Abstract
Objective
To explore relevant mechanisms of miR-139-5p in alleviating the metastasis of non-small cell lung cancer cells (NSCLC) and their resistance against cisplatin.
Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot (WB) assays were carried out to determine the protein levels of miR-139-5p and YAF2, and cisplatin (DDP)-resistant NSCLC cell strains were established. Subsequently, an MTT assay was employed to evaluate the viability of the cell strains, a Transwell assay to evaluate cell invasion activity, and flow cytometry to analyze cell apoptosis rate. Finally, a Western blot assay was carried out to determine the protein levels of P-PI3K and p-p38.
Results
NSCLC tissues showed lower miR-139-5p expression and higher YAF2 expression than paracancerous tissues and human normal lung epithelial cells, and miR-139-5p was related to the prognosis of NSCLC patients. Overexpression of miR-139-5p or knock-down of YAF2 inhibited the proliferation and invasion of NSCLC cells and induced their apoptosis. Additionally, the dual-luciferase reporter assay verified a targeting relationship between miR-139-5p and YAF2. Overexpression of miR-139-5p and knockdown of YAF2 reversed the resistance of A549/DDP cells against DDP, inactivated p38 and Akt proteins, and inhibited the AKT/p38 MAPK signaling pathway. Furthermore, inhibiting the AKT/p38 MAPK signaling pathway with MK2206 resisted the effects of knock-down of miR-139-5p on DDP resistance in NSCLC cells.
Conclusion
MiR-139-5p targetedly regulates YAF2 and mediates the AKT/p38 MAPK signaling pathway to alleviate the metastasis of NSCLC cells and their resistance against cisplatin, which may be a novel target for improving the therapeutic effect on NSCLC.
Keywords: miR-139-5p, YAF2, AKT/p38 MAPK, non-small cell lung cancer, cisplatin
Introduction
Lung cancer, as a malignant tumor with high incidence in the world, is the primary cause of tumor-related death.1,2 Non-small cell lung cancer (NSCLC) is the main pathological category of lung carcinoma, accounting for about eighty percent of all lung carcinoma cases.3 At present, lung cancer is mostly treated by traditional surgery plus radiotherapy and chemotherapy. Although the therapeutic effect on lung cancer patients has improved as the progress of medical technology, there are still many patients with poor prognosis, and the overall 5-year survival rate of patients is not more than 20%.4 The occurrence of lung cancer is a very complicated multi-step process, which is mainly identified through genetic and epigenetic changes, so many lung cancers are relatively insensitive to radiotherapy and chemotherapy.5 Thus, it is pivotal to excavate the precise molecular biological mechanism of lung cancer and further clarify the possible targets for preventing the development of lung cancer to develop effective treatment methods.
In the past dozen years, microRNAs (miRNAs) have been widely studied as a suppressor or oncogene in tumors, which mainly regulate the expression of target genes by binding to the 3ʹUTR of target mRNA.6,7 Reportedly, miRNAs abnormally expressed in many human cancers may be important for the occurrence and metastasis of human tumors, such as NSCLC.8 MiR-139-5p, a miRNA in the 11q13.4 region of human chromosome, has been confirmed to play a crucial part in various tumors.9 For example, one research has revealed that miR-139-5p can inhibit the growth of cervical cancer cells by targeting TCF4,10 and one other study has revealed the strong link between abnormality of miR-139-5p in colon cancer cells and cell invasion and migration.11 In addition, one study has pointed out that miR-139-5p is strongly linked to the resistance of ovarian cancer against cisplatin (DDP).12 However, there has been no research and discussion on the mechanism of miR-139-5p in NSCLC and its relationship with cisplatin resistance. YY1 associated factor 2 (YAF2) protein is a YY1-related factor encoded by YAF2 gene, which has been found to be significantly up-regulated in the process of muscle cell generation and is believed to be closely related to cell apoptosis.13 We found binding sites between miR-139-5p and YAF2 through biological information prediction, so we speculated that miR-139-5p might affect the function of NSCLC cells by regulating YAF2.
In this study, we explored the influences of miR-139-5p on cell proliferation, invasion, and chemical resistance to DDP in NSCLC and related mechanisms, with the goal of providing a new target direction for improving the therapeutic effect on NSCLC.
Materials and Methods
Clinical Data
A total of 95 NSCLC patients (average age of (62.26±4.21) years) admitted to Harbin Medical University Tumer Hospital from March 2015 to January 2017 were enrolled as research objects, and 95 NSCLC tissue specimens and 95 paracancerous tissue specimens were sampled from them during surgery with their permission (Table 1). The inclusion criteria of the participants: Patients confirmed with NSCLC according to pathological diagnosis and those with estimated survival time longer than3 months. The exclusion criteria of them: Patients with severe hepatic or renal dysfunction, patients with other comorbid malignant tumors, patients who had received any related treatment before the experiment, and those with immune system disorder. All patients signed informed consent forms, and this study was performed with permission from the Ethics Committee of Harbin Medical University Tumer Hospital.
Table 1.
Baseline Data [n (%)]
| Factors | NSCLC Patients (n=94) |
|---|---|
| Sex | |
| Male | 55 (58.51) |
| Female | 39 (41.49) |
| Age (year) | |
| ≤62 | 44 (46.81) |
| >62 | 50 (53.19) |
| BMI (kg/m2) | |
| ≤23 | 46 (48.94) |
| >23 | 48 (51.06) |
| Smoking history | |
| Yes | 51 (54.26) |
| No | 43 (45.74) |
| Tumor size | |
| ≥3cm | 31 (32.98) |
| <3cm | 63 (67.02) |
| TNM staging | |
| Stage I–II | 64 (68.09) |
| stage III | 30 (31.91) |
| Differentiation | |
| Low differentiation | 56 (59.57) |
| Medium + high differentiation | 38 (40.43) |
| Lymphatic metastasis | |
| Yes | 37 (36.17) |
| No | 57 (60.64) |
Cell Culturing and Transfection
H1975, A549, PC-9, and SPCA-1, and human normal lung epithelial cell strains (BEAS-2B) (Cell lines were purchased from ATCC) were subjected to 37°C incubation in dulbecco’s modified eagle medium (DMEM; Invitrogen Company, USA) containing 10% phosphate buffer saline (PBS), streptomycin (100 U/mL), and penicillin (100 U/mL) (Invitrogen, Carlsbad, CA, USA) under 5% CO2. Upon reaching 85% confluency in adherent growing, the cells were digested through 25% trypsin, and then incubated in the medium for passage. Subsequently, H1975 and A549 cells were transfected with targetedly inhibited sequence of miR-139-5p (miR-139-5p-inhibitor), miR-139-5p mimic sequence (miR-139-5p-mimics), miR negative control (miR-NC), sequence for knock-down of YAF2 (Si-YAF2), and control sequence (Si-NC) through a Lipofectamine™ 2000 Kit (Invitrogen, CA, USA), separately.
Construction of Drug-Resistant Cell Strains
Drug-resistant cell strains were constructed by increasing the DDP (Sigma) concentration. A549 cells at the logarithmic phase were transferred to a culture solution containing DDP with an initial concentration of 0.5μm. After 48 hours, the solution was replaced with fresh solution, and the cells were digested. After digestion, 1 μm DDP was added to treat the cells for 48 hours. Finally, cell strains tolerant to 10 μm DDP were acquired by changing the solution with gradually increased DDP concentration and named A549/DDP.
RT-PCR
Total RNA was extracted from tissues and cells using the Trizol kit (Invitrogen Company, USA), and then 5 μg total RNA (5 μg) was sampled from the extracted total RNAs, respectively, and reversely transcribed into cDNA under the kit instructions (TaKaRa company, Dalian, China). Subsequently, 1 μL synthetic cDNA was taken for amplification after reverse transcription. The amplification system consisted of 20 μL total volume containing 1 μL cDNA, 0.4 μL upstream primers, 0.4 μL downstream primers (the primer concentration was 0.5uM), 10 μL 2X TransScript® Tip Green qPCR SuperMix, 0.4 μL Passive Reference Dye (50X), and Nuclease-free water added to adjust the volume under 95°C for 60s, followed by 40 cycles 95°C for 40s, and 60°C for 40s. 2-ΔΔcq was employed to analyze data of this experiment with U6 and GAPD as internal references for miR-139-5p and YAF2, respectively. Primer sequences are shown in Table 2.
Table 2.
Primer Sequences
| Factors | Upstream Primer (5ʹ-3ʹ) | Downstream Primer (5ʹ-3ʹ) |
|---|---|---|
| MiR-139-5p | GCCTCTACAGTGCACGTGTCTC | CGCTGTTCTCATCTGTCTCGCGC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| YAF2 | GTTACTGGGACTGTAGCGTCT | TTCCGCACATCGCACATCAT |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
MTT Assay
The cells transfected for 24 h were collected according to the MTT kit instructions (Beyotime Biotechnology Co., Ltd., Shanghai, China), and seeded into a 96-well plate at 4*103 cells/well. Then, the plate was put with 20μL MTT solution (5 μ mg/mL) at 0, 24, 48, and 72 h after 37°C incubation, separately, and then the cells were incubated at the same temperature for 4 h. Each well was added with 200 μL dimethyl sulfoxide, and the optical density of cells from different groups at 570 nm was measured via a spectrophotometer.
Evaluation of Drug Resistance of Cells
Transfected cells were transferred to 96 well plates at 1x105 per well, and the plates were added with 2 mL 0 μmol/L DDP, 2 mL 1 μmol/L DDP, 2 mL 2 μmol/L DDP, 2 mL 4 μmol/L DDP, 2 mL 8 μmol/L DDP, and 2 mL 12 μmol/L DDP, respectively, followed by 48 h incubation. After incubation, the medium was replaced with fresh medium, and 10 μL MTT solution was added into each well, followed by incubation for 2 h. Subsequently, the OD of each well at 570 mm was detected using a SpectraMax M5 microplate reader for cell proliferation determination, and the assay was repeated three times. Finally, 50% inhibitive concentration (IC50) of the cells was calculated based on the cell survival.
Transwell Assay
After 24 hours of transfection, the cells were transferred to a 24-well plate at 3*104 cells/well, trypsinized, and then moved to the upper compartment, followed by the addition of 200 μ l RPMI 1640. The bottom compartment was added with 600 mL RPMI1640 containing 10% FBS. Afterwards, the chamber was cultured at 37°C for48 hours, and the substrates and cells that did not penetrate the membrane were wiped off. The membrane was washed with PBS three times, immobilized through paraformaldehyde for 10 minutes, and rinsed through double distilled water three times again, and it was then stained with 0.5% crystal violet after being dried out naturally. Finally, cell migration was analyzed using a microscope.
Apoptosis Assay
The cells were trypsinized through 0.25% trypsin, and then washed with PBS twice, and added with binding buffer (100μL) to prepare 1*106 cells/mL suspension. The suspension was added with AnnexinV-FITC and PI in order, followed by 5 min incubation with the dark surrounding at indoor temperature, and finally determined using the FC500MCL flow cytometer system. The above assay steps were repeated three times, and the obtained data were averaged.
Western Blot (WB) Assay
Total protein was extracted from collected cells in each group through the radio Immunoprecipitation Assay (RIPA) lysis method (Thermo Scientific, USA), and its concentration was measured using the bicinchoninic acid method (Thermo Scientific, USA), and adjusted to 4μg/μL. After being isolated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the total protein was transferred to a polyvinylidene fluoride membrane, stained with Ponceau’s stain liquid, soaked in phosphate buffer saline with Tween (PBST) for 5 min for washing. Then, the total protein was immersed in 5% non-fat milk for 2 hours, and added with Caspase-3 (1: 500), Bax (1: 500), Bcl-2 (1: 500), p-AKT (1: 500), p-p38 (1: 500), and β-Actin (1: 500) (Cell Signaling Technology), and blocked at 4°Covernight. The membrane was washed to remove the primary antibody, added with secondary antibody (1: 1000) (Abcam Company, USA), cultured at 37°C for 1 hour, rinsed with PBS three times, 5 min each time. A filter paper was used for the membrane to remove excess liquid on it, and then the membrane was made to be luminescent with ECL and developed. The gray value was analyzed.
Dual-Luciferase Reporter (DLR) Assay
The targeted genes of miR-139-5p were predicted using the TargetScan website, and 3ʹ-UTR of YAF2 with miR-139-5p putative binding loci was subcloned into pGL3 luciferase promoter vector after amplification. The vector and miR-139-5p mimics were co-transfected into lung cells, and 48 h later, the cells were acquired and their relative luciferase activity was analyzed through a DLR assay kit (Promega Corporation) under the kit instructions. All experiments were repeated 3 times.
Statistical Analyses
In this study, the obtained data were processed using SPSS20.0 and visualized into required figures using GraphPad 7. Data were compared between groups using the independent t test, and compared among multiple groups using the one-way ANOVA. Post hoc pairwise comparison was conducted using the LSD-t test, and expression comparison at different time points was conducted using the repeated measures analysis of variance, and Bonferroni post hoc test was adopted. P< 0.05 implies a significant difference.
Results
Low miR-139-5p Level in NSCLC
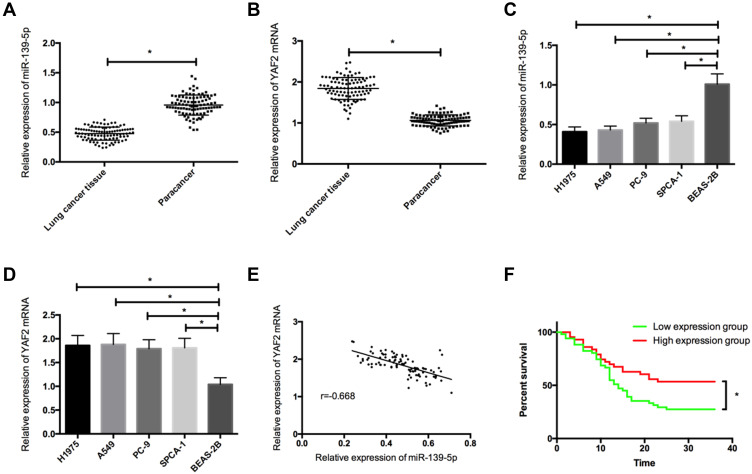
The qRT-PCR assay revealed that NSCLC tissues showed significantly lower miR-139-5p expression and significantly higher YAF2 mRNA expression than paracancerous tissues (both P<0.05), and NSCLC cell strains also showed lower miR-139-5p expression and higher YAF2 expression than normal lung epithelial cells (both P<0.05). Additionally, the analysis on correlation uncovered a negative correlation of miR-139-5p with YAF2 in NSCLC tissues (P<0.05). The patients were assigned to high and low expression groups (n=43 and n=51, respectively) based on the mean miR-139-5p expression (1.83), and the high expression group presented a significantly higher 3-year survival rate than the low expression group (P< 0.05) (Figure 1).
Figure 1.
The expression of miR-139-5p and YAF2mRNA in NSCLC. (A), The expression of miR-139-5p in NSCLC tissues. (B), The expression of YAF2 mRNA in NSCLC tissues. (C), The expression of miR-139-5p in NSCLC cells. (D), The expression of YAF2mRNA in NSCLC cells. (E), Correlation analysis between miR-139-5p and YAF2 in NSCLC. (F), Effects of miR-139-5p on the prognosis of NSCLC patients. *Indicates P<0.05.
Influences of miR-139-5p on the Proliferation, Invasion, and Apoptosis of NSCLC Cells
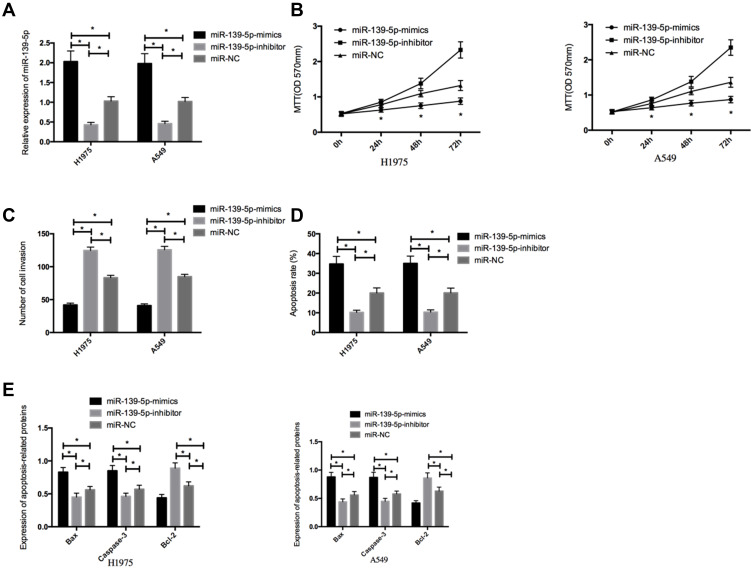
Compared with H1975 and A549 cells transfected with miR-139-5p-mimics, those transfected with miR-NC presented a remarkable up-regulation in miR-139-5p, and those transfected with miR-139-5p-inhibitor showed a remarkable decrease in it (both P<0.05). Additionally, in contrast to the miR-NC group, cells transfected with miR-139-5p-mimics presented significantly decreased proliferation and invasion abilities and significantly increased apoptosis rate, significantly down-regulated Bcl-2, and significantly up-regulated Caspase-3and Bax (all P<0.05), and those transfected with miR-139-5p-inhibitor showed opposite results (all P<0.05) (Figure 2).
Figure 2.
Effects of miR-139-5p on proliferation, invasion, and apoptosis of NSCLC cells. (A), The expression of miR-139-5p in transfected NSCLC cells. (B), Effects of miR-139-5p on proliferation of NSCLC cells. (C), Effects of miR-139-5p on invasion of NSCLC cells. (D), Effects of miR-139-5p on apoptosis of NSCLC cells. (E), Effects of miR-139-5p on apoptosis-related proteins in NSCLC cells. *Indicates P<0.05.
Effects of miR-139-5p on Resistance of NSCLC Cells Against DDP
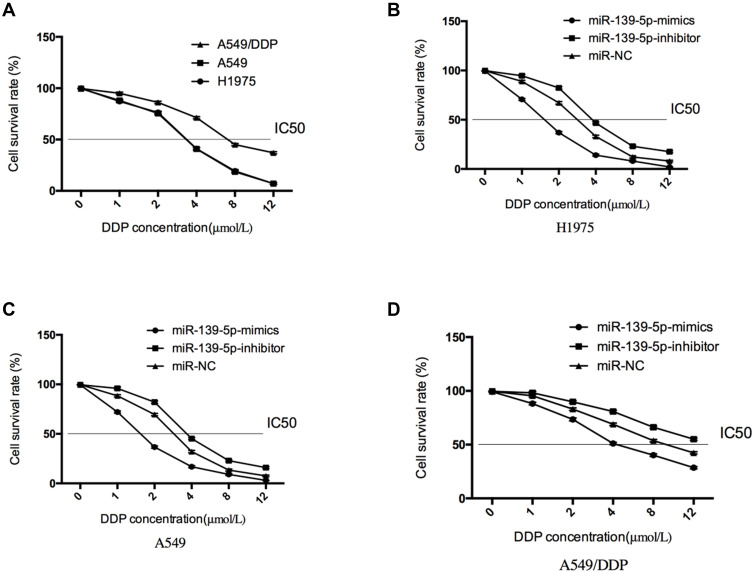
We carried out DDP chemical treatment on H1975, A549, and A549/DDP cells. It came out that DDP strongly suppressed the proliferation of H1975 and A549 cells (both P<0.05), in a dose-dependent way, and the IC50 of DDP against A549/DDP cells was thicker than that against H1975 and A549 cells (P<0.05). Additionally, transfection of miR-139-5p mimics strongly enhanced the sensitivity of H1975 and A549 cells to DDP, reversed the DDP resistance of A549/DDP cells, and reduced the IC50 of DDP against cells (all P<0.05), and transfecting miR-139-5p-inhibitor exerted opposite effects (all P<0.05) (Figure 3).
Figure 3.
Effects of miR-139-5p on the resistance against DDP. (A), IC50 of DDP against H1975, A549, and A549/DDP cells. (B), IC50 of DDP against H1975 cells after regulation of miR-139-5p. (C), IC50 of DDP againstA549 cells after regulation of miR-139-5p. (D), IC50 of DDP against A549/DDP cells after regulation of miR-139-5p.
Targeting of miR-139-5p to YAF2
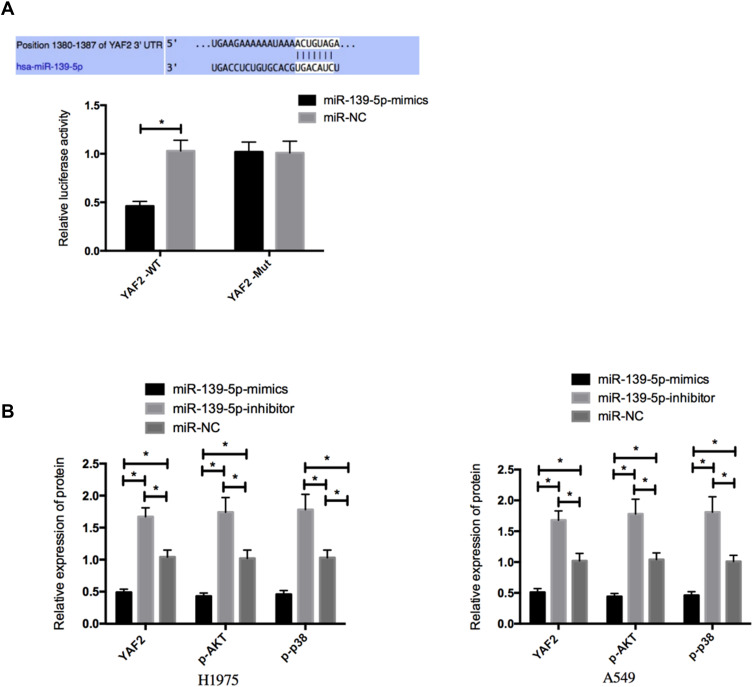
For the purpose of exploring the potential mechanism of miR-139-5p in NSCLC, a bioinformatics analysis was conducted for prediction of the target genes of miR-139-5p. YAF2 was confirmed to be the target gene of miR-139-5p. To verify whether 3ʹUTR of YAF2 can be directly targeted by miR-139-5p, we carried out a DLR assay. It came out that overexpressing miR-139-5p lowered the luciferase activity of YAF23ʹ UTR Wt (P<0.05), but did not exert influence on YAF23ʹ UTR Mut. Moreover, the WB assay uncovered that H1975 and A549 cells transfected with miR-139-5p mimics presented down-regulated YAF2 protein, p-AKT, and p-p38, but NSCLC cells with miR-139-5p-inhibitor presented an up-regulation in them (all P <0.05) (Figure 4).
Figure 4.
Targeting of miR-139-5p to YAF2. (A), DLR assay. (B), Effects of miR-139-5p on the YAF2 protein and AKT/p38 MAPK signaling pathway in NSCLC cells. *Indicates P<0.05.
Influences of Down-Regulating YAF2 on the Growth and DDP Resistance of NSCLC Cells
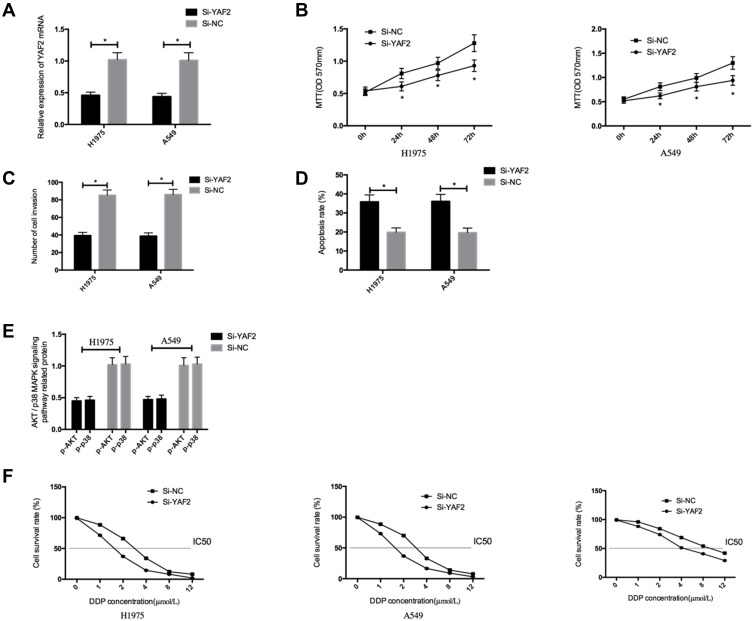
H1975 and A549 cells transfected with Si-YAF2 presented significantly higher YAF2 expression than those with Si-NC (P<0.05). In contrast to the Si-NC group, cells with Si-YAF2 showed significantly weakened cell proliferation and invasion, significantly enhanced apoptosis, significantly down-regulated Bcl-2, p-AKT, and p-p38, and significantly up-regulated Caspase-3 and Bax proteins (all P<0.05), and transfection of Si-YAF2 strongly enhanced the sensitivity of H1975 and A549 cells to DDP, reversed the DDP resistance of A549/DDP cells, and lowered the IC50 of DDP against cells (all P<0.05) (Figure 5).
Figure 5.
Effects of down-regulating YAF2 on proliferation, invasion, apoptosis and DDP resistance of NSCLC cells. (A), The expression of YAF2 in transfected NSCLC cells. (B), Effects of YAF2on the proliferation of NSCLC cells. (C), Effects of YAF2on the invasion of NSCLC cells. (D), Effects of YAF2on the apoptosis of NSCLC cells. (E), Effects of YAF2on the apoptosis-related proteins of NSCLC cells. (F), Effects of YAF2 on the DDP resistance of H1975, A549, and A549/DDP cells. *Indicates P<0.05.
Effects of Inhibiting the AKT/P38 MAPK Signaling Pathway on NSCLC Cells
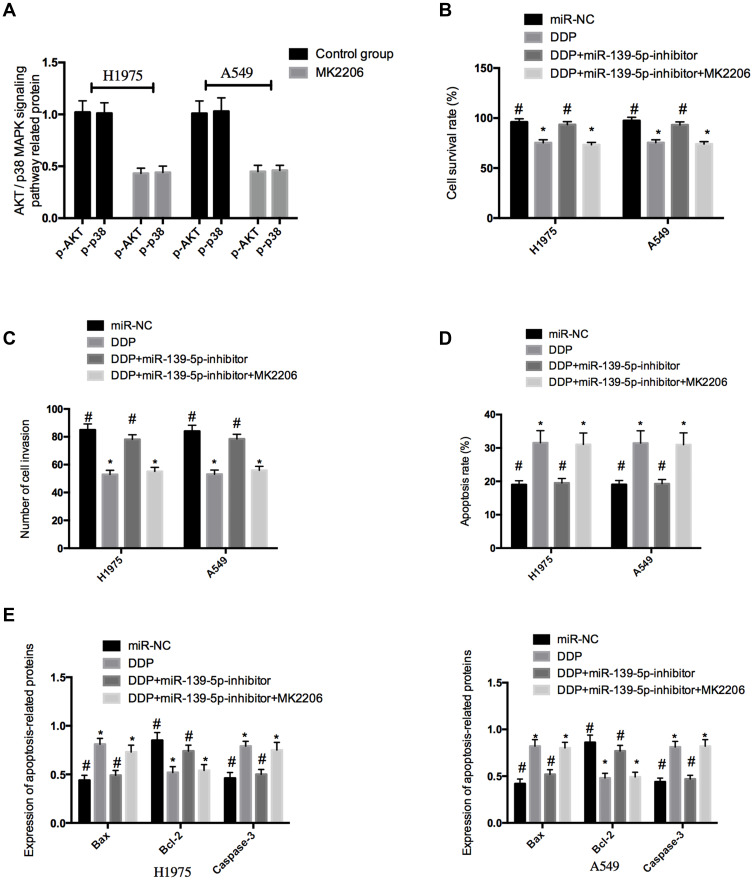
H1975 and A549 cells were treated with 5μL MK2206 (inhibitor of AKT/p38 MAPK signaling pathway), and it was found that compared with H1975 and A549 cells untreated with MK2206, those treated with MK2206 showed significantly down-regulated p-Akt and p-PI3K (both P<0.05). Then, cells transfected with miR-139-5p-inhibitor were exposed to 0.5 μmol/L DDP and then exposed to 5μL MK2206 for 48 h. It came out that miR-139-5p-inhibitor restored the inhibiting influence of DDP on the proliferation and invasion of H1975 and A549 cells, and inhibited apoptosis of the cells induced by DDP, but the inhibition was reversed after the addition of MK2206 (Figure 6).
Figure 6.
Effects of inhibiting the AKT/p38 MAPK signaling pathway on NSCLC cells. (A), Effects of MK2206 on the AKT/p38 MAPK signaling pathway in NSCLC cells. (B), Comparison of cell survival rate. (C), Comparison of cell invasion ability. (D), Comparison of cell apoptosis rate. (E), The expression of apoptosis-related proteins in NSCLC cells. *Indicates P<0.05; #indicates P<0.05.
Discussion
miRNA has been confirmed to be strongly linked to the development and progression of many malignant tumors, and the important role of miRNA in tumors promotes the exploration of miRNA-based targeted therapy programs in turn.14,15 Some NSCLC patients still have unfavorable prognosis due to metastasis and recurrence of NSCLC even after surgery, and chemotherapy based on DDP is usually adopted to further consolidate the curative effect after surgery.16,17 However, the occurrence of resistance to chemotherapy is one of the main causes of treatment failure,18 so how to solve the resistance to chemotherapy is of great clinical value for more effective therapy of patients.
In our research, we firstly quantified miR-139-5p in NSCLC tissues and cells and found its down-regulation in the tissues, which indicated that downregulating miR-139-5p may take an crucial part in the development of NSCLC. One previous study has also revealed the down-regulation of miR-139-5p in NSCLC,19 which is similar to our results. One other previous study has pointed out that miR-139-5p plays the role of anti-oncogene in NSCLC and can regulate the invasion and migration of NSCLC cells by regulating MMP2.20 In addition, miR-139-5p has also been reported to be able to inhibit the viability, migration, and invasion of NSCLC cells by targeting the Hepatoma Derived Growth Factor (HDGF) of the cells.21 All these studies have discussed the possible mechanism of miR-139-5p in NSCLC, but there is no much explanation about its role in chemotherapy resistance of NSCLC and whether there are other targets. Therefore, we further regulated miR-139-5p in NSCLC cells, and it was turned out that up-regulation of miR-139-5p significantly inhibited the proliferation and invasion of NSCLC cells and accelerated their apoptosis, while its down-regulation caused opposite cell phenotypes, which suggested that miR-139-5p was a tumor suppressor gene in NSCLC. One previous study has pointed out that miR-139-5p can suppress the development and progression of esophageal carcinoma through regulating VEGFR and downstream signaling pathways.22 There is also one study showing that miR-139-5p targets HOXA10 transcript and inhibits the growth and migration of endometrial carcinoma cells,23 which also verifies that miR-139-5p acts as a tumor suppressor in tumors. The role of miRNA in chemotherapy resistance has been extensively explored in the past, and miR-139-5p has also been verified to be related to the chemotherapy resistance in various tumors. For example, one study has revealed that miR-139-5p can reverse CD44+/CD133+-related multidrug resistance by downregulating NOTCH1 in colorectal cancer cells.24 Therefore, we explored the correlation of miR-139-5p with DDP resistance of NSCLC cells by establishing a DDP-resistant NSCLC cell model. It came out that overexpressing miR-139-5p could strongly enhance the sensitivity of NSCLC cells to DDP, and could also reverse the DDP resistance of NSCLC drug-resistant cell strains. The above results implied that miR-139-5p might be a promising target for improving DDP-based chemotherapy for NSCLC.
YAF2 is a protein that interacts with transcription factor yin and yang 1 (YY1) during muscle differentiation and development, which can play a pivotal role in regulating p53-mediated apoptosis by interacting with key protein programmed cell death 5 (PDCD5).25,26 Moreover, we found that the expression of YAF2 in NSCLC was abnormally high. Subsequently, we found a targeting relationship between miR-139-5p and YAF2 through website prediction and verified the targeting relationship with a DLR assay. Therefore, we suspected that miR-139-5p might take a part in NSCLC cells. Our results implied that inhibiting the YAF2 expression in NSCLC significantly inhibited the proliferation and invasion of NSCLC cells, intensified their apoptosis, enhanced their sensitivity to DDP, and reversed the resistance of NSCLC drug-resistant cell strains against DDP. One previous study has uncovered that downregulating YAF2 could suppress the development and progression of NSCLC,27 which is similar to our conclusion, but this is the first time that we have found the correlation between YAF2 and NSCLC. Moreover, interestingly, we also acquired that upregulating miR-139-5p or downregulating YAF2 could suppress the activation of the AKT/p38 MAPK signaling pathway. AKT/p38 MAPK signaling pathway, as a classical signaling pathway in tumors, has been recognized to be related to the early cycle arrest and apoptosis of tumor cells.28 One previous study has pointed out that the AKT/p38 MAPK signaling pathway takes part in the development of sensitivity and resistance of NSCLC cells to DDP,29 and our study results also showed that after inhibition on the AKT/p38 MAPK signaling pathway, the sensitivity of NSCLC cells to DDP was enhanced, and the DDP resistance of NSCLC drug-resistant cell strains was also reversed. According to these findings, miR-139-5p hinders the NSCLC cell proliferation and invasion and accelerates cell apoptosis by targetedly affecting the YAF2/AKT/p38 MAPK axis, and can also improve NSCLC cell sensitivity to DDP. However, the downstream mechanisms of miR-139-5p need further exploration.
Conclusion
MiR-139-5p is under-expressed in NSCLC tissues and cells, and YAF2 is up-regulated in them, and miR-139-5p inhibits the progression of NSCLC through targetedly regulating the YAF2/AKT/p38 MAPK axis and alleviates the chemotherapy resistance of it. Therefore, miR-139-5p might become a treatment target of NSCLC. However, there are still some deficiencies in this study. For example, we have not carried out in vivo tumor-forming experiments to verify whether miR-139-5p can regulate the sensitivity of solid tumors to chemotherapy, and we are also not clear about whether miR-139-5p has other targets. We will carry out further basic experiments for the above deficiencies in subsequent studies.
Acknowledgments
This study was financially supported by Haiyan Science Foundation, No. JJQN2018-12; Natural Science Foundation of Heilongjiang Province, No. LH2019H097.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 4.Wood SL, Pernemalm M, Crosbie PA, Whetton AD. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev. 2015;41(4):361–375. [DOI] [PubMed] [Google Scholar]
- 5.Cekaite L, Eide PW, Lind GE, Skotheim RI, Lothe RA. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7(6):6476–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abba ML, Patil N, Leupold JH, et al. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94. [DOI] [PubMed] [Google Scholar]
- 7.Mazza T, Gioffreda D, Fontana A, et al. Clinical Significance of Circulating miR-1273g-3p and miR-122-5p in Pancreatic Cancer. Front Oncol. 2020;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarredar H, Ansarin K, Baradaran B, et al. Critical microRNAs in Lung Cancer: recent Advances and Potential Applications. Anticancer Agents Med Chem. 2018;18(14):1991–2005. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Hu P, Xu F. LINC00152/miR-139-5p regulates gastric cancer cell aerobic glycolysis by targeting PRKAA1. Biomed Pharmacother. 2018;97:1296–1302. [DOI] [PubMed] [Google Scholar]
- 10.Ji X, Guo H, Yin S, Du H. miR-139-5p functions as a tumor suppressor in cervical cancer by targeting TCF4 and inhibiting Wnt/beta-catenin signaling. Onco Targets Ther. 2019;12:7739–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao T, Feng DU, Tingyu LI, Yuanyuan LU, Zhao X. The miR-139-5p regulates proliferation, migration and invasion in KRAS-mutated colon cancer cells. Theranostics. 2017;10:7335. [Google Scholar]
- 12.Jiang Y, Jiang J, Jia H, Qiao Z, Zhang J. Recovery of miR-139-5p in Ovarian Cancer Reverses Cisplatin Resistance by Targeting C-Jun. Cell Physiol Biochem. 2018;51(1):129–141. [DOI] [PubMed] [Google Scholar]
- 13.Hou C, Ma X, Chen H, Huang B, Chen D. YAF2 promotes tumor cell proliferation via targeting cyclin D1 expression. Basic Clin Med. 2018;38(6):764–770. [Google Scholar]
- 14.Wang V, Wu W. MicroRNA-based therapeutics for cancer. BioDrugs. 2009;23(1):15–23. [DOI] [PubMed] [Google Scholar]
- 15.Duz MB, Karatas OF, Guzel E, et al. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. Cell Oncol. 2016;39(2):187–193. [DOI] [PubMed] [Google Scholar]
- 16.Tan W, Liao Y, Qiu Y, et al. miRNA 146a promotes chemotherapy resistance in lung cancer cells by targeting DNA damage inducible transcript 3 (CHOP). Cancer Lett. 2018;428:55–68. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Li X, Ren Y, et al. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019;110(5):1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye L, Pu C, Tang J, et al. Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir Res. 2019;20(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong-Hao Y, Xian-Guo W, Ming X, Jin-Ping Z. Expression and clinical significance of miR-139-5p in non-small cell lung cancer. J Int Med Res. 2019;47(2):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng F, Yu N, Han Y, Ainiwaer J. The long non-coding RNA MIAT/miR-139-5p/MMP2 axis regulates cell migration and invasion in non-small-cell lung cancer. J Biosci. 2020;45(1):1–12. [PubMed] [Google Scholar]
- 21.Zhang Z, Li W, Jiang D, Liu C, Lai Z. MicroRNA-139-5p inhibits cell viability, migration and invasion and suppresses tumor growth by targeting HDGF in non-small cell lung cancer. Oncol Lett. 2020;19(3):1806–1814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Jiao W, Zhang J, Wei Y, et al. MiR-139-5p regulates VEGFR and downstream signaling pathways to inhibit the development of esophageal cancer. Dig Liver Dis. 2019;51(1):149–156. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Li C, Jiang Y, Wan Y, Zhou S, Cheng W. Tumor-suppressor role of miR-139-5p in endometrial cancer. Cancer Cell Int. 2018;18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu K, Shen K, Liang X, et al. MiR-139-5p reverses CD44+/CD133+-associated multidrug resistance by downregulating NOTCH1 in colorectal carcinoma cells. Oncotarget. 2016;7(46):75118–75129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Liu M, Ji H, et al. The polycomb group protein Yaf2 regulates the pluripotency of embryonic stem cells in a phosphorylation-dependent manner. J Biol Chem. 2018;293(33):12793–12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Wilkinson FH, Colavita K, Fennelly C, Atchison ML. YY1 DNA binding and interaction with YAF2 is essential for Polycomb recruitment. Nucleic Acids Res. 2014;42(4):2208–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang XF, Zhao LX, Guo SP, Wei S, Zhai JF, Zhou QH. miR-34b inhibits the migration/invasion and promotes apoptosis of non-small-cell lung cancer cells by YAF2. Eur Rev Med Pharmacol Sci. 2019;23(5):2038–2046. [DOI] [PubMed] [Google Scholar]
- 28.Nagappan A, Lee WS, Yun JW, et al. Tetraarsenic hexoxide induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt suppression and p38 MAPK activation in SW620 human colon cancer cells. PLoS One. 2017;12(3):e0174591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das U, Manna K, Adhikary A, et al. Ferulic acid enhances the radiation sensitivity of lung and liver carcinoma cells by collapsing redox homeostasis: mechanistic involvement of Akt/p38 MAPK signalling pathway. Free Radic Res. 2019;53(9–10):944–967. [DOI] [PubMed] [Google Scholar]