs, trunk, and total body. The FMR was calculated as the ratio between the percentage of the trunk fat mass and the percentage of the lower limb fat mass. 36 For the definition of lipodystrophy using FMR, we used the cut‐off values of 1.961 for men and 1.329 for women. 30
The quantification of total, visceral, and peripheral fat was performed using a 64‐slice computed tomography (CT) scanner. Based on a coronal topogram, a single 10‐mm slice was obtained in all patients at the level of the transverse processes of L4. Radiological evidence of fat was considered as any area with an attenuation value comprised between −190 and −30 Hounsfield units. 37 , 38 Total fat area was calculated by drawing a region of interest (ROI) including all the abdomen and subcutaneous tissue. Visceral fat area was calculated by manually drawing a ROI along the internal contour of the abdominal wall muscles, anterior border of the psoas muscles, and anterior contour of the L4 vertebral body. Peripheral fat area was obtained by subtracting the value of visceral fat area from total fat area. All values were expressed in cm2 rounded to the nearest centesimal. 37 , 38 The CD4+ cell count was determined by flow cytometry, and plasma HIV‐1 RNA load was measured by a quantitative reverse transcriptase polymerase chain reaction, which has a lower limit of detection of 50 copies/mL.
Statistical Analysis
Statistical analysis was performed using SPSS version 17.0 software (SPSS Inc, Chicago, IL). All probabilities were two tailed and P values <.05 were regarded as significant. Data were described as mean and standard deviation (SD) for quantitative variables and compared using the Student t test or the Mann‐Whitney test as appropriate. Kruskal‐Wallis test was used for the comparison between the 4 groups of fat distribution. Categoric variables were described as counts and proportions and compared using the chi‐square or Fisher exact test.
Odds ratios (ORs) and respective 95% confidence intervals (95% CIs) were computed using unconditional logistic regression for estimating the association between hypertension and anthropometric measures and insulin resistance.
Results
Characteristics of the Population
In this sample of 364 HIV‐1–infected patients (250 men and 114 women) taking antiretroviral therapy (cART), 59.1% patients presented with CL. Table I shows the characteristics of the study sample according to the presence of CL. Patients with CL were more frequently male, older, had longer HIV infection duration and length of cART, and presented a lower mean of all the anthropometric measures, with the exception of height and neck circumference. In addition, patients with CL had higher FMRs. Patients with CL had a significantly higher mean CD4+ cell count and viral suppression rate. No differences were observed between patients with and without CL regarding the cART regimens. Also, no differences between patients with or without CL were observed regarding the frequency of antihypertensive medication use.
Table I.
Sample Characteristics According to the Presence of Clinical Lipodystrophy
| Without CL | With CL | P Value | |
| No. (%) | 149 (40.9) | 215 (59.1) | |
| Sex, No. (%) | |||
| Male | 87 (58.4) | 163 (75.8) | <.001 |
| Female | 62 (41.6) | 52 (24.2) | |
| Age, mean (SD), y | 44.4 (12.0) | 47.0 (11.0) | .010 |
| Duration, mean (SD) | 6.7 (3.9) | 9.2 (4.1) | <.001 |
| cART, mean (SD) | 5.0 (3.8) | 8.0 (4.0) | <.001 |
| Weight, mean (SD), kg | 73.7 (14.3) | 66.2 (12.5) | <.001 |
| Height, mean (SD), m | 1.65 (0.09) | 1.66 (0.09) | .383 |
| BMI, mean (SD), kg/m2 | 27.1 (5.1) | 24.0 (3.9) | <.001 |
| Waist circumference, mean (SD), cm | 95.5 (13.3) | 89.5 (10.6) | <.001 |
| Hip circumference, mean (SD), cm | 100.7 (9.4) | 91.6 (6.2) | <.001 |
| Thigh circumference, mean (SD), cm | 50.4 (6.2) | 46.1 (6.1) | <.001 |
| Arm circumference, mean (SD), cm | 28.2 (3.4) | 26.6 (4.0) | <.001 |
| Neck circumference, mean (SD), cm | 37.0 (3.9) | 37.2 (4.0) | .856 |
| Waist/hip circumference ratio, mean (SD) | 0.95 (0.09) | 0.98 (0.08) | .006 |
| CD4 cell count, mean (SD), cells/mm3 | 503.5 (311.6) | 573.4 (324.0) | .010 |
| HIV RNA (<50), No. (%) | 121 (83.4) | 197 (91.2) | .026 |
| HIV risk factor, No. (%) | |||
| Inject | 31 (20.8) | 63 (29.4) | .002 |
| Homosexual | 12 (8.1) | 30 (14.0) | |
| Heterosexual | 105 (70.5) | 111 (51.9) | |
| Other | 1 (0.7) | 10 (4.7) | |
| CDC, No. (%) | |||
| A | 82 (55.4) | 118 (55.7) | .056 |
| B | 6 (4.1) | 1 (0.5) | |
| C | 60 (40.5) | 93 (43.9) | |
| ART, No. (%) | |||
| PI | 88 (61.1) | 116 (54.2) | .263 |
| NNRTI | 63 (44.1) | 104 (48.8) | .438 |
| NRTI | 137 (95.8) | 210 (98.6) | .064 |
| Body fat mass by quantitative CT | |||
| Total fat, mean (SD), cm2 | 345.8 (163.2) | 230.9 (133.1) | <.001 |
| Central fat, mean (SD), cm2 | 131.2 (86.5) | 127.1 (79.9) | .886 |
| Peripheral fat, mean (SD), cm2 | 214.6 (125.0) | 103.8 (82.9) | <.001 |
| Central/peripheral, mean (SD) | 0.82 (0.84) | 2.06 (2.41) | <.001 |
| FMR by DXA, mean (SD) | 1.24 (0.53) | 1.98 (0.98) | <.001 |
| Systolic blood pressure, mean (SD) | 120.6 (19.0) | 120.5 (17.2) | .978 |
| Diastolic blood pressure, mean (SD) | 75.6 (13.3) | 75.6 (10.9) | .945 |
| Taking antihypertensive medications, No. (%) | 21 (14.1%) | 44 (20.6%) | .149 |
| Smoking history, No. (%) | |||
| Never | 66 (45.2) | 71 (34.5) | .103 |
| Current | 59 (40.4) | 94 (45.6) | |
| Former | 21 (14.4) | 41 (19.9%) | |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; cART, combination antiretroviral therapy; CDC, Centers for Disease Control and Prevention criteria for staging of human immunodeficiency virus (HIV) infection; CL, clinical lipodystrophy; CT, computed tomography; DXA, dual‐energy x‐ray absorptiometry; FMR, fat mass ratio; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
Prevalence of Hypertension
Of the 364 patients, 34.3% were hypertensive. No significant differences were observed regarding the prevalence of hypertension in patients with and without lipodystrophy, regardless of sex or the definition that was used (Table II). In addition, no differences in mean systolic BP (SBP) or diastolic BP (DBP) between patients with or without lipodystrophy were found (Table I), defined either by clinical criteria or by FMR (SBP: with lipodystrophy 123.7 mm Hg [16.8] vs without 119.4 mm Hg [18.3], P=.068; DBP: with lipodystrophy 76.8 mm Hg [9.9] vs without 76.0 mm Hg [12.5]. P=.411).
Table II.
Prevalence of HTN According to Lipodystrophy Defined Clinically and by FMR
| HTN | Lipodystrophy Defined Clinically | P Value | Lipodystrophy Defined by FMR | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Without CL | With CL | Total | Without L | With L | |||
| N=364 | n=149 | n=215 | N=221 | n=118 | n=103 | |||
| Without HTN, No. (%) | 239 (65.7) | 100 (67.1) | 139 (64.7) | .708 | 140 (63.3) | 80 (67.8) | 60 (58.3) | .184 |
| With HTN, No. (%) | 125 (34.3) | 49 (32.9) | 76 (35.3) | 91 (36.7) | 38 (32.2) | 43 (41.7) | ||
| No. | 250 | 87 | 163 | 147 | 57 | 90 | ||
| Men | ||||||||
| Without HTN, No. (%) | 157 (62.8) | 55 (63.2) | 102 (62.6) | 1.000 | 92 (62.6) | 41 (71.9) | 51 (56.7) | .091 |
| With HTN, No. (%) | 93 (37.2) | 32 (36.8) | 61 (37.4) | 55 (37.4) | 16 (28.1) | 39 (43.3) | ||
| No. | 114 | 62 | 52 | 74 | 61 | 13 | ||
| Women | ||||||||
| Without HTN, No. (%) | 82 (71.9) | 45 (72.6) | 37 (71.2) | 1.000 | 48 (64.9) | 39 (63.9) | 9 (69.2) | 1.000 |
| With HTN, No. (%) | 32 (28.1) | 17 (27.4) | 15 (28.8) | 26 (35.1) | 22 (36.1) | 4 (30.8) | ||
Abbreviations: CL, clinical lipodystrophy; FMR, fat mass ratio; HTN, hypertension; L, lipodystrophy.
When patients were classified into 4 categories of fat distribution as previously described, the presence of hypertension was more prevalent in patients with isolated central fat accumulation and mixed forms of lipodystrophy (patients with abdominal prominence regardless of the presence or absence of lipoatrophy) both in men and in the total sample (Table III).
Table III.
Prevalence of Hypertension by Body Composition According to 4 Groups of Fat Distribution and Sex
| HTN | No Lipodystrophy | Isolated Central Fat Accumulation | Isolated Lipoatrophy | Mixed Forms of Lipodystrophy | P Value |
|---|---|---|---|---|---|
| Total | |||||
| Without HTN, No. (%) | 39 (76.5) | 54 (52.8) | 80 (72.7) | 53 (56.4) | .029 |
| With HTN, No. (%) | 12 (23.5) | 32 (37.2) | 30 (27.3) | 41 (43.6) | |
| Men | |||||
| Without HTN, No. (%) | 31 (73.8) | 23 (56.1) | 67 (69.8) | 29 (51.8) | .049 |
| With HTN, No. (%) | 11 (26.1) | 18 (43.9) | 29 (30.2) | 27 (48.2) | |
| Women | |||||
| Without HTN, No. (%) | 8 (88.9) | 31 (68.9) | 13 (92.9) | 24 (63.2) | .119 |
| With HTN, No. (%) | 1 (11.1) | 14 (31.1) | 1 (7.1) | 14 (36.8) | |
Abbreviation: HTN, hypertension.
Patient Characteristics According the Presence of Hypertension
Patients with hypertension were older; had higher mean weight, body mass index (BMI), waist, arm, and neck circumferences; and higher waist/hip circumference ratio. They also had higher total and central fat and central/peripheral fat ratio without difference in the peripheral fat on CT at abdominal level (Table IV and figure ). There was no difference between patients with or without hypertension in sex, duration of HIV infection, length of cART, hip and thigh circumferences, CD4 cell count, and presence of lipodystrophy (clinically or FMR‐defined).
Table IV.
Sample Characteristics According to the Presence of HTN
| Without HTN | With HTN | P Value | |
| No. (%) | 239 (65.7) | 125 (34.3) | |
| Sex, No. (%) | |||
| Male | 157 (65.7) | 93 (74.4) | .114 |
| Female | 82 (34.3) | 32 (25.6) | |
| Age, mean (SD), y | 42.0 (9.3) | 53.5 (11.6) | <.001 |
| Duration, mean (SD), y | 8.2 (4.1) | 8.0 (4.6) | .394 |
| ART, mean (SD), y | 6.8 (4.0) | 6.7 (4.4) | .739 |
| Weight, mean (SD), kg | 67.8 (13.3) | 72.1 (14.2) | .009 |
| Height, mean (SD), m | 1.66 (0.09) | 1.65 (0.08) | .388 |
| BMI, mean (SD), kg/m2 | 24.6 (4.5) | 26.5 (4.8) | <.001 |
| Waist circumference, mean (SD), cm | 89.9 (11.9) | 96.0 (8.9) | <.001 |
| Hip circumference, mean (SD), cm | 94.9 (8.8) | 96.2 (8.9) | .151 |
| Thigh circumference, mean (SD), cm | 48.3 (6.7) | 47.0 (6.0) | .214 |
| Arm circumference, mean (SD), cm | 27.0 (4.1) | 27.8 (3.3) | .031 |
| Neck circumference, mean (SD), cm | 36.5 (3.8) | 38.2 (3.9) | .002 |
| Ratio waist/hip circumference, mean (SD) | 0.95 (0.08) | 1.00 (0.08) | <.001 |
| CD4 cell count, mean (SD), cells/mm3 | 554.2 (326.1) | 526.8 (309.8) | .402 |
| HIV risk factor, No. (%) | |||
| Inject | 85 (35.7) | 9 (7.2) | <.001 |
| Homosexual | 23 (9.7) | 19 (15.2) | |
| Heterosexual | 122 (51.3) | 94 (75.2) | |
| Other | 8 (3.4) | 3 (2.4) | |
| CDC, No. (%) | |||
| A | 126 (53.4) | 74 (59.7) | .031 |
| B | 6 (2.5) | 1 (0.8) | |
| C | 104 (44.1) | 49 (39.5) | |
| ART, No. (%) | |||
| PI | 140 (59.8) | 64 (51.6) | .167 |
| NNRTI | 106 (45.3) | 61 (50.0) | .464 |
| NRTI | 228 (97.9) | 119 (96.4) | .664 |
| Body fat mass by quantitative CT | |||
| Total fat, mean (SD), cm2 | 241.9 (139.4) | 329.0 (165.7) | <.001 |
| Central fat, mean (SD), cm2 | 107.2 (72.7) | 167.4 (72.7) | <.001 |
| Peripheral fat, mean (SD), cm2 | 134.7 (102.6) | 161.5 (129.7) | .184 |
| Central/peripheral, mean (SD) | 1.30 (1.36) | 2.16 (2.90) | .005 |
| Clinical lipodystrophy, No. (%) | |||
| Without | 100 (41.8) | 49 (39.2) | .708 |
| With | 139 (58.2) | 76 (60.8) | |
| Lipodystrophy FMR defined, No. (%) | |||
| Without | 80 (57.1) | 38 (46.9) | .184 |
| With | 60 (42.9) | 43 (53.1) | |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CDC, Centers for Disease Control and Prevention criteria for staging of human immunodeficiency virus (HIV) infection; CT, computed tomography; FMR, fat mass ratio; HTN, hypertension; PI, protease inhibitor; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; SD, standard deviation.
Figure FIGURE.
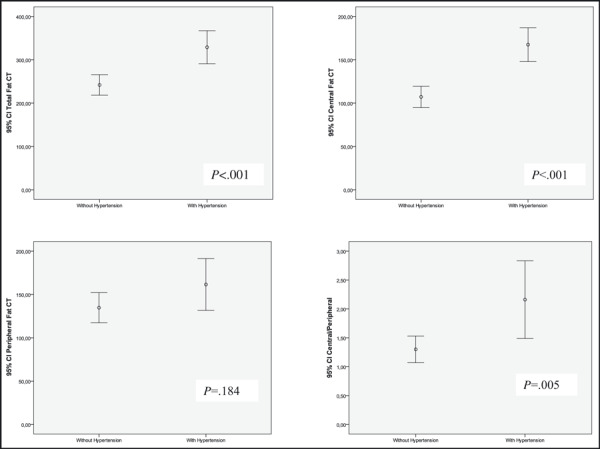
Mean of body fat mass by quantitative computed tomography (CT) according to the presence of hypertension. CI indicates confidence interval.
Anthropometric Characteristics and the Presence of Hypertension
After adjustment for age, sex, smoking history, and BMI, hypertension was not associated with either insulin resistance or the presence of clinically or FMR‐defined lipodystrophy. Also, total abdominal and central and peripheral fat mass evaluated by CT scan were not associated with hypertension. On the other hand, central/peripheral fat mass ratio was significantly associated with hypertension, even after adjustment for the previously mentioned variables (OR, 1.26; 95% CI, 1.01–1.57). Total body, arm, and trunk fat mass, assessed by DXA, were not associated with hypertension, independent of age, sex, and smoking history (Table V).
Table V.
Association Between Anthropometric Characteristics and the Presence of Hypertension
| Crude OR (95% CI) | Adjusted OR (95% CI)a | Adjusted OR (95% CI)b | |
| HOMA score | |||
| <4 | 1 | 1 | 1 |
| ≥4 | 2.13 (1.26–3.60) | 1.95 (1.07–3.56) | 1.80 (0.97–3.31) |
| Clinical lipodystrophy | 1.12 (0.72–1.74) | 0.86 (0.51–1.46) | 1.12 (0.64–1.96) |
| Lipodystrophy defined by FMR | 1.51 (0.87–2.62) | 0.95 (0.46–1.94) | 0.97 (0.47–2.01) |
| Body fat mass by quantitative CT | |||
| Total fat, mean (SD), cm2 | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.00) |
| Central fat, mean (SD), cm2 | 1.01 (1.01–1.01) | 1.01 (1.00–1.01) | 1.00 (0.99–1.00) |
| Peripheral fat, mean (SD), cm2 | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.00) |
| Central/peripheral ratio, mean (SD) | 1.27 (1.06–1.52) | 1.15 (0.94–1.40) | 1.26 (1.01–1.57) |
| DXA | |||
| Total body fat mass, % | 1.03 (1.00–1.05) | 1.05 (1.01–1.09) | 1.02 (0.96–1.08) |
| Leg body fat mass, % | 1.01 (0.99–1.03) | 1.02 (0.99–1.05) | 1.00 (0.96–1.04) |
| Arm body fat mass, % | 1.01 (0.98–1.02) | 1.04 (1.01–1.08) | 1.03 (0.98–1.07) |
| Trunk body fat mass, % | 1.04 (1.10–2.00) | 1.04 (1.01–1.08) | 1.01 (0.96–1.07) |
| FMR by DXA, mean (SD) | 1.48 (1.10–2.00) | 1.17 (0.79–1.74) | 1.16 (0.78–1.73) |
Abbreviations: CI, confidence interval; CT, computed tomography; DXA, dual‐energy x‐ray absorptiometry; FMR, fat mass ratio; HOMA, Homeostatic Model Assessment; SD, standard deviation. aOdds ratio (OR) adjusted for age, sex, and smoking history. bOR adjusted for age, sex, smoking history and body mass index (BMI).
Discussion
Prevalence of Hypertension in HIV‐Infected Patients
In this study, 34.3% of the HIV‐1–infected patients (with a mean age of 45.9 [11.5] years) taking cART were hypertensive. This prevalence is lower than the 42.1% (18–90 years) and 47.1% (35–64 years) in our country’s general population. 39
There are few and discrepant studies on hypertension in HIV‐infected patients. In a study by Bergersen and colleagues, 19 the prevalence of hypertension was 21% in patients taking cART, 13% (P=.20) in antiretroviral‐naive patients, and 24% in HIV‐negative controls (P=.28). Similar results were observed in patients taking cART and in HIV‐negative controls. These observations led to the perception that influence of cART on the prevalence of hypertension appears to be minor. The authors 19 argued that the most probable explanation for those findings was that HIV‐positive patients in general have a lower BMI and thus have a lower BP than the general population.
Gazzaruso and coworkers 16 studied 287 HIV‐positive patients taking cART, with a mean age of 41.1 years, and the prevalence of hypertension was similar (34.2%) to our results (34.3%). By using multiple logistic regression analysis, it was shown that family history of hypertension, metabolic syndrome, lipodystrophy, and Homeostatic Model Assessment (HOMA) were predictors of hypertension in HIV‐infected patients.
Hypertension, Insulin Resistance, and Lipodystrophy
There is scarce information available on hypertension in HIV‐infected patients according to the presence of lipodystrophy. 10 , 11 , 12 , 13 We aimed to evaluate whether the loss of subcutaneous adipose tissue shown by lipodystrophy has any effect on BP. In our study, the prevalence of hypertension in patients with or without lipodystrophy clinically or FMR‐defined was similar, but in men and using the 4 groups of body composition, we observed an increase in prevalence of hypertension in those with lipoatrophy when compared with those without any fat distribution abnormality, even though patients with central fat accumulation and mixed forms had higher prevalence.
Hadigan 10 and Sattler 11 and colleagues showed that HIV patients with lipodystrophy had high BP. In addition, Crane and coworkers 40 found that lipoatrophy and lipohypertrophy were significantly associated with hypertension in HIV‐infected patients. Gazzaruso and colleagues 16 showed that lipodystrophy and HOMA were predictors of hypertension in HIV‐infected patients and that insulin resistance could be involved in the development of both lipodystrophy and hypertension, suggesting that the presence or the development of lipodystrophy in an HIV‐infected patient should be an indication to check for hypertension. In our sample, after adjustment for age, sex, and smoking history, hypertension remained associated with insulin resistance, but, after including BMI in the model, no significant association remained. Total body, arm, and trunk fat mass assessed by DXA were not associated with hypertension, independent of age, sex, and smoking history. In addition, total abdominal, central, and peripheral fat mass evaluated by CT scan were not associated with hypertension, but central/peripheral fat mass ratio was significantly associated with hypertension even after adjustment for the above‐mentioned variables. Patients with hypertension had higher total, central, and central/peripheral fat mass ratio. Adipose tissue produces angiotensinogen, which could lead to activation of renin‐angiotensin‐aldosterone, an important endocrine BP regulatory system. 41 , 42 But, the loss of subcutaneous peripheral adipose tissue can also contribute to hypertension through insulin resistance. 16 The presence of hypertension was more prevalent in patients with isolated central fat accumulation and mixed forms of lipodystrophy (patients with abdominal prominence regardless of the presence or absence of lipoatrophy). This could mean that abdominal prominence, as in the general population, is more important in determining hypertension; thus, the central/peripheral fat mass ratio combining the effect of central fat accumulation with loss of subcutaneous adipose tissue is more strongly associated with hypertension.
Another important aspect is the association between hypertension and smoking. According to our data, smokers had a significantly lower prevalence of hypertension. 43 , 44 , 45 , 46 , 47 However, after adjustment for age, no significant differences remained in the occurrence of hypertension according to smoking history.
Study Limitations Although all patients referred by the infectious diseases department to our department were included, bias in the referral cannot be excluded, since some patients could have been referred because they had some degree of glucose intolerance or dyslipidemia. Nevertheless, the aim of our study was to evaluate the influence of HIV lipodystrophy on the prevalence of hypertension. It was not our aim to determine the national prevalence of hypertension in the HIV population, because these patients are not representative of the general HIV population in our country. Our results are cross‐sectional and therefore do not permit causal inference.
Study Strengths The strengths of the current investigation include the following: the study was performed in a unit that is highly experienced in the assessment of metabolic and body fat abnormalities in HIV‐infected patients; the clinical assessment of lipodystrophy was performed by the same investigator (PF); an objective definition of lipodystrophy was used (fat mass ratio by DXA), visceral fat by CT, and consistent results with both definitions of lipodystrophy were observed.
Conclusions
The prevalence of hypertension was not higher in HIV‐cART lipodystrophic patients compared with patients without lipodystrophy. HIV‐isolated lipoatrophy was not significantly associated with hypertension, but patients with isolated central fat accumulation and mixed forms of lipodystrophy had a significantly higher prevalence of hypertension. Hypertensive HIV patients had significantly higher total fat, central, and central/peripheral mass ratio than normotensive patients. After adjustment for age, sex, smoking, and BMI, hypertension remained significantly associated with central/peripheral fat mass ratio.
Transparency declaration: None to declare.
Acknowledgments
Acknowledgments and disclosures: We thank Alberto Santos for performing the DXA measurements. Research Fellowship Dr Manuel Almeida Ruas, Portuguese Society of Diabetology. Research Fellowship of the Portuguese Association for Clinical Study of AIDS. Research grant to support doctoral studies in the area of HIV/AIDS Foundation GlaxoSmithKline of Health Sciences.
References
- 1. Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. [DOI] [PubMed] [Google Scholar]
- 2. Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. [DOI] [PubMed] [Google Scholar]
- 3. Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019–1026. [DOI] [PubMed] [Google Scholar]
- 4. Cattelan AM, Trevenzoli M, Sasset L, et al. Indinavir and systemic hypertension. AIDS. 2001;15:805–807. [DOI] [PubMed] [Google Scholar]
- 5. Galli M, Ridolfo AL, Gervasoni C. Cardiovascular disease risk factors in HIV‐infected patients in the HAART era. Ann N Y Acad Sci. 2001;946:200–213. [DOI] [PubMed] [Google Scholar]
- 6. Fantoni M, Del Borgo C, Autore C, Barbaro G. Metabolic disorders and cardiovascular risk in HIV‐infected patients treated with antiretroviral agents. Ital Heart J. 2002;3:294–299. [PubMed] [Google Scholar]
- 7. Duong M, Buisson M, Cottin Y, et al. Coronary heart disease associated with the use of human immunodeficiency virus (HIV)‐1 protease inhibitors: report of four cases and review. Clin Cardiol. 2001;24:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grinspoon S. Insulin resistance in the HIV‐lipodystrophy syndrome. Trends Endocrinol Metab. 2001;12:413–419. [DOI] [PubMed] [Google Scholar]
- 9. Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus‐1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. [DOI] [PubMed] [Google Scholar]
- 10. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. [DOI] [PubMed] [Google Scholar]
- 11. Sattler FR, Qian D, Louie S, et al. Elevated blood pressure in subjects with lipodystrophy. AIDS. 2001;15:2001–2010. [DOI] [PubMed] [Google Scholar]
- 12. Mattana J, Siegal FP, Sankaran RT, Singhal PC. Absence of age‐related increase in systolic blood pressure in ambulatory patients with HIV infection. Am J Med Sci. 1999;317:232–237. [DOI] [PubMed] [Google Scholar]
- 13. Gazzaruso C, Sacchi P, Garzaniti A, et al. Prevalence of metabolic syndrome among HIV patients. Diabetes Care. 2002;25:1253–1254. [DOI] [PubMed] [Google Scholar]
- 14. Lundgren JD, Behrens G, Collins S, et al. Guidelines, prevention and management of non‐infectious co‐morbidities in HIV. EACS guidelines‐Version 5‐2, 2009.
- 15. Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV‐infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7:10–15. [DOI] [PubMed] [Google Scholar]
- 16. Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382. [DOI] [PubMed] [Google Scholar]
- 17. Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. [DOI] [PubMed] [Google Scholar]
- 18. Thiebaut R, El‐Sadr WM, Friis‐Moller N, et al. Predictors of hypertension and changes of blood pressure in HIV‐infected patients. Antivir Ther. 2005;10:811–823. [DOI] [PubMed] [Google Scholar]
- 19. Bergersen BM, Sandvik L, Dunlop O, et al. Prevalence of hypertension in HIV‐positive patients on highly active retroviral therapy (HAART) compared with HAART‐naive and HIV‐negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22:731–736. [DOI] [PubMed] [Google Scholar]
- 20. Jerico C, Knobel H, Montero M, et al. Hypertension in HIV‐infected patients: prevalence and related factors. Am J Hypertens. 2005;18:1396–1401. [DOI] [PubMed] [Google Scholar]
- 21. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. [DOI] [PubMed] [Google Scholar]
- 22. Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. [DOI] [PubMed] [Google Scholar]
- 23. Bjornstad PG, Semb BK, Trygstad O, Seip M. Echocardiographic assessment of cardiac function and morphology in patients with generalised lipodystrophy. Eur J Pediatr. 1985;144:355–359. [DOI] [PubMed] [Google Scholar]
- 24. Bjornstad PG, Foerster A, Ihlen H. Cardiac findings in generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:39–43. [DOI] [PubMed] [Google Scholar]
- 25. Hegele RA, Anderson CM, Wang J, et al. Association between nuclear lamin A/C R482Q muation and partial lipodystrophy with hyperinsulinemia, dyslipidemia, hypertension and diabetes. Genome Res. 2000;10:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Human immunodeficiency virus (HIV) infection codes and new codes for Kaposi’s sarcoma. MMWR Recomm Rep. 1991;40(RR‐9):1–18. [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 28. WHO . Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committe. Geneva: WHO; 1995. [PubMed] [Google Scholar]
- 29. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 30. Freitas P, Santos AC, Carvalho D, et al. Fat mass ratio: an objective tool to define lipodystrophy in HIV‐infected patients under antiretroviral therapy. J Clin Densitom. 2010;13:197–203. [DOI] [PubMed] [Google Scholar]
- 31. Freitas P, Carvalho D, Souto S, et al. Impact of lipodystrophy on the prevalence and components of metabolic syndrome in HIV‐infected patients. BMC Infect Dis. 2011;11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freitas P, Carvalho D, Santos AC, et al. Assessment of body fat composition disturbances by bioimpedance analysis in HIV‐infected adults. J Endocrinol Invest. 2011;34(10): e321–e329. [DOI] [PubMed] [Google Scholar]
- 33. Freitas P, Carvalho D, Santos AC, et al. Prevalence of obesity and its relationship to clinical lipodystrophy in HIV‐infected adults on anti‐retroviral therapy. J Endocrinol Invest. 2011;2011 Dec 16. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 35. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 36. Bonnet E, Delpierre C, Sommet A, et al. Total body composition by DXA of 241 HIV‐negative men and 162 HIV‐infected men: proposal of reference values for defining lipodystrophy. J Clin Densitom. 2005;8:287–292. [DOI] [PubMed] [Google Scholar]
- 37. van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17:187–196. [PubMed] [Google Scholar]
- 38. Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. [DOI] [PubMed] [Google Scholar]
- 39. De Macedo ME, Lima MJ, Silva AO, et al. Prevalence, awareness, treatment and control of hypertension in Portugal. The PAP study. Rev Port Cardiol. 2007;26:21–39. [PubMed] [Google Scholar]
- 40. Crane HM, Grunfeld C, Harrington RD, Kitahata MM. Lipoatrophy and lipohypertrophy are independently associated with hypertension. HIV Med. 2009;10:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarzani R, Salvi F, Dessi‐Fulgheri P, Rappelli A. Renin‐angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26:831–843. [DOI] [PubMed] [Google Scholar]
- 42. Karlsson C, Lindell K, Ottosson M, et al. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–3929. [DOI] [PubMed] [Google Scholar]
- 43. Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J. 1986;111:932–940. [DOI] [PubMed] [Google Scholar]
- 44. Mikkelsen KL, Wiinberg N, Hoegholm A, et al. Smoking related to 24‐h ambulatory blood pressure and heart rate: a study in 352 normotensive Danish subjects. Am J Hypertens. 1997;1(Pt 1):483–491. [DOI] [PubMed] [Google Scholar]
- 45. Lee DH, Ha MH, Kim JR, et al. Effects of smoking cessation on changes in blood pressure and incidence of hypertension: a 4‐year follow‐up study. Hypertension. 2001;37:194–198. [DOI] [PubMed] [Google Scholar]
- 46. Okubo Y, Suwazono Y, Kobayashi E, Nogawa K. An association between smoking habits and blood pressure in normotensive Japanese men: a 5‐year follow‐up study. Drug Alcohol Depend. 2004;73:167–174. [DOI] [PubMed] [Google Scholar]
- 47. Omvik P. How smoking affects blood pressure. Blood Press. 1996;5:71–77. [DOI] [PubMed] [Google Scholar]


