Abstract
Albuminuria is an important risk marker for adverse cardiovascular (CV) and renal outcomes and mortality. The relationship between albuminuria and risk is continuous and linear, like that of blood pressure and cardiovascular risk. Evidence now supports increased risk even at levels traditionally considered within normal limits. In high‐risk patients, routine annual screening can detect changes in urine albumin excretion and improve the timely identification of albuminuria, and therefore should be considered in patients with diabetes, hypertension, and chronic kidney disease. Preferred simple screening methods appropriate for use in the primary care setting include microalbumin‐specific dipsticks and urinary albumin:creatinine ratio determination (from a spot urine sample). Cornerstones of albuminuria treatment include risk factor management, ongoing monitoring, and, in patients with hypertension, chronic kidney disease, or diabetes, the use of renin‐angiotensin‐aldosterone system (RAAS)–blocking agents. Both angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have demonstrated utility in this regard; data from studies of direct renin inhibition are promising. The combined use of an ACE inhibitor and ARB was once considered a viable option for the treatment of albuminuria; however, results of the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) raised important questions regarding the benefits and limitations of dual RAAS blockade. Ongoing studies should provide important insight into the effects of this approach on renal outcomes. J Clin Hypertens (Greenwich). 2011;13:438–449. ©2011 Wiley Periodicals, Inc.
Albuminuria, the presence of abnormal levels of the protein albumin in the urine, occurs in as many as 10% of representative samples of US adults. 1 , 2 As hypertension, diabetes, and obesity are each associated with albuminuria, it should not be surprising that the increased prevalence of these disorders has resulted in a concomitant increase in the prevalence of albuminuria. 2 Primary care clinicians (PCCs; physicians, nurse practitioners, and physician assistants) readily recognize that elevated urinary albumin excretion (UAE) is an easily identifiable marker of kidney glomerular disease; however, it is not commonly appreciated that elevated UAE is a strong marker for adverse cardiovascular (CV) outcomes and mortality. Moreover, albuminuria is more common in older individuals and in Hispanic and African American populations in general, and the rates of end‐stage kidney disease also are higher in these groups. Currently established laboratory standards suggest that healthy individuals excrete <30 mg albumin/24 h. Even at levels below this generally accepted cutoff, adverse events are more common in persons with “high normal” than “low normal” UAE. 3 Unfortunately, PCCs do not consistently screen for elevated UAE even in at‐risk patients. 4 , 5 Equally problematic is the commonplace uncertainty about the best management of elevated UAE, once identified. Treatment of albuminuria has been shown to provide salutary outcomes in reference to reduction in albuminuria, improved renal function, and decreased end‐stage renal disease and it is even associated with reductions in CV events. 6 , 7 , 8 , 9 , 10
PCCs typically are the first point of contact for patients with hypertension, diabetes, and kidney disease, those most at risk for target organ damage and most likely to have elevated UAE. Thus, proper identification and treatment of elevated UAE in these patients is important in the primary care setting. This article reviews the risks associated with increased UAE, provides a primer on the pathophysiology of albuminuria, reviews simple methods for detection and monitoring UAE, and includes a screening and treatment algorithm that can easily be implemented by PCCs in order to enhance care of the at‐risk patient.
Albuminuria, Microalbuminuria, Macroalbuminuria: What is the Difference?
Albumin is a negatively charged, water‐soluble molecule produced in the liver. This approximately 69‐kDa globular protein is the most abundant plasma protein and is involved in supporting oncotic pressure and blood volume. In healthy persons, small amounts of albumin (<30 mg/24 h) are excreted into the urine daily. Albuminuria is simply defined as an abnormal amount of albumin in the urine, with the level of albumin having been loosely defined as either microalbuminuria or macroalbuminuria for the past 2 decades (Table I). 9 The terms micro and macro have nothing to do with the size of the particles excreted, but rather to the amount of albumin excreted, as all albumin molecules are the same size. Microalbuminuria is the presence of persistent UAE in the range of 30 mg/24 h to 299 mg/24 h, demonstrated on ≥2 occasions in a first morning urine sample in the absence of certain stressors (see Confounding Factors), or urinary albumin:creatinine ratio (UACR) 30 μg/mg to 299 μg/mg. Macroalbuminuria is defined as UAE ≥300 mg/24 h or UACR ≥300 μg/mg. The National Kidney Foundation’s (NFK’s) Kidney Disease Outcomes Quality Initiative (K/DOQI) recommends classification based on UACR rather than UAE, as the former corrects for variations in urine concentration due to hydration. 11 The predictive value of UACR appears to be valid despite variability in creatinine excretion due to age, sex, race, and body size. 11
Table I.
Definitions of Albuminuria
| Normal | Microalbuminuria | Macroalbuminuria | |
|---|---|---|---|
| Urinary albumin excretion, mg/24 h | <30 | 30–299 | ≥300 |
| Spot collection, urinary albumin‐creatinine ratio, μg/mg | <30 | 30–299 | ≥300 |
Adapted from the American Diabetes Association. 9
The distinction between microalbuminuria and macroalbuminuria is more than academic. Whereas the glomerulus tolerates small volumes of albumin passage through the basement membrane with little recognized consequence (microalbuminuria), the heavy trafficking of these large, highly charged molecules both reflects and induces substantial glomerular damage, ultimately resulting in glomerular dysfunction and demise. The end result of continued macroalbuminuria appears to be progressive decline in renal function, eventually leading to end‐stage renal disease. When early elevation of UAE is identified (microalbuminuria), it is often possible to stop, and even reverse, renal function decline. However, by the time macroalbuminuria occurs, treatment can only slow the process of declining renal function as it cannot be fully forestalled. Hence, early identification, preferably before the advent of macroalbuminuria, is paramount.
PCCs commonly assess urine protein by dipstick urinalysis. The absence of protein on routine dipstick urinalysis is not sufficient evidence that important levels of elevated UAE are absent. Indeed, typical office urinalysis dipsticks are designed to detect UAE only at levels of ≥500 mg/24 h (ie, patients who are already experiencing macroalbuminuria). Point‐of‐care UAE devices specifically designed to detect microalbuminuria are inexpensively available.
The degree of elevation of UAE is associated with different risks to end organs. Microalbuminuria is more predictive of reaching CV end points than kidney end points. Conversely, macroalbuminuria is more predictive of reaching kidney end points. 12 Microalbuminuria occurs 9 times more often than macroalbuminuria in stages 1 and 2 chronic kidney disease (CKD). In stage 4 CKD, macroalbuminuria is more common than microalbuminuria (42% vs 24% of patients). 2 Equally important is the observation that even levels of albumin below the microalbuminuria threshold (so‐called “high normal”) are associated with increased risk for CV outcomes. 13 This finding is similar to our understanding of the relationship between blood pressure (BP) and risk: albuminuria is a continuous and linear variable with no specific cutoff. While no literature guidance is available to suggest optimum management of UAE levels below the traditionally established normal cutoff (<30 mg/24 h), prudence would indicate periodic follow‐up of such individuals.
Albuminuria: Why Should Primary Care Physicians Bother?
The presence of albumin in the urine as a strong marker of CV and kidney risk has been understood for some time. The Multiple Risk Factor Intervention Trial (MRFIT), which enrolled 12,866 middle‐aged men between 1973 and 1975, established a correlation between macroalbuminuria (as detected by office dipstick testing) and increased risk of CV death (Figure 1). 14 Macroalbuminuria was associated with a 2‐ to 3‐fold increased risk of death. The Heart Outcomes Prevention Evaluation (HOPE) study, which followed 9043 patients aged 55 years and older with a history of CV disease or diabetes and an additional CV risk factor, found that any level of albuminuria is a CV risk marker, even at levels well below the defined microalbuminuria threshold (Figure 2). 15 As depicted, the risk for CV events begins to increase linearly and continuously at levels of albuminuria well below microalbuminuria. In the HOPE study, the presence of baseline microalbuminuria predicted subsequent progression to macroalbuminuria 17‐fold. 8 A growing body of evidence suggests that albuminuria is an independent risk factor that has additional prognostic value when used in concert with traditional risk‐assessment tools (eg, Framingham Risk Score). 16 , 17 , 18 , 19
Figure 1.
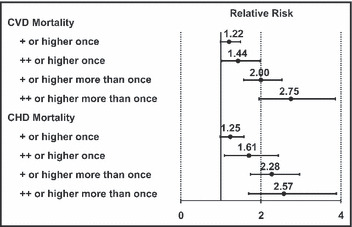
The relative risk of death by levels of proteinuria determined by positive urine protein dipstick (Ames Labstix, Elkhart, IN). The relative risk was established against patients without proteinuria and adjusted for age, cholesterol, diastolic blood pressure, smoking, antihypertensive use, change in glomerular filtration rate, study group, and race. With permission from Grimm and colleagues. 14 CVD indicates cardiovascular disease; CHD, coronary heart disease.
Figure 2.
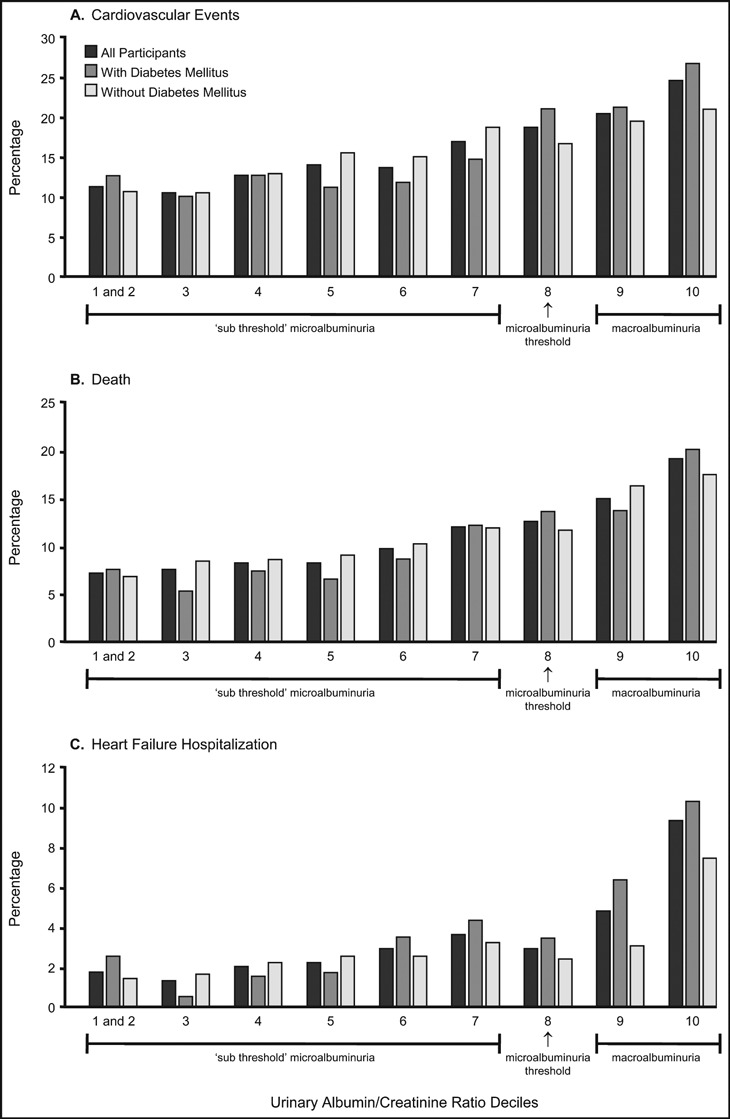
The risk for cardiovascular events begins to occur even at levels of microalbuminuria well below the current cutoff definition: resultsfrom the Heart Outcomes Prevention Evaluation (HOPE) trial. In this study, the eighth decile was considered the cutoff for microalbuminuria (urinaryalbumin‐creatinine ratio of approximately 17.7 lg / mg). Adapted with permission from Gerstein and colleagues. 15
Back to Basics: Physiology of the Kidney
The kidney is the only organ that has a dual capillary/filtration system, the primary system being the glomerulus and the secondary being peritubular (Figure 3). The glomerular filtration barrier (GFB) separates the blood (flowing in the glomerular capillaries) from the urine (filtered into Bowman’s space) and retains plasma macromolecules in the blood based on size, shape, and charge, allowing the passage of small molecules but almost completely restricting the passage of molecules the size of albumin or larger (Figure 4). 20 , 21 The GFB prevents leakage of molecules, including albumin, out of the blood and into urine by 3 layers through which molecules must pass sequentially. Each layer has characteristics that selectively restrict particles based on size, configuration, and/or chemical charge. Smaller plasma proteins can readily pass through the GFB; however, because they are much less abundant than albumin, the filtered load is small. Additionally, with healthy renal function, smaller proteins and any albumin that are able to make it into the glomerular filtrate are then reabsorbed/degraded as they pass through the proximal tubules (Figure 3). 21 Given these multiple and complex filtering mechanisms, in the absence of renal disease (or some transient stressors) only very small amounts of albumin (<30 mg/24 h) are excreted into the urine.
Figure 3.
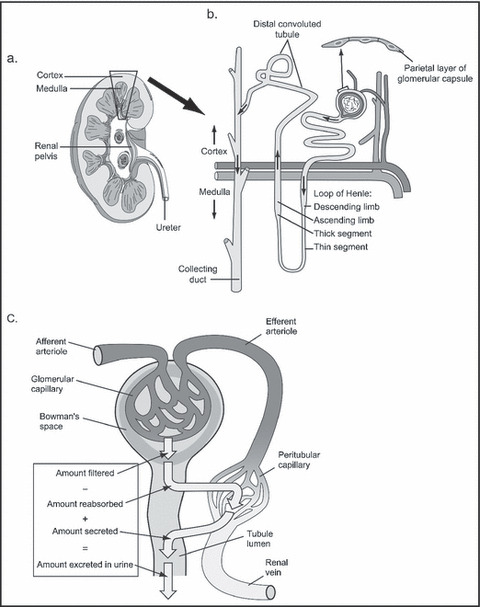
Normal physiology of the kidney. (a) Vertical section of right kidney showing cortex and medulla. Inset (b) diagrammatic representation of a nephron within the cortex and medulla. (c) Diagrammatic representation of renal circulation showing dual capillary beds allowing filtration and reabsorption.
Figure 4.
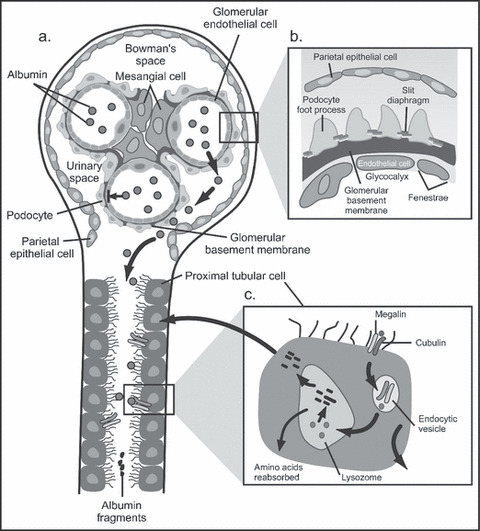
Normal processing of albumin by the kidney. (a) Normal glomerulus and proximal tubule. Albumin (spheres) normally is retained withinthe glomerular capillaries and does not enter Bowman's space. Inset (b) shows the components of the glomerular filtration barrier. (c) Albumin thatis filtered by the glomerulus and enters Bowman's space is taken up by the megalin / cubulin receptor lining of the proximal tubular brush bordercells; from there albumin fragments are either reabsorbed or secreted back into the tubular lumen. Adapted with permission from Jefferson andcolleagues. 21
Pathophysiology of Microalbuminuria
The pathophysiologic mechanisms resulting in persistent microalbuminuria are not completely understood and may reflect a number of mechanisms. Defects in any of the layers of the GFB could be suspect, as well as failure of the tubulointerstitium to perform appropriate resorption of molecules that have slipped through the GFB. 21 Conditions that are associated with endothelial dysfunction (eg, diabetes, hypertension, vascular disease, inflammation, and insulin resistance) also may manifest as glomerular endothelial dysfunction (ie, altered permeability), such that microalbuminuria reflects a state of impaired vascular function, with the kidney being a window to the vasculature in other tissue compartments. 22 Additionally, there is a genetic predisposition (expressed on podocytes) for some patients to develop albuminuria. 23 In any case, vasculopathy manifested as albuminuria appears to reflect vasculopathy diffusely throughout the body and is especially pertinent to the cardiac vasculature.
Testing for Albuminuria
Test Methods
Urinary albumin is a more sensitive marker for CKD due to diabetes, glomerular disease, and hypertension than is total urinary protein excretion. 24 “Standard” office dipstick urinalysis tools are not useful for detecting microalbuminuria because they detect total protein in the urine only at high levels (>500 mg/24 h). Both the NFK and the American Diabetes Association recommend using a microalbumin‐sensitive dipstick or UACR in a spot urine sample for screening adults at risk for CKD and UACR for monitoring adult patients with CKD. 9 , 24 First morning urine samples are preferred: timed or 24‐hour urine samples are not necessary for screening and are much more cumbersome for patients.
There are a number of inexpensive easy‐to‐use methods for testing UAE in the primary care clinic that provide point‐of‐care results (Table II). The advantage of point of care testing is early identification of microalbuminuria, while it is still reversible, and the ability to modify treatment based on immediately available results. The authors believe that each of the currently available methodologies for urinary albumin screening provides a major step forward in promptly identifying elevated UAE. At the time of writing this communication, testing costs <$10 per test with the dipstick methodologies but insurance reimbursement has been erratic.
Table II.
In‐Office Albumin Tests
| Product Name | Test Description |
|---|---|
| HemoCue albumin 201 system (HemoCue, Angelholm, Sweden) | Urine is drawn into a reagent‐lined microcuvette and read in analyzer in 90 s; range of albuminuria detection is 7–150 mg/L |
| Chemstrip Micral test strips (Roche Diagnostics, Indianapolis, IN) | 1‐Min dipstick reagent strip; color match result |
| Clinitek microalbumin reagent strips (Siemens Medical Solutions, Deerfield, IL) | Strips have 2 reagent areas (albumin and creatinine) making UACR calculation possible; used in Clinitek analyzers; hard copy report provided; results in 1 min |
| ImmunoDip test for microalbuminuria (Diagnostic Chemicals, San Diego, CA) | 3‐Min dipstick in a patented housing that allows for recording patient identification; color match result |
Abbreviation: UACR, urinary albumin‐creatinine ratio.
Confounding Factors
UAE can be elevated in certain conditions (eg, dehydration, hyperglycemia, systolic heart failure, and inflammatory states). 25 , 26 , 27 It also is transiently increased as a result of strenuous exercise and during fever/infection. 28 For example, 30 minutes following maximal exercise on a stationary bicycle, UAE is increased 20‐fold from baseline in men aged 20 to 30 years and 3‐ to 7‐fold in older men. 29 UAE is increased during the febrile period but declines significantly 2 days postnormalization of body temperature. 30 Interestingly, asymptomatic urinary tract infections are not associated with albuminuria, whereas symptomatic urinary tract infections are commonly associated with albuminuria. 31 Consequently, to confirm the diagnosis of clinically significant microalbuminuria, clinicians should always ascertain elevated UAE measurements by rechecking a subsequent first morning urine sample, taking care that no confounding factors influence the UAE levels and obtaining at least 2 test results on separate occasions.
Ruling Out Other Disease States that Could Be Causing Albuminuria
Clinical experience suggests that all patients with hypertension, CKD, or diabetes should have UAE assessed at least annually. Because albuminuria can be caused by conditions other than hypertension, CKD, or diabetes, consideration of other disorders, such as immunoglobulin A nephritis is appropriate for patients with symptoms or signs that suggest consideration of an alternative diagnosis (Table III). 32 , 33
Table III.
Differential Diagnoses in the Etiology of Albuminuria in Patients With Hypertension, Diabetes, or Evidence of Target Organ Damage
| Diagnosis | Concurrent Conditions | Further Workup |
|---|---|---|
| Diabetic nephropathy | Retinopathy | HgbA1c |
| Neuropathy | Ophthalmology examination | |
| Duration of diabetes | Refer to nephrologist if albuminuria worsens or renal function declines | |
| Hypertensive nephrosclerosis | Typically <1 g/d proteinuria No other UA abnormalities | Refer to nephrologist if albuminuria worsens of renal function declines |
| Focal segmental glomerulosclerosis | Viral infection (HIV, parvovirus) | HIV antibody test |
| Bisphosphonate use | ||
| Minimal change disease (much less likely diagnosis in adults) | Chronic urinary reflux | |
| Obesity | ||
| Glomerulonephritis | Hematuria | ANA |
| Autoimmune disease (rash, joint pain) | dsDNA C3, C4 | |
| Hepatitis | Rheumatoid factor | |
| Vasculitis | Hepatitis C antibody | |
| IgA nephropathy (recent URI?) | Serum cryoglobulins c‐ANCA, p‐ANCA | |
| Membranous glomerulopathy | Hepatitis B/C | Hepatitis B surface antigen |
| Syphilis | RPR | |
| Malignancy | ||
| Drugs (Gold/penicillamine/NSAID) | ||
| Lymphoproliferative disease (multiple myeloma, amyloid) | Bone pain + lytic lesions | Urine protein:creatinine ratio |
| Hypercalcemia | Urine/serum protein immunoelectrophoresis Bone marrow/fat pad biopsy | |
| Polycystic kidney disease | Family history | Renal ultrasound |
| Hematuria |
Abbreviations: ANA, antinuclear antibodies; c‐ANCA, classical antineutrophil cytoplasmic antibodies; dsDNA, double‐stranded DNA; HgbA1c, hemoglobin A1c; HIV, human immunodeficiency virus; IgA, immunoglobulin A; NSAID, nonsteroidal anti‐inflammatory disease; pANCA, protoplasmic‐staining antineutrophil cytoplasmic antibodies; RPR, rapid plasma reagin; UA, urinary albumin; URI, urinary infection.
Diabetes is the most common cause of albuminuria and the leading cause of end‐stage renal disease in the United States. Diabetic nephropathy is characterized by hypertension, progressive albuminuria, and glomerulosclerosis, and can lead to a decline in glomerular filtration and ultimately to end‐stage kidney disease if untreated. Renal biopsy is the gold standard for evaluating glomerular and tubular structure to establish the etiology of albuminuria, but is usually reserved for patients in whom a nondiabetic cause is suspected. In diabetics, because vascular basement membrane damage in the glomerulus usually occurs at the same time vascular damage in the retina is evolving (diabetic retinopathy), the absence of eye findings should direct consideration of other etiologies for albuminuria than simple diabetes. Findings that suggest a nondiabetic etiology for persistent albuminuria in a patient with diabetes include: (1) lack of retinopathy, (2) lack of autonomic neuropathy, and (3) presence of albuminuria at the time of diagnosis of diabetes (ie, albuminuria is more commonly seen in long‐standing diabetes, rather than at the time of initial diagnosis). 32
In patients with diabetes, differentiating between diabetes and less common etiologies of albuminuria requires a workup that can be initiated in the primary care setting. A careful patient history and physical examination may provide information that suggests alternative causes: rashes (autoimmune or systemic inflammatory disease), thrush (immunocompromised patients), or findings of liver disease (eg, ascites, splenomegaly) that may be associated with viral hepatitis. Initial laboratory testing should include a routine urinalysis with microscopic examination. The hematuria, pyuria, or casts may indicate either a glomerular or interstitial nephritis. Complete metabolic profile is appropriate to provide estimated glomerular filtration rate, creatinine (for CKD), and electrolytes. Complete blood cell count, liver function tests, and hemoglobin A1c level assessments should also be performed. In patients with nephrotic range albuminuria (urine albumin to creatinine ratio >3000 mg/g) or otherwise abnormal urinalysis, a serologic evaluation should include human immunodeficiency virus (HIV) antibody test (to assess for possible HIV‐associated nephropathy), hepatitis B surface antigen, and hepatitis C antibody because either viral hepatitis can cause glomerulonephritis. Evidence also exists that hepatitis C is independently associated with albuminuria in older adults. 34 An autoimmune workup including serum antinuclear antibody, antidouble strand DNA, complement 3, complement 4, and rheumatoid factor may help establish the diagnosis of systemic lupus erythematosus. Albuminuria is a universal finding in lupus nephritis, which occurs in almost 25% of patients with systemic lupus erythematosus. Other rare causes of albuminuria/proteinuria include paraproteinemias such as myeloma and amyloidosis. Supranormal serum total protein, especially when the serum albumin level is low, is indicative of excessive plasma globulin. In this setting, serum protein electrophoresis and 24‐hour urine monitoring for protein electrophoresis and immunofixation are indicated to exclude multiple myeloma or other serious paraproteinemic states. Patients with abnormalities on these tests should be considered for referral to definitive diagnosis and treatment.
Autosomal dominant polycystic kidney disease is a relatively common cause of CKD and can present with hypertension and albuminuria. Family history and ultrasound examination of the kidney that reveals multiple bilateral cysts strongly suggests the presence of this condition.
In general, referral to a nephrologist for further evaluation should be considered whenever the PCC desires a second opinion on the etiology of albuminuria. Patients with abnormal laboratory data, as listed above, or deviation from the typical clinical course of diabetic nephropathy are more likely to have a nondiabetic etiology for albuminuria.
Failure to decrease proteinuria after treatment with an angiotensin‐converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) does not distinguish between the various proteinuric diseases but may be an indication of progressing renal disease that requires the involvement of a nephrologist. Patients with albumin excretion in the range of nephrotic syndrome and patients with stage 4 kidney disease should be referred promptly to a nephrologist for further evaluation. These groups may require more specialized testing and are more likely to develop complications that require further management considerations (eg, severe edema, anemia, hyperparathyroidism).
Treatment Algorithm for Managing Albuminuria
An algorithm for evaluating and managing albuminuria is depicted in Figure 5. Optimizing risk factor management by minimizing other risk factors for CV and kidney disease goes hand in hand with addressing microalbuminuria (Figure 6). Indeed, controlling these risk factors is the first line of microalbuminuria management in patients with glomerular filtration rates >60 mL/min. 35
Figure 5.
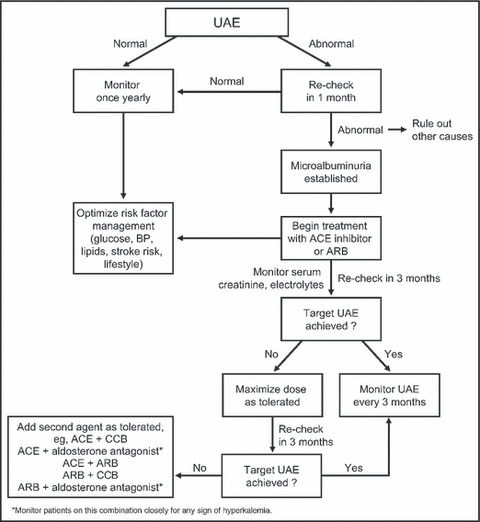
An algorithm for screening and treating albuminuria in the primary care setting. UAE indicates urinary albumin excretion; BP, blood pressure; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, nondihydropyridine calcium channel blocker (verapamil or diltiazem).
Figure 6.
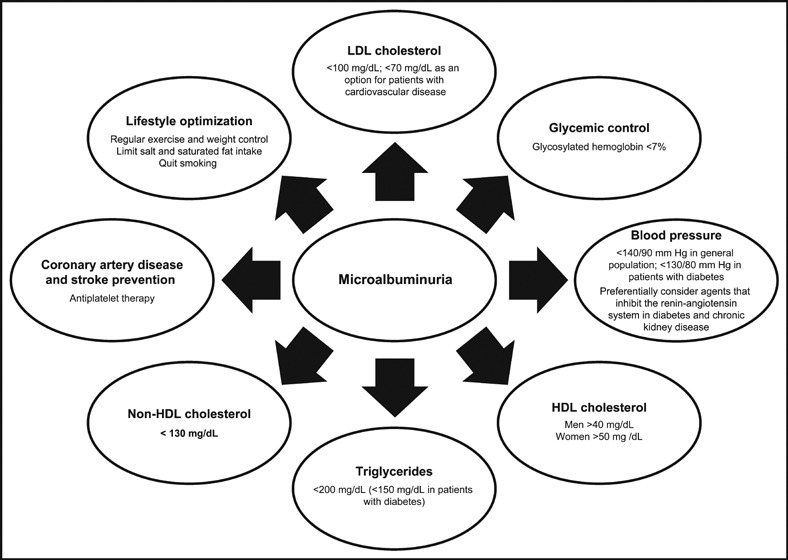
Optimization of cardiovascular and kidney risk factors in patients with microalbuminuria: hypertension and diabetes recommendations. 9 , 36 LDL indicates low‐density lipoprotein; HDL, high‐density lipoprotein.
Agents that block the RAAS (specifically ACE inhibitors and ARBs) are the cornerstone of therapy for the hypertensive patient with CKD or diabetes. 36 While other antihypertensives also can reduce microalbuminuria, a large body of outcomes data supports the efficacy of ACE inhibitors and ARBs in this regard. As a result, these agents are preferentially recommended by the American Diabetes Association for the treatment of patients with type 1 or type 2 diabetes, hypertension, and microalbuminuria or macroalbuminuria, given their ability to delay progression to worsening albuminuria and kidney function. 9 Similarly, the NFK considers them preferred agents based on their ability to reduce albuminuria and slow progression of both diabetic and nondiabetic kidney disease 37 as demonstrated in at‐risk microalbuminuric patients in the HOPE study. 7
There are few head‐to‐head trials to distinguish one ACE inhibitor or ARB from another for use in patients with diabetes and elevated UAE. A single randomized, double‐blind, parallel‐group trial compared the use of telmisartan 80 mg with losartan 100 mg/d for a year in 860 patients with diabetic nephropathy. 38 Limited additional antihypertensive medication could be given to achieve the BP target <130/80 mm Hg. At the end of the study, there was a significantly greater reduction (P=.04) in geometric mean UACR (35.5%; from 1426.1 mg/g creatinine at baseline to 952.5 mg/g creatinine; P<.0001) in the telmisartan‐based regimen than in the losartan‐based regimen (27.0%; from 1390.5 mg/g creatinine to 1054.9 mg/g creatinine; P<.0001). The results of the trial demonstrated that in patients with diabetic nephropathy, a telmisartan‐based regimen was superior to a losartan‐based regimen for reducing albuminuria, given similar levels of BP reduction at 1 year. 38
Aldosterone blockers (spironolactone and eplerenone) also reduce microalbuminuria, although there are far fewer studies demonstrating their efficacy in this regard than there are for ACE inhibitors and ARBs. 39 Direct renin inhibitors represent the newest class of RAAS inhibitors, but only one agent in this class has been approved to date (aliskiren). The limited data available to date regarding aliskiren efficacy in microalbuminuria reduction is promising. 40 , 41
Initiating Therapy With an ACE Inhibitor or ARB
In patients in whom BP is controlled on maximal doses of another antihypertensive but microalbuminuria is present, an ACE inhibitor or ARB should be added to therapy. To lessen the risk for hypotension and other potential tolerability concerns, therapy should be initiated at a very low dose (eg, ramipril 1.25 mg daily, telmisartan 20 mg) with consideration to tapering other antihypertensives. Doses of the ACE inhibitor/ARB can be doubled at 2‐week intervals to the maximally tolerated dose.
In patients not receiving antihypertensive therapy, a low dose of an ACE inhibitor or ARB also should be employed as initial therapy, particularly in patients with glomerular filtration rates ≤60 mL/min. 35 Doses should be titrated upward at 2‐week intervals as tolerated. Table IV lists doses of ACE inhibitors and ARBs recommended by the NFK. 37 No ceiling doses have been established for the treatment of microalbuminuria. Since there is little difference in tolerability throughout the dosing range of ACE inhibitors and ARBs, and there may be additional benefit to higher doses of either class, clinicians may consider using the highest tolerated doses of either. Some time may be needed for albuminuria reductions to become maximal on a given agent; 42 therefore, an adequate period is necessary (clinical experience suggests 3 months) before changing therapies, titrating dose, or adding on other agents.
Table IV.
Usual Dose Ranges of ACE Inhibitors and ARBs for the Treatment of Hypertension
| Agent | Usual Total Daily Dose Range, mg | Frequency Per Day |
|---|---|---|
| ACE inhibitors | ||
| Benazepril | 20–40 | 1–2 |
| Captopril | 25–150 | 2–3 |
| Enalapril | 10–40 | 1–2 |
| Fosinopril | 20–40 | 1–2 |
| Lisinopril | 20–40 | 1 |
| Moexipril | 7.5–30 | 1–2 |
| Perindopril | 4–8 | 1–2 |
| Quinapril | 20–80 | 1–2 |
| Ramipril | 2.5–20 | 1–2 |
| Trandolapril | 2–4 | 1 |
| ARBs | ||
| Candesartan | 16–32 | 1 |
| Eprosartan | 400–800 | 1–2 |
| Irbesartan | 150–300 | 1 |
| Losartan | 50–100 | 1–2 |
| Olmesartan | 20–40 | 1 |
| Telmisartan | 40–80 | 1 |
| Valsartan | 80–320 | 1 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker. With permission from the National Kidney Foundation. 37
Either an ARB or ACE inhibitor is appropriate for treatment of albuminuria, with no clear advantage of one over the other. Because tolerability issues such as cough, angioedema, or rash may limit use of ACE inhibitors, ARBs would be preferred over ACE inhibitors on that basis. However, there is a larger data base supportive of ACE inhibitors in CV disease risk reduction than there is for ARBs, and ACE inhibitors are currently more economical, thus individual patient circumstance may dictate which therapy is initiated. 43 For albuminuria, the degree of reduction was similar between ACE inhibitors and ARBs in a meta‐analysis comparing the two classes of agents, 44 although recent head‐to‐head studies of ARBs suggest some agents may be better than others in this regard. In a 1‐year study of 860 hypertensive patients with type 2 diabetes and proteinuria, telmisartan 80 mg/d reduced mean urinary albumin‐creatinine ratio to a greater degree than losartan 100 mg/d (30% vs 21%; P=.03) at comparable BP reductions. 38 In another study, albuminuria reductions with telmisartan were comparable with those with valsartan. 45 Thus, if considerable albuminuria reductions do not occur after adequate therapy, it would be reasonable to try a different RAAS blocker before using other pharmacologic therapies.
Monitoring Kidney Function
Early studies with RAAS inhibitors excluded patients with advanced renal insufficiency (creatinine clearance >3.0 mg/dL) on the basis that these agents could further increase serum creatinine or electrolytes (ie, potassium). 46 , 47 However, more recent studies have shown that RAAS inhibitors continue to confer renoprotective benefits in patients with advanced renal insufficiency (serum creatinine >3.0 mg/dL) 48 , 49 and at higher‐than‐usual doses, 42 while infrequently incurring hyperkalemia. Therefore, a preexisting elevated serum creatinine level should not deter clinicians from using ACE inhibitors or ARBs. An increase of up to 30% in serum creatinine within the first few weeks of RAAS blockade is acceptable, provided serum potassium is <5.5 mmol/L. 46 , 50 However, if serum creatinine increases >30% from baseline within the first 2 months of treatment, or if serum phosphorus or potassium exceeds 5.6 mmol/L, the dosage of RAAS inhibitor should be reduced by 50% or discontinued entirely, 46 as this change in serum creatinine or electrolytes may indicate a reduction in kidney function sufficient to interfere with metabolic functions of the kidney. Further increases in serum creatinine are not expected after the first month of ACE inhibitor or ARB therapy with the following exceptions: concomitant diuretic therapy has been initiated or increased, nonsteroidal anti‐inflammatory drug use has been initiated, or the patient has experienced volume depletion by nondiuretic causes (eg, gastroenteritis, dehydration). 46
Clinical experience suggests that BP, serum creatinine, and electrolytes are monitored 2 weeks after ACE inhibitor or ARB initiation, and then 2 weeks after each upward titration to ensure that potential changes in kidney function or unacceptable elevations of potassium, which usually occur promptly, are detected. Once a patient is on a stable dose of ACE inhibitor/ARB, 6‐monthly or annual monitoring of blood urea nitrogen, creatinine, and electrolytes should be sufficient.
Dual RAAS Blockade
The combined use of an ACE inhibitor and ARB was considered a viable option for the treatment of albuminuria, as supported by a meta‐analysis of 49 trials that included more than 6100 patients with microalbuminuria or macroalbuminuria in which the combination was associated with better albuminuria reductions than either ACE inhibitor or ARB monotherapy. 44 Similarly, a few studies observed further microalbuminuria reductions when spironolactone was added to ACE inhibitor or ARB therapy. 39 However, the results of the recent Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) have made physicians apprehensive about prescribing the combination of RAAS inhibitors. In ONTARGET, 25,620 patients at high vascular risk (atherosclerotic vascular disease or diabetes with end‐organ damage) were randomized to receive either telmisartan, ramipril, or the combination for more than 4 years. 51 Although combination therapy had benefits on UACR compared with ramipril alone, it failed to lower CV and kidney events relative to monotherapy and was associated with greater morbidity. 51 , 52 It is important to note that ONTARGET was not powered to detect differences in kidney outcomes. Further, UAE was not assessed annually and the need for dialysis was established arbitrarily, with no predetermined protocol, and the data was evaluated post hoc. 53 Thus, definitive conclusions cannot be made on the basis of ONTARGET. The results of two ongoing studies (Diabetes In Nephropathy Study [VA NEPHRON‐D] and Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints [ALTITUDE]), both of which are powered to assess the role of dual RAAS blockade on kidney outcomes in patients with type 2 diabetes at high risk for CV and kidney events, are eagerly awaited.
The results of the ONTARGET trial present a difficult therapeutic conundrum: how can it be that a combined ACE inhibitor/ARB was associated with an increased incidence of renal end points but also improved proteinuria? 52 Although the full explanation for this observation remains to be confirmed, the authors believe that it is likely that there is a subgroup of patients who, when an ACE inhibitor and an ARB are combined, undergo glomerular hypoperfusion, with subsequent decline in renal function. If indeed this is the explanation for the increased renal end points in ONTARGET, it should still be acceptable to treat patients who achieve insufficient improvements in UAE on monotherapy with dual RAAS inhibition therapy. However, regular monitoring for signs of decline in renal function (monthly until stable, then twice yearly) would be essential, and if serum creatinine levels increase by >30%, dual RAAS inhibition should be discontinued.
Continual Monitoring of UAE
Once patients are on a stable dose of an ACE inhibitor or ARB for 3 months, UAE should be re‐checked. Quarterly UAE assessments work well for patients with or at risk for diabetes because they coincide with glycosylated hemoglobin assessments. If microalbuminuria reduction is stabilized but still above normal on a consistent dose of ACE inhibitor or ARB, a second antihypertensive can be considered. The nondihydropyridine calcium antagonists verapamil and diltiazem have been shown to significantly reduce albuminuria even further when added to RAAS inhibitor therapy. 54 However, in a recent head‐to‐head comparison neither agent was superior in lowering albuminuria in a macroalbuminuric cohort of patients with type 2 diabetes. 55 The diuretic indapamide also significantly reduced albuminuria. 56 , 57 When adding on a second agent, kidney function should be monitored in a similar manner to that used when an ACE inhibitor or an ARB is initiated, as the second agent may have effects that can also lead to glomerular hypoperfusion and hyperkalemia.
Conclusions
Microalbuminuria is an important risk marker for both CV and kidney risks in patients with diabetes, hypertension, and/or CKD. PCCs have the most frequent contact with these at‐risk patients and therefore have the greatest potential to favorably affect their health. UAE can be measured in the physician’s office and should be part of routine annual health assessments among at‐risk persons. PCCs can dramatically improve the utilization of this simple, easy‐to‐use, and inexpensive tool to make a great impact on CV and kidney outcomes. Similar to the use of targets for BP and blood glucose levels, a UAE “goal” can reinforce the status of kidney and CV function and serve as an additional educational tool to encourage risk factor management and medication adherence.
Acknowledgments
Acknowledgments: The authors wish to thank Jacqueline Connor Bailey, PharmD, of Oxford PharmaGenesis, Inc, for editorial assistance in preparing the first draft under the guidance of the authors, creating the Figures and Tables, and styling the manuscript to the journal requirements. All authors reviewed and revised the manuscript critically for intellectual content. All authors approved the final version of the manuscript submitted for publication.
Disclosures:
Louis Kuritzky, MD, has served as an advisor or consultant for Eli Lilly and Company; Takeda Pharmaceuticals North America, Inc; Endo Pharmaceuticals; and Ortho‐McNeil‐Janssen Pharmaceuticals, Inc. He has served as a speaker, or member of a speakers’ bureau, for Eli Lilly and Company; Takeda Pharmaceuticals North America, Inc; Endo Pharmaceuticals; and Ortho‐McNeil‐Janssen Pharmaceuticals, Inc. Drs Toto and Van Buren have no disclosures to declare. This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
References
- 1. Foster MC, Hwang SJ, Larson MG, et al. Cross‐classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med. 2007;167:1386–1392. [DOI] [PubMed] [Google Scholar]
- 2. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 3. Danziger J. Importance of low‐grade albuminuria. Mayo Clin Proc. 2008;83:806–812. [DOI] [PubMed] [Google Scholar]
- 4. Kraft SK, Lazaridis EN, Qiu C, et al. Screening and treatment of diabetic nephropathy by primary care physicians. J Gen Intern Med. 1999;14:88–97. [DOI] [PubMed] [Google Scholar]
- 5. Frazee LA, Samandari S, Tanphaichitr N, et al. Screening for nephropathy and antiangiotensin use among diabetic patients in an academic community medical center. Am J Ther. 2006;13:18–23. [DOI] [PubMed] [Google Scholar]
- 6. Zandbergen AA, Vogt L, de Zeeuw D, et al. Change in albuminuria is predictive of cardiovascular outcome in normotensive patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:3119–3121. [DOI] [PubMed] [Google Scholar]
- 7. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 8. Mann JF, Gerstein HC, Yi QL, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. J Am Soc Nephrol. 2003;14:641–647. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Standards of medical care in diabetes – 2009. Diabetes Care. 2009;32(suppl 1):S13–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makino H, Haneda M, Babazono T, et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30:1577–1578. [DOI] [PubMed] [Google Scholar]
- 11. National Kidney Foundation . KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm .
- 12. de Jong PE, Gansevoort RT, Bakker SJ. Macroalbuminuria and microalbuminuria: do both predict renal and cardiovascular events with similar strength? J Nephrol. 2007;20:375–380. [PubMed] [Google Scholar]
- 13. Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. [DOI] [PubMed] [Google Scholar]
- 14. Grimm RH Jr, Svendsen KH, Kasiske B, et al. Proteinuria is a risk factor for mortality over 10 years of follow‐up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int Suppl. 1997;63:S10–S14. [PubMed] [Google Scholar]
- 15. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 16. Asselbergs FW, Hillege HL, Van Gilst WH. Framingham score and microalbuminuria: combined future targets for primary prevention? Kidney Int Suppl. 2004;(92):S111–S114. [DOI] [PubMed] [Google Scholar]
- 17. Ärnlöv J, Evans JC, Meigs JB, et al. Low‐grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. [DOI] [PubMed] [Google Scholar]
- 18. Sciarretta S, Valenti V, Tocci G, et al. Association of renal damage with cardiovascular diseases is independent of individual cardiovascular risk profile in hypertension: data from the Italy – developing education and awareness on microalbuminuria in patients with hypertensive disease study. J Hypertens. 2010;28:251–258. [DOI] [PubMed] [Google Scholar]
- 19. Cao JJ, Biggs ML, Barzilay J, et al. Cardiovascular and mortality risk prediction and stratification using urinary albumin excretion in older adults ages 68–102: the Cardiovascular Health Study. Atherosclerosis. 2008;197:806–813. [DOI] [PubMed] [Google Scholar]
- 20. Reiser J, Pollak MR. Inherited disorders of podocyte function. In: Brenner BM, ed. Brenner & Rector’s The Kidney, 8th ed. Philadelphia, PA: Saunders, Inc; 2007. [Google Scholar]
- 21. Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. [DOI] [PubMed] [Google Scholar]
- 22. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. [DOI] [PubMed] [Google Scholar]
- 23. Freedman BI, Kopp JB, Winkler CA, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (myh9) are associated with albuminuria in hypertensive African Americans: the HyperGEN Study. Am J Nephrol. 2009;29:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 25. Meigs JB, Nathan DM, D’Agostino RB Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. [DOI] [PubMed] [Google Scholar]
- 26. Mahmud N, McDonald GS, Kelleher D, et al. Microalbuminuria correlates with intestinal histopathological grading in patients with inflammatory bowel disease. Gut. 1996;38:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Figueiredo EL, Leão FV, Oliveira LV, et al. Microalbuminuria in nondiabetic and nonhypertensive systolic heart failure patients. Congest Heart Fail. 2008;14:234–238. [DOI] [PubMed] [Google Scholar]
- 28. Lillehoj EP, Poulik MD. Normal and abnormal aspects of proteinuria. Part I: mechanisms, characteristics and analyses of urinary protein. Part II: clinical considerations. Exp Pathol. 1986;29:1–28. [DOI] [PubMed] [Google Scholar]
- 29. Poortmans JR, Ouchinsky M. Glomerular filtration rate and albumin excretion after maximal exercise in aging sedentary and active men. J Gerontol A Biol Sci Med Sci. 2006;61:1181–1185. [DOI] [PubMed] [Google Scholar]
- 30. Sølling J, Solling K, Mogensen CE. Patterns of proteinuria and circulating immune complexes in febrile patients. Acta Med Scand. 1982;212:167–169. [DOI] [PubMed] [Google Scholar]
- 31. Carter JL, Tomson CR, Stevens PE, et al. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant. 2006;21:3031–3037. [DOI] [PubMed] [Google Scholar]
- 32. Parving HH, Gall MA, Skott P, et al. Prevalence and causes of albuminuria in non‐insulin‐dependent diabetic patients. Kidney Int. 1992;41:758–762. [DOI] [PubMed] [Google Scholar]
- 33. Christensen PK, Larsen S, Horn T, et al. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719–1731. [DOI] [PubMed] [Google Scholar]
- 34. Tsui JI, Vittinghoff E, Shlipak MG, et al. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–1174. [DOI] [PubMed] [Google Scholar]
- 35. Bakris GL. ACE inhibitors and ARBs: are they better than other agents to slow nephropathy progression? J Clin Hypertens (Greenwich). 2007;9:413–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 37. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–S290. [PubMed] [Google Scholar]
- 38. Bakris G, Burgess E, Weir M, et al. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int. 2008;74:364–369. [DOI] [PubMed] [Google Scholar]
- 39. Bomback AS, Kshirsagar AV, Amamoo MA, et al. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211. [DOI] [PubMed] [Google Scholar]
- 40. Parving HH, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. [DOI] [PubMed] [Google Scholar]
- 41. Persson F, Rossing P, Schjoedt KJ, et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73:1419–1425. [DOI] [PubMed] [Google Scholar]
- 42. Hollenberg NK, Parving HH, Viberti G, et al. Albuminuria response to very high‐dose valsartan in type 2 diabetes mellitus. J Hypertens. 2007;25:1921–1926. [DOI] [PubMed] [Google Scholar]
- 43. Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin‐converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 44. Kunz R, Friedrich C, Wolbers M, et al. Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. [DOI] [PubMed] [Google Scholar]
- 45. Galle J, Schwedhelm E, Pinnetti S, et al. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant. 2008;23:3174–3183. [DOI] [PubMed] [Google Scholar]
- 46. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor‐associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. [DOI] [PubMed] [Google Scholar]
- 47. Thorp ML, Ditmer DG, Nash MK, et al. A study of the prevalence of significant increases in serum creatinine following angiotension‐converting enzyme inhibitor administration. J Hum Hypertens. 2005;19:389–392. [DOI] [PubMed] [Google Scholar]
- 48. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. [DOI] [PubMed] [Google Scholar]
- 49. Hou FF, Xie D, Zhang X, et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007;18:1889–1898. [DOI] [PubMed] [Google Scholar]
- 50. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin‐angiotensin‐aldosterone system. N Engl J Med. 2004;351:585–592. [DOI] [PubMed] [Google Scholar]
- 51. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 52. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double‐blind, controlled trial. Lancet. 2008;372:547–553. [DOI] [PubMed] [Google Scholar]
- 53. Sarafidis PA, Bakris GL. Renin‐angiotensin blockade and kidney disease. Lancet. 2008;372:511–512. [DOI] [PubMed] [Google Scholar]
- 54. Bakris GL, Weir MR, DeQuattro V, et al. Effects of an ACE inhibitor/calcium antagonist combination on proteinuria in diabetic nephropathy. Kidney Int. 1998;54:1283–1289. [DOI] [PubMed] [Google Scholar]
- 55. Toto RD, Tian M, Fakouhi K, et al. Effects of calcium channel blockers on proteinuria in patients with diabetic nephropathy. J Clin Hypertens (Greenwich). 2008;10:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Molyneaux LM, Willey KA, Yue DK. Indapamide is as effective as captopril in the control of microalbuminuria in diabetes. J Cardiovasc Pharmacol. 1996;27:424–427. [DOI] [PubMed] [Google Scholar]
- 57. Marre M, Puig JG, Kokot F, et al. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. J Hypertens. 2004;22:1613–1622. [DOI] [PubMed] [Google Scholar]


